A Case of Pulmonary Fibrosis and COVID-19-Related Pneumonia in a Pembrolizumab-Treated Patient
Abstract
1. Introduction
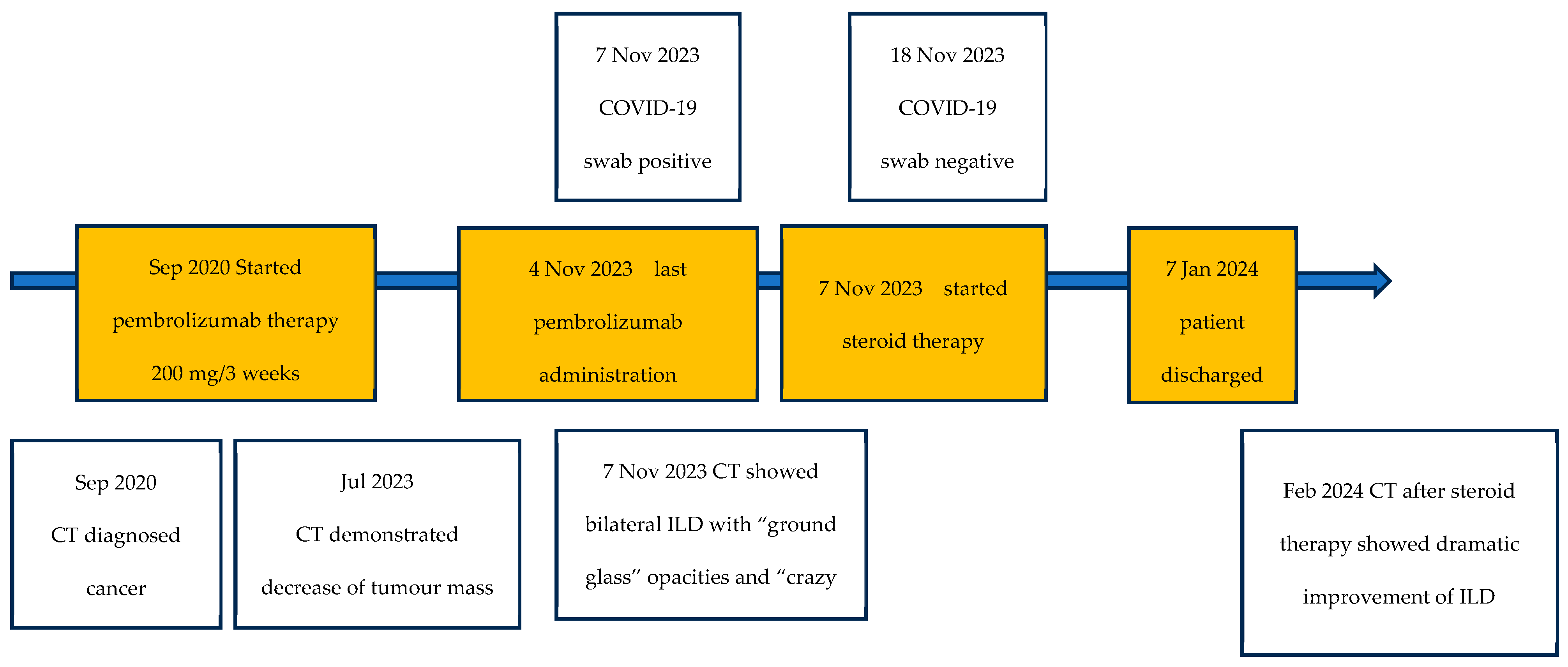
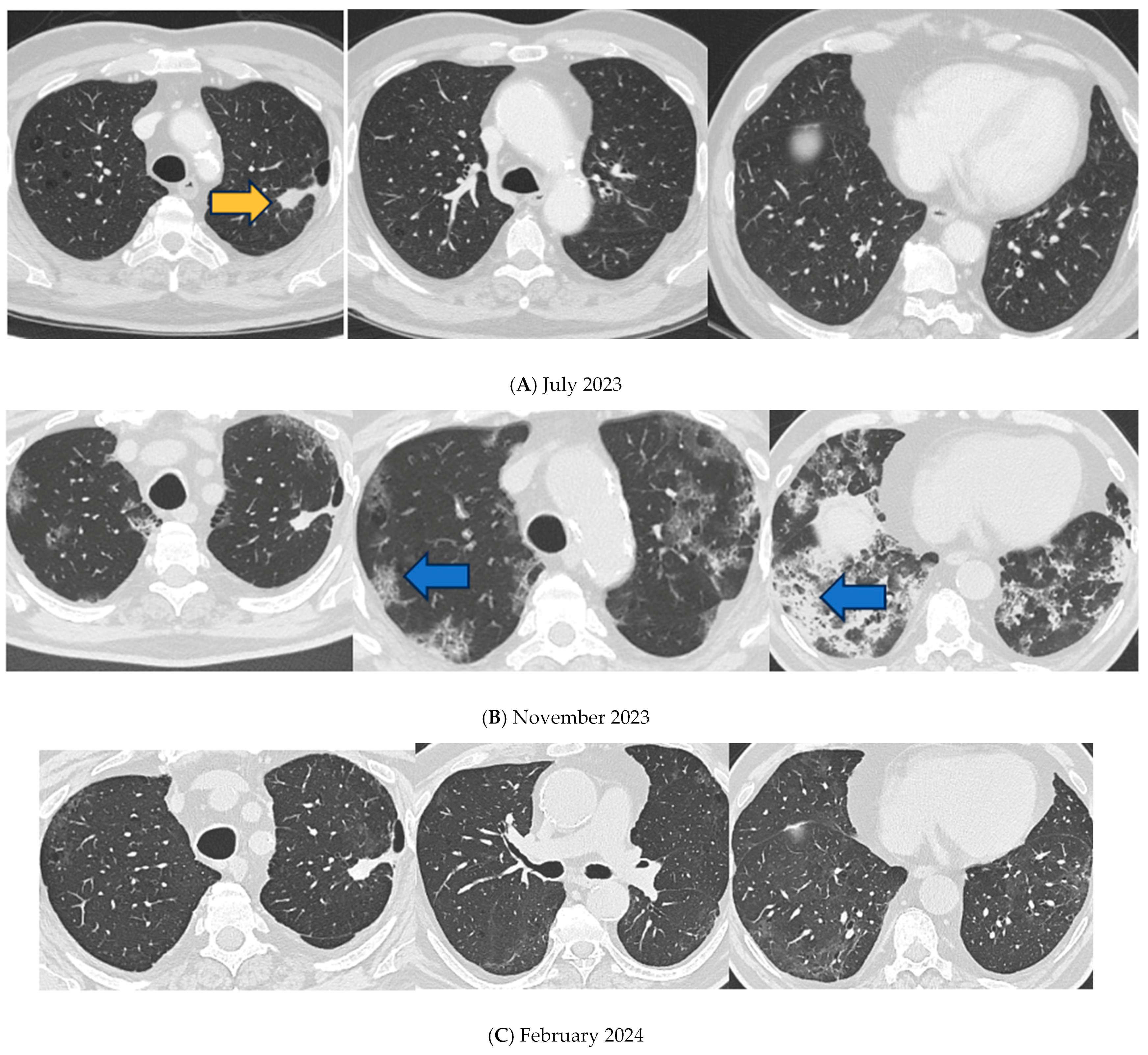
2. Detailed Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | Acute respiratory distress syndrome |
| CIP | Check-point inhibitor-related pneumonitis |
| COVID-19 | Coronavirus disease 2019 |
| CRS | Cytokine release syndrome |
| CT | Computed tomography |
| ICIs | Immune checkpoint inhibitors |
| IFN | Interferon |
| IL-6 | Interleukin-6 |
| ILD | Interstitial lung disease |
| irAEs | Immune-related adverse events |
| NIV | Non-invasive ventilation |
| NPS | Naso-pharyngeal swab |
| NSCLC | Non-small cell lung cancer |
| P/F | PO2/FiO2 |
| PCR | Polymerase chain reaction |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed Death Ligand 1 |
| PF | Pulmonary fibrosis |
| RCP | Reactive C protein |
| RT-PCR | Reverse-transcriptase polymerase-chain reaction |
| SARS CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
References
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. Pd-Loma: A Cancer Entity with a Shared Sensitivity to the PD-1/PD-L1 Pathway Blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab Versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.N.; Behera, M.; Owonikoko, T.K.; Kamphorst, A.O.; Pakkala, S.; Belani, C.P.; Khuri, F.R.; Ahmed, R.; Ramalingam, S.S. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018, 124, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, A.; Yokoyama, T.; Fukuda, Y.; Ishida, T. Impact of interstitial lung disease associated with immune checkpoint inhibitors on prognosis in patients with non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2021, 87, 251–258. [Google Scholar] [CrossRef]
- Ono, K.; Ono, H.; Toi, Y.; Sugisaka, J.; Aso, M.; Saito, R.; Kawana, S.; Aiba, T.; Odaka, T.; Matsuda, S.; et al. Association of immune-related pneumonitis with clinical benefit of anti-programmed cell death-1 monotherapy in advanced non-small cell lung cancer. Cancer Med. 2021, 10, 4796–4804. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Miao, R.; Ahmed, Z.; Yu, J.; Guan, J.; Ahmad, S.; Zhou, S.; Grove, A.; Manoucheri, M.; et al. A Single Center Retrospective Study of the Impact of COVID-19 Infection on Immune-related Adverse Events in Cancer Patients Receiving Immune Checkpoint Inhibitors. J. Immunother. 2022, 45, 389–395. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Bui, A.-T.N.; Tyan, K.; Giobbie-Hurder, A.; Klein, I.A.; Manos, M.P.; Zubiri, L.; Reynolds, K.; Grover, S.; Weinhouse, G.L.; Ott, P.A.; et al. Impact of COVID-19 on Patients with Cancer Receiving Immune Checkpoint Inhibitors. J. Immunother. Precis. Oncol. 2021, 4, 35–44. [Google Scholar] [CrossRef]
- Rogiers, A.; da Silva, I.P.; Tentori, C.; Tondini, C.A.; Grimes, J.M.; Trager, M.H.; Nahm, S.; Zubiri, L.; Manos, M.; Bowling, P.; et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J. Immunother. Cancer 2021, 9, e001931, Erratum in: J. Immunother. Cancer 2021, 9, e001931corr1. https://doi.org/10.1136/jitc-2020-001931corr1. Erratum in: J. Immunother. Cancer 2022, 10, e001931corr2. https://doi.org/10.1136/jitc-2020-001931corr2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Satici, M.O.; Islam, M.M.; Satici, C.; Uygun, C.N.; Ademoglu, E.; Altunok, I.; Aksel, G.; Eroglu, S.E. The role of a noninvasive index ‘Spo2/ Fio2’ in predicting mortality among patients with COVID-19 pneumonia. Am. J. Emerg. Med. 2022, 57, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Golden, E.; Freire, A.X.; Taylor, E.; Zaman, M.; Carson, S.J.; Gibson, M.; Umberger, R. Methylprednisolone infusion in early severe ards: Results of a randomized controlled trial. Chest 2007, 131, 954–963. [Google Scholar] [CrossRef]
- Hu, H.; Wei, S.; Huang, J.; Sharma, L.; Chang, D. Beneficial effects of early low-dose methylprednisolone with long-term treatment in ARDS. Eur. J. Intern. Med. 2024, 129, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Duong-Quy, S.; Vo-Pham-Minh, T.; Tran-Xuan, Q.; Huynh-Anh, T.; Vo-Van, T.; Vu-Tran-Thien, Q.; Nguyen-Nhu, V. Post-COVID-19 Pulmonary Fibrosis: Facts—Challenges and Futures: A Narrative Review. Pulm. Ther. 2023, 20, 295–307. [Google Scholar] [CrossRef]
- Ponnalagu, V.; Kwan, E.L.K.; Sadasiv, M.S.; Teo, H.L.; Low, H.M. Pembrolizumab-related pneumonitis in a patient with COVID-19 infection. Singap. Med. J. 2022, 64, 454–458. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, S.; Zhang, Y.; Li, X.; Zhao, Z.; Liu, P.; Du, Y. A false alarm of COVID-19 pneumonia in lung cancer with anti-PD-1 related pneumonitis: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 41. [Google Scholar] [CrossRef]
- Abid, M.B. Overlap of immunotherapy-related pneumonitis and COVID-19 pneumonia: Diagnostic and vaccine considerations. J. Immunother. Cancer 2021, 9, e002307. [Google Scholar] [CrossRef]
- Moffat, G.T.; Hanna, L.; Hopman, W.; Fung, A.S.; Gaudreau, P.-O. An assessment of extended pembrolizumab dosing in advanced non-small-cell lung cancer in the COVID-19 pandemic. Immunotherapy 2023, 15, 921–932. [Google Scholar] [CrossRef]
- Che, K.; Hong, C.; He, Y.; Peng, D.; Zeng, Z.; Liu, A. Association of immune-related adverse events with COVID-19 pneumonia in lung cancer patients receiving immune checkpoint inhibitors: A cross-sectional study in China. BMC Cancer 2023, 23, 1069. [Google Scholar] [CrossRef]
- Tanvetyanon, T.; Chen, D.-T.; Gray, J.E. Impact of COVID-19 Pandemic on Frontline Pembrolizumab-Based Treatment for Advanced Lung Cancer. J. Clin. Med. 2023, 12, 1611. [Google Scholar] [CrossRef] [PubMed]
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: A position paper from the fleischner society. Radiology 2021, 298, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Madahar, P.; Capaccione, K.M.; Salvatore, M.M.; Short, B.; Wahab, R.; Abrams, D.; Parekh, M.; Geleris, J.D.; Furfaro, D.; Anderson, M.R.; et al. Fibrotic-Like Pulmonary Radiographic Patterns Are Not Associated With Adverse Outcomes in COVID-19 Chronic Critical Illness. Crit. Care Med. 2023, 51, e209–e220. [Google Scholar] [CrossRef]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT imaging features of 2019 novel coronavirus (2019 nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolezzi, A.; Gualano, G.; Mastrobattista, A.; Vittozzi, P.; Di Bari, V.; Cerva, C.; Mosti, S.; Lugini, A.; Albarello, F.; Di Stefano, F.; et al. A Case of Pulmonary Fibrosis and COVID-19-Related Pneumonia in a Pembrolizumab-Treated Patient. Infect. Dis. Rep. 2025, 17, 53. https://doi.org/10.3390/idr17030053
Zolezzi A, Gualano G, Mastrobattista A, Vittozzi P, Di Bari V, Cerva C, Mosti S, Lugini A, Albarello F, Di Stefano F, et al. A Case of Pulmonary Fibrosis and COVID-19-Related Pneumonia in a Pembrolizumab-Treated Patient. Infectious Disease Reports. 2025; 17(3):53. https://doi.org/10.3390/idr17030053
Chicago/Turabian StyleZolezzi, Alberto, Gina Gualano, Annelisa Mastrobattista, Pietro Vittozzi, Virginia Di Bari, Carlotta Cerva, Silvia Mosti, Antonio Lugini, Fabrizio Albarello, Federica Di Stefano, and et al. 2025. "A Case of Pulmonary Fibrosis and COVID-19-Related Pneumonia in a Pembrolizumab-Treated Patient" Infectious Disease Reports 17, no. 3: 53. https://doi.org/10.3390/idr17030053
APA StyleZolezzi, A., Gualano, G., Mastrobattista, A., Vittozzi, P., Di Bari, V., Cerva, C., Mosti, S., Lugini, A., Albarello, F., Di Stefano, F., Valli, M. B., & Palmieri, F. (2025). A Case of Pulmonary Fibrosis and COVID-19-Related Pneumonia in a Pembrolizumab-Treated Patient. Infectious Disease Reports, 17(3), 53. https://doi.org/10.3390/idr17030053







