Cytomegalovirus Blood DNAemia in Patients with Severe SARS-CoV-2 Pneumonia
Abstract
1. Introduction
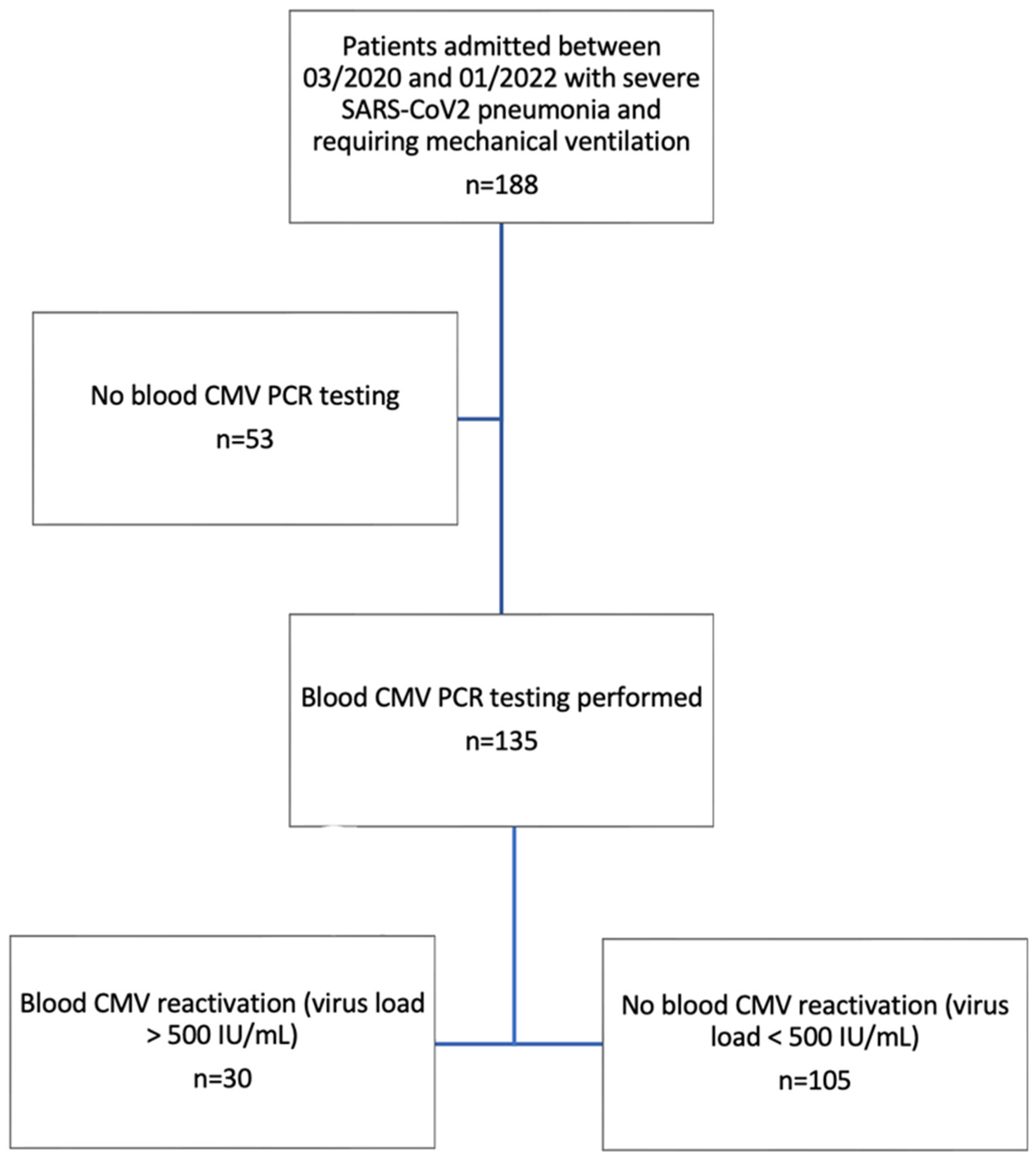
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMV | Cytomegalovirus |
| SARS-CoV-2 | Severe acute respiratory syndrome-coronavirus2 |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
| ICU | Intensive care unit |
| LOS | Length of stay |
| ECMO | Extra-corporeal membrane oxygenation |
| IMV | Invasive mechanical ventilation |
| ARDS | Acute Respiratory Distress Syndrome |
References
- Zhang, Z.; Liu, X.; Sang, L.; Chen, S.; Wu, Z.; Zhang, J.; Sun, Y.; Huang, Y.; Xu, Y.; He, W.; et al. Cytomegalovirus reactivation in immunocompetent mechanical ventilation patients: A prospective observational study. BMC Infect. Dis. 2021, 21, 1026. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.; Singh, N. Cytomegalovirus infection in critically ill patients: A systematic review. Crit. Care 2009, 13, R68. [Google Scholar] [CrossRef] [PubMed]
- Lachance, P.; Chen, J.; Featherstone, R.; Sligl, W.I. Association Between Cytomegalovirus Reactivation and Clinical Outcomes in Immunocompetent Critically Ill Patients: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2017, 4, ofx029. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.S.; Armstrong, W.S.; Caliendo, A.M. Interpreting quantitative cytomegalovirus DNA testing: Understanding the laboratory perspective. Clin. Infect. Dis. 2012, 54, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jacob, J.; Mayer, D.; Eckardt, P.A. Cytomegalovirus reactivation in critically-ill Coronavirus Disease 2019 patients: A case series of 11 patients. IDCases 2022, 27, e01402. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Hraiech, S.; Lehingue, S.; Roch, A.; Chiche, L.; Wiramus, S.; Forel, J.-M. Cytomegalovirus reactivation in ICU patients. Intensive Care Med. 2016, 42, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Gatto, I.; Biagioni, E.; Coloretti, I.; Farinelli, C.; Avoni, C.; Caciagli, V.; Busani, S.; Sarti, M.; Pecorari, M.; Gennari, W.; et al. Cytomegalovirus blood reactivation in COVID-19 critically ill patients: Risk factors and impact on mortality. Intensive Care Med. 2022, 48, 706–713. [Google Scholar] [CrossRef]
- Pérez-Granda, M.J.; Catalán, P.; Muñoz, P.; Aldámiz, T.; Barrios, J.C.; Ramírez, C.; García-Martínez, R.; Villalba, M.V.; Puente, L.; Bouza, E. Cytomegalovirus reactivation in patients diagnosed with severe COVID-19: A point prevalence study in a general hospital. Rev. Esp. Quimioter. 2022, 36, 45–51. [Google Scholar] [CrossRef]
- Tassaneeyasin, T.; Sungkanuparph, S.; Srichatrapimuk, S.; Charoensri, A.; Thammavaranucupt, K.; Jayanama, K.; Kirdlarp, S. Prevalence and risk factors of cytomegalovirus reactivation in critically Ill patients with COVID-19 pneumonia. PLoS ONE 2024, 19, e0303995. [Google Scholar] [CrossRef] [PubMed]
- Boers, L.S.; Gréve, F.v.S.; van Hattem, J.M.; de Brabander, J.; Zwaan, T.; van Willigen, H.; Cornelissen, M.; de Jong, M.; van der Poll, T.; Duitman, J.; et al. Pulmonary herpes simplex virus and cytomegalovirus in patients with acute respiratory distress syndrome related to COVID-19. Intensive Care Med. 2024, 50, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Hraiech, S.; Bonnardel, E.; Guervilly, C.; Fabre, C.; Loundou, A.; Forel, J.-M.; Adda, M.; Parzy, G.; Cavaille, G.; Coiffard, B.; et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann. Intensive Care 2019, 9, 142. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Burrel, S.; Mokrani, D.; de Chambrun, M.P.; Luyt, D.; Chommeloux, J.; Guiraud, V.; Bréchot, N.; Schmidt, M.; Hekimian, G.; et al. Herpesviridae lung reactivation and infection in patients with severe COVID-19 or influenza virus pneumonia: A comparative study. Ann. Intensive Care 2022, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Caciagli, V.; Coloretti, I.; Talamonti, M.; Farinelli, C.; Gatto, I.; Biagioni, E.; Sarti, M.; Franceschini, E.; Meschiari, M.; Mussini, C.; et al. Association between Pulmonary Aspergillosis and Cytomegalovirus Reactivation in Critically Ill COVID-19 Patients: A Prospective Observational Cohort Study. Viruses 2023, 15, 2260. [Google Scholar] [CrossRef]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Jaber, S.; Hraiech, S.; Baumstarck, K.; Cayot-Constantin, S.; Aissaoui, N.; Jung, B.; Leone, M.; Souweine, B.; Schwebel, C.; et al. Preemptive ganciclovir for mechanically ventilated patients with cytomegalovirus reactivation. Ann. Intensive Care 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Chiche, L.; Forel, J.-M.; Roch, A.; Guervilly, C.; Pauly, V.; Allardet-Servent, J.; Gainnier, M.; Zandotti, C.; Papazian, L. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit. Care Med. 2009, 37, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Chanques, G.; Borry, J.; Souche, B.; Verdier, R.; Perrigault, P.F.; Eledjam, J.J. Cytomegalovirus infection in critically ill patients: Associated factors and consequences. Chest 2005, 127, 233–241. [Google Scholar] [CrossRef]
- Zamora, M.R. DNA viruses (CMV, EBV, and the herpesviruses). Semin. Respir. Crit. Care Med. 2011, 32, 454–470. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Enrile, E.M.; Dentone, C.; Vena, A.; Robba, C.; Ball, L.; Bartoletti, M.; Coloretti, I.; Di Bella, S.; et al. Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study. J. Clin. Med. 2021, 10, 555. [Google Scholar] [CrossRef]
- Guiouillier, F.; Derely, J.; Salvadori, A.; Pochard, J.; Le Goff, J.; Martinez, T.; Raffin, F.; Laitselart, P.; Beaucreux, C.; Priou, S.; et al. Reactivation of Epstein-Barr virus among intensive care patients: A prospective observational study. Intensive Care Med. 2024, 50, 418–426. [Google Scholar] [CrossRef] [PubMed]
| CMV Negative (n = 105) | CMV Positive (n = 30) | p Value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age, years | 63 (54; 70) | 64 (55; 69) | 0.97 |
| Male (%) | 75/105 (28.6) | 10/30 (33.3) | 0.61 |
| BMI | 28 (25; 32) | 30 (26; 32) | 0.57 |
| Hypertension (%) | 54 (51.4) | 21 (70) | 0.071 |
| Diabetes mellitus (%) | 37 (35.2) | 8 (26.7) | 0.38 |
| Cardiomyopathy (%) | 22 (21) | 4 (13.3) | 0.35 |
| Chronic kidney disease (%) | 10 (9.5) | 2 (6.7) | 0.63 |
| Immunosuppression (%) | 20 (19.1) | 6 (20) | 0.91 |
| COPD (%) | 7 (6.7) | 0 (0) | 0.15 |
| Neoplasia <2 years (%) | 9 (8.6) | 0 (0) | 0.097 |
| SOFA | 6 (4; 7) | 6 (5; 8) | 0.55 |
| APACHE II | 15 (13; 18) | 14 (12; 19) | 0.28 |
| Biological parameters | |||
| Admission CRP, mg/dL | 139 (90; 216) | 145 (65; 212) | 0.66 |
| Highest CRP, mg/dL | 331 (266; 380) | 344 (303; 413) | 0.21 |
| Admission LDH, IU/L | 546 (440; 657) | 485 (398; 563) | 0.10 |
| Highest LDH, IU/L | 699 (583; 934) | 712 (533; 1194) | 0.85 |
| Admission lymphocytes,/mcL | 550 (385; 940) | 610 (368; 920) | 0.75 |
| Lowest lymphocytes,/mcL | 370 (190; 540) | 290 (165; 420) | 0.14 |
| Admission platelet, 103/mcL | 222 (169; 306) | 232 (157; 277) | 0.89 |
| Highest D-dimer, ng/mL | 1554 (915; 5840) | 1253 (580; 2562) | 0.025 |
| Admission fibrinogen, mg/dL | 636 (503; 802) | 670 (526; 793) | 0.59 |
| PaO2/FiO2 (admission) | 68 (53; 84) | 64 (54; 78) | 0.59 |
| Lowest PaO2/FiO2 | 48 (42; 51) | 49 (45; 53) | 0.23 |
| Treatment | |||
| Dexamethasone (%) | 77 (73.3) | 28 (93.3) | 0.020 |
| Hydroxychloroquine (%) | 23 (21.9) | 3 (10) | 0.15 |
| ECMO (%) | 29 (27.6) | 11 (36.7) | 0.34 |
| RRT (%) | 28 (26.7) | 13 (43.3) | 0.08 |
| CMV Negative (n = 105) | CMV Positive (n = 30) | p Value | |
|---|---|---|---|
| ICU mortality (%) | 71 (64.8) | 17 (56.7) | 0.42 |
| ICU length of stay, days | 23 (14; 47) | 68 (48; 98) | <0.0001 |
| Hospital length of stay, days | 31 (20; 70) | 70 (50; 115) | <0.0001 |
| Duration of ventilation, days | 20 (12; 37) | 62.5 (41; 83) | <0.0001 |
| Duration of ECMO, days (n = 40) | 26 (11; 47) (n = 29) | 54 (34; 105) (n = 11) | 0.0076 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | IC 95 | p | Adjusted Hazard Ratio | IC 95 | p | |
| SOFA score | 0.91 | 0.736–1.125 | 0.38 | 0.915 | 0.744–1.126 | 0.403 |
| Diabetes | 0.509 | 0.206–1.262 | 0.145 | 0.593 | 0.261–1.343 | 0.21 |
| Immunosuppressive therapy | 1.188 | 0.438–3.219 | 0.735 | 1.164 | 0.46–2.941 | 0.75 |
| Chronic kidney disease | 0.877 | 0.181–4.261 | 0.87 | 0.875 | 0.18–4.247 | 0.87 |
| Dexamethasone | 6.047 | 1.31–27.87 | 0.021 | 4.23 | 1.006–17.792 | 0.049 |
| Male sex | 0.642 | 0.271–1.524 | 0.31 | 0.645 | 0.285–1.46 | 0.392 |
| Age | 1.022 | 0.988–1.058 | 0.21 | 1.014 | 0.982–1.046 | 0.394 |
| Body mass index | 0.998 | 0.94–1.059 | 0.94 | 0.998 | 0.94–1.059 | 0.937 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesland, J.-B.; Collienne, C.; Montiel, V.; Werion, A.; Hantson, P.; Wittebole, X.; Laterre, P.-F.; Gerard, L. Cytomegalovirus Blood DNAemia in Patients with Severe SARS-CoV-2 Pneumonia. Infect. Dis. Rep. 2025, 17, 8. https://doi.org/10.3390/idr17010008
Mesland J-B, Collienne C, Montiel V, Werion A, Hantson P, Wittebole X, Laterre P-F, Gerard L. Cytomegalovirus Blood DNAemia in Patients with Severe SARS-CoV-2 Pneumonia. Infectious Disease Reports. 2025; 17(1):8. https://doi.org/10.3390/idr17010008
Chicago/Turabian StyleMesland, Jean-Baptiste, Christine Collienne, Virginie Montiel, Alexis Werion, Philippe Hantson, Xavier Wittebole, Pierre-François Laterre, and Ludovic Gerard. 2025. "Cytomegalovirus Blood DNAemia in Patients with Severe SARS-CoV-2 Pneumonia" Infectious Disease Reports 17, no. 1: 8. https://doi.org/10.3390/idr17010008
APA StyleMesland, J.-B., Collienne, C., Montiel, V., Werion, A., Hantson, P., Wittebole, X., Laterre, P.-F., & Gerard, L. (2025). Cytomegalovirus Blood DNAemia in Patients with Severe SARS-CoV-2 Pneumonia. Infectious Disease Reports, 17(1), 8. https://doi.org/10.3390/idr17010008






