Molecular Identification of Etiological Agents in Fungal and Bacterial Skin Infections: United States, 2020–2024
Abstract
1. Introduction
2. Materials and Methods
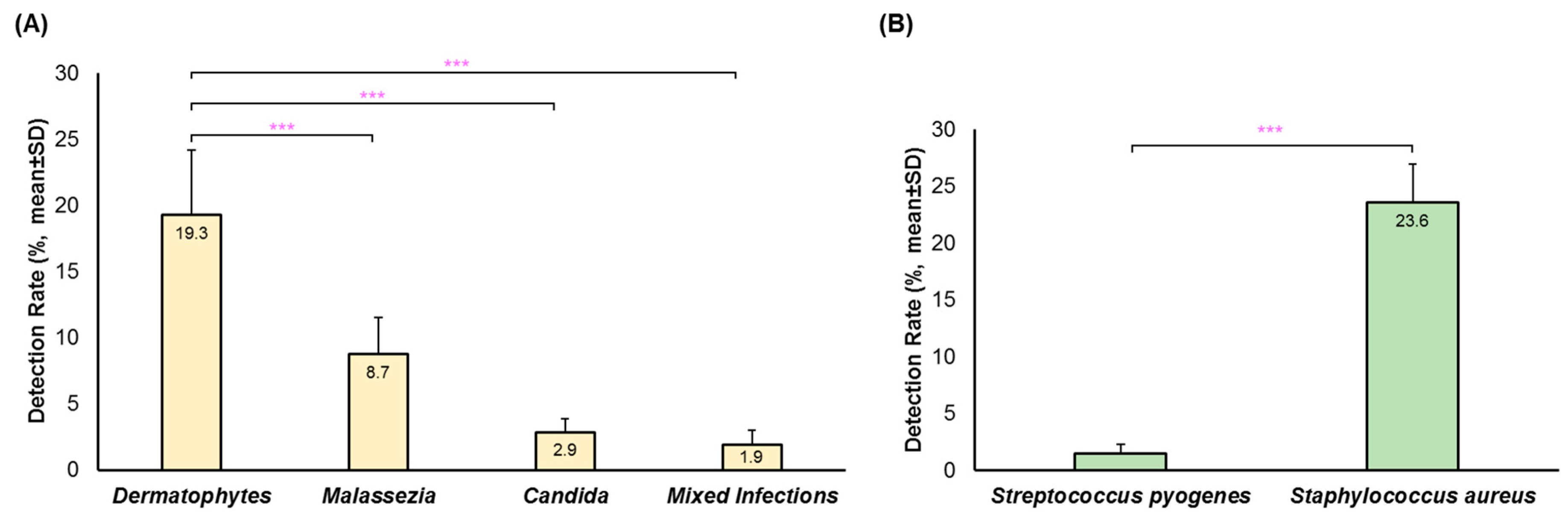
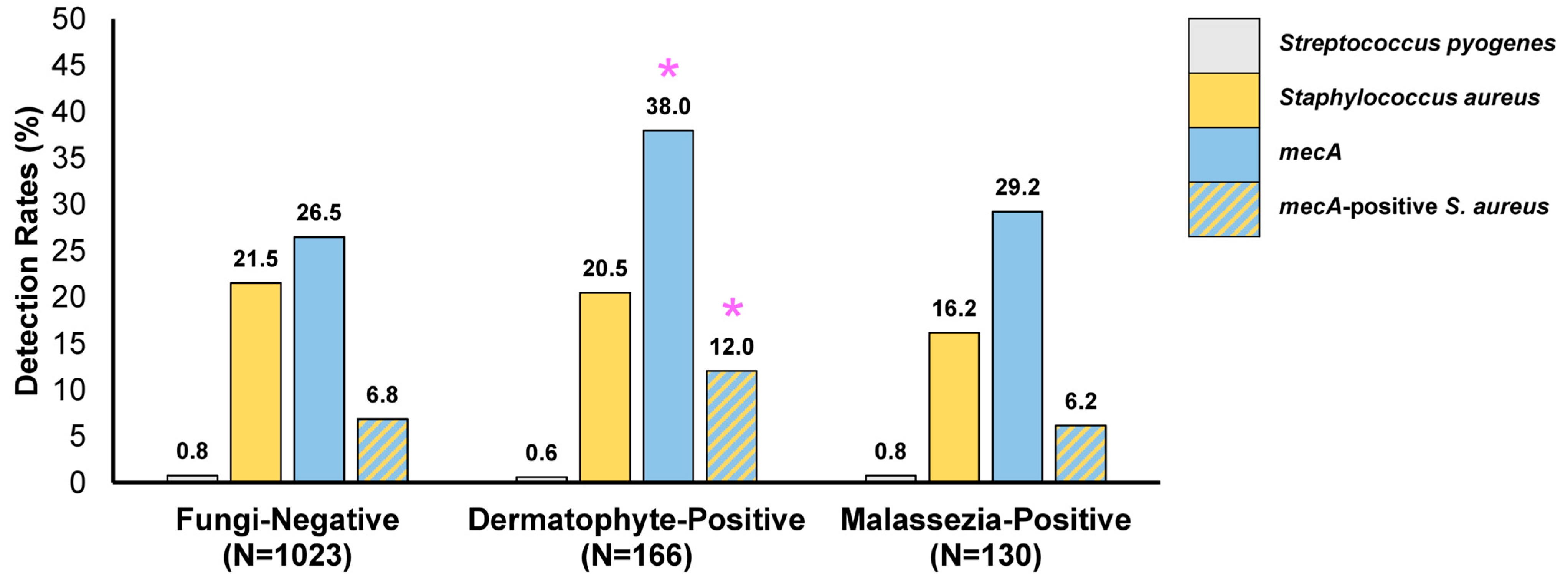
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The Burden of Skin and Subcutaneous Diseases: Findings from the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef]
- Del Giudice, P. Skin Infections Caused by Staphylococcus Aureus. Acta Derm. Venereol. 2020, 100, adv00110. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, J.; Xu, B.-N.; Li, Y.; Bao, W.; Cheng, X.L.; He, Y.; Xu, C.P.; Ren, J.; Zheng, Y.R.; et al. Global Burden of Bacterial Skin Diseases: A Systematic Analysis Combined With Sociodemographic Index, 1990–2019. Front. Med. 2022, 9, 861115. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.C.; Caplan, A.S.; Elewski, B.; Gold, J.A.W.; Lockhart, S.R.; Smith, D.J.; Lipner, S.R. Expert Panel Review of Skin and Hair Dermatophytoses in an Era of Antifungal Resistance. Am. J. Clin. Dermatol. 2024, 25, 359–389. [Google Scholar] [CrossRef]
- Gupta, A.K.; Hall, D.C.; Cooper, E.A.; Ghannoum, M.A. Diagnosing Onychomycosis: What’s New? J. Fungi 2022, 8, 464. [Google Scholar] [CrossRef]
- Hatlen, T.J.; Miller, L.G. Staphylococcal Skin and Soft Tissue Infections. Infect. Dis. Clin. N. Am. 2021, 35, 81–105. [Google Scholar] [CrossRef]
- Furuya, H.; Ogura, K.; Takemoto, N.; Watanabe, S.; Yamazaki, A.; Ogai, K.; Sugama, J.; Okamoto, S. A Multilocus Sequence Typing Method of Staphylococcus Aureus DNAs in a Sample from Human Skin. Microbiol. Immunol. 2023, 67, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Marsh, P.; Ahmad, N. Cryptic Resistance in Staphylococcus Aureus: A Risk for the Treatment of Skin Infection? Curr. Opin. Infect. Dis. 2014, 27, 130–136. [Google Scholar] [CrossRef]
- Palavecino, E.L. Clinical, Epidemiologic, and Laboratory Aspects of Methicillin-Resistant Staphylococcus Aureus Infections. Methods Mol. Biol. 2020, 2069, 1–28. [Google Scholar] [CrossRef]
- Zabielinski, M.; McLeod, M.P.; Aber, C.; Izakovic, J.; Schachner, L.A. Trends and Antibiotic Susceptibility Patterns of Methicillin-Resistant and Methicillin-Sensitive Staphylococcus Aureus in an Outpatient Dermatology Facility. JAMA Dermatol. 2013, 149, 427–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonesso, M.F.; Marques, S.A.; Camargo, C.H.; Fortaleza, C.M.C.B.; da Cunha, M.d.L.R.d.S. Community-Associated Methicillin-Resistant Staphylococcus Aureus in Non-Outbreak Skin Infections. Braz. J. Microbiol. 2014, 45, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Jiménez, I.G.; Ruiz-Hernández, F.G.; Carmona-Cruz, S.A.; Pastrana-Arellano, E.; Aquino-Andrade, A.; Romo-González, C.; Arias-de la Garza, E.; Álvarez-Villalobos, N.A.; García-Romero, M.T. Global Antimicrobial Susceptibility Patterns of Staphylococcus Aureus in Atopic Dermatitis: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2024, e243360. [Google Scholar] [CrossRef] [PubMed]
- Saheb Kashaf, S.; Harkins, C.P.; Deming, C.; Joglekar, P.; Conlan, S.; Holmes, C.J.; NISC Comparative Sequencing Program; Almeida, A.; Finn, R.D.; Segre, J.A.; et al. Staphylococcal Diversity in Atopic Dermatitis from an Individual to a Global Scale. Cell Host Microbe 2023, 31, 578–592.e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, K.Y.; Jin, T.; Kim, J.H.; Seo, S.J. Rapid Detection of Staphylococcus Aureus and Methicillin-Resistant S. aureus in Atopic Dermatitis by Using the BD Max StaphSR Assay. Ann. Lab. Med. 2017, 37, 320–322. [Google Scholar] [CrossRef][Green Version]
- Balci, D.D.; Duran, N.; Ozer, B.; Gunesacar, R.; Onlen, Y.; Yenin, J.Z. High Prevalence of Staphylococcus Aureus Cultivation and Superantigen Production in Patients with Psoriasis. Eur. J. Dermatol. 2009, 19, 238–242. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. How to Obtain the P Value from a Confidence Interval. BMJ 2011, 343, d2304. [Google Scholar] [CrossRef]
- Ogawa, T.; Matsuda, A.; Ogawa, Y.; Tanaka, R. Risk Factors for the Development of Tinea Pedis and Onychomycosis: Real-World Evidence from a Single-Podiatry Center, Large-Scale Database in Japan. J. Dermatol. 2024, 51, 30–39. [Google Scholar] [CrossRef]
- Gupta, A.K.; Chaudhry, M.; Elewski, B. Tinea Corporis, Tinea Cruris, Tinea Nigra, and Piedra. Dermatol. Clin. 2003, 21, 395–400. [Google Scholar] [CrossRef]
- Jabet, A.; Dellière, S.; Seang, S.; Chermak, A.; Schneider, L.; Chiarabini, T.; Teboul, A.; Hickman, G.; Bozonnat, A.; Brin, C.; et al. Sexually Transmitted Trichophyton Mentagrophytes Genotype VII Infection among Men Who Have Sex with Men. Emerg. Infect. Dis. 2023, 29, 1411–1414. [Google Scholar] [CrossRef]
- Kupsch, C.; Czaika, V.-A.; Deutsch, C.; Gräser, Y. Trichophyton Mentagrophytes—A New Genotype of Zoophilic Dermatophyte Causes Sexually Transmitted Infections. J. Dtsch. Dermatol. Ges. 2019, 17, 493–501. [Google Scholar] [CrossRef] [PubMed]
- White, T.C.; Findley, K.; Dawson, T.L.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the Skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef] [PubMed]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia Species in Healthy Skin and in Dermatological Conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Bluhm, R.; Summerbell, R. Pityriasis Versicolor. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 19–33. [Google Scholar] [CrossRef]
- Al Bataineh, M.T.; Cacciatore, S.; Semreen, M.H.; Dash, N.R.; Soares, N.C.; Zhu, X.; Mousa, M.K.; Salam, J.S.A.; Zerbini, L.F.; Hajjo, R.; et al. Exploring the Effect of Estrogen on Candida Albicans Hyphal Cell Wall Glycans and Ergosterol Synthesis. Front. Cell. Infect. Microbiol. 2022, 12, 977157. [Google Scholar] [CrossRef]
- Kumwenda, P.; Cottier, F.; Hendry, A.C.; Kneafsey, D.; Keevan, B.; Gallagher, H.; Tsai, H.-J.; Hall, R.A. Estrogen Promotes Innate Immune Evasion of Candida Albicans through Inactivation of the Alternative Complement System. Cell Rep. 2022, 38, 110183. [Google Scholar] [CrossRef]
- Salah, L.A.; Faergemann, J. A Retrospective Analysis of Skin Bacterial Colonisation, Susceptibility and Resistance in Atopic Dermatitis and Impetigo Patients. Acta Derm. Venereol. 2015, 95, 532–535. [Google Scholar] [CrossRef]
- Bowen, A.C.; Tong, S.Y.C.; Chatfield, M.D.; Carapetis, J.R. The Microbiology of Impetigo in Indigenous Children: Associations between Streptococcus Pyogenes, Staphylococcus Aureus, Scabies, and Nasal Carriage. BMC Infect. Dis. 2014, 14, 727. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K. Impetigo. Adv. Emerg. Nurs. J. 2020, 42, 262–269. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus Aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- Schöfer, H.; Bruns, R.; Effendy, I.; Hartmann, M.; Jappe, U.; Plettenberg, A.; Reimann, H.; Seifert, H.; Shah, P.; Sunderkötter, C.; et al. Diagnosis and Treatment of Staphylococcus Aureus Infections of the Skin and Mucous Membranes. J. Dtsch. Dermatol. Ges. 2011, 9, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Najar-Peerayeh, S.; Mahrooghi, M.; Mansouri, P.; Bakhshi, B. Methicillin-Resistant Staphylococcus Aureus Colonization of Infectious and Non-Infectious Skin and Soft Tissue Lesions in Patients in Tehran. BMC Microbiol. 2021, 21, 282. [Google Scholar] [CrossRef] [PubMed]
- Blömer, R.-H.; Keilani, N.; Faber, A.; Rodeck, B.; Krüger, C.; Uhrlaß, S.; Gräser, Y.; Nenoff, P. Tinea Capitis Profunda Due to Trichophyton Verrucosum with CMRSA Superinfection in an Infant. Hautarzt 2012, 63, 648–652. [Google Scholar] [CrossRef]
- Sardana, K.; Gupta, A.; Mathachan, S.R. Immunopathogenesis of Dermatophytoses and Factors Leading to Recalcitrant Infections. Indian Dermatol. Online J. 2021, 12, 389–399. [Google Scholar] [CrossRef]
- Youssef, N.; Wyborn, C.H.; Holt, G.; Noble, W.C.; Clayton, Y.M. Ecological Effects of Antibiotic Production by Dermatophyte Fungi. J. Hyg. 1979, 82, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ryall, C.; Holt, G.; Noble, W.C. Interactions between Dermatophyte Fungi and Staphylococci or Brevibacterium in Vitro. J. Investig. Dermatol. 1981, 76, 21–23. [Google Scholar] [CrossRef]
- Larsen, J.; Raisen, C.L.; Ba, X.; Sadgrove, N.J.; Padilla-González, G.F.; Simmonds, M.S.J.; Loncaric, I.; Kerschner, H.; Apfalter, P.; Hartl, R.; et al. Emergence of Methicillin Resistance Predates the Clinical Use of Antibiotics. Nature 2022, 602, 135–141. [Google Scholar] [CrossRef]
| Parameter | Dermatophyte | Malassezia | Candida | Mixed Detection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | OR (95% CI) | N | % | OR (95% CI) | N | % | OR (95% CI) | N | % | OR (95% CI) | ||
| Sex | |||||||||||||
| Male | 382 | 19.6 | 1.4 (1.2, 1.6) | 263 | 13.5 | 2.1 (1.7, 2.6) | 38 | 1.9 | 0.6 (0.4, 0.9) | 58 | 3.0 | 2.0 (1.3, 3.1) | |
| Female | 343 | 15.0 | Referent | 156 | 6.8 | Referent | 76 | 3.3 | Referent | 34 | 1.5 | Referent | |
| Age Group | |||||||||||||
| <18 | 57 | 11.3 | 0.8 (0.6, 1.1) | 61 | 12.1 | 0.9 (0.6, 1.2) | 8 | 1.6 | 0.7 (0.3, 1.5) | 4 | 0.8 | 0.5 (0.2, 1.5) | |
| 18–44 | 209 | 14.0 | Referent | 204 | 13.6 | Referent | 35 | 2.3 | Referent | 23 | 1.5 | Referent | |
| 45–64 | 225 | 18.4 | 1.4 (1.1, 1.7) | 99 | 8.1 | 0.6 (0.4, 0.7) | 35 | 2.9 | 1.2 (0.8, 2.0) | 22 | 1.8 | 1.2 (0.6, 2.1) | |
| ≥65 | 236 | 22.8 | 1.8 (1.5, 2.2) | 58 | 5.6 | 0.4 (0.3, 0.5) | 36 | 3.5 | 1.5 (0.9, 2.4) | 44 | 4.3 | 2.8 (1.7, 4.7) | |
| Region | |||||||||||||
| Northeast | 237 | 14.9 | 0.7 (0.5, 0.9) | 150 | 9.5 | 1.1 (0.8, 1.7) | 40 | 2.5 | 0.8 (0.4, 1.6) | 26 | 1.6 | 0.5 (0.3, 1.1) | |
| Midwest | 76 | 21.1 | Referent | 30 | 8.3 | Referent | 11 | 3.1 | Referent | 11 | 3.1 | Referent | |
| South | 318 | 17.1 | 0.8 (0.6, 1.0) | 204 | 10.9 | 1.4 (0.9, 2.0) | 49 | 2.6 | 0.9 (0.4, 1.7) | 45 | 2.4 | 0.8 (0.4, 1.5) | |
| West | 93 | 23.6 | 1.2 (0.8, 1.6) | 34 | 8.6 | 1.0 (0.6, 1.7) | 11 | 2.8 | 0.9 (0.4, 2.1) | 9 | 2.3 | 0.7 (0.3, 1.8) | |
| Parameter | S. aureus | mecA | Co-Detection S. aureus and mecA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | OR (95% CI) | N | % | OR (95% CI) | N | % | OR (95% CI) | |
| Sex | |||||||||
| Male | 204 | 26.2 | 1.5 (1.2, 1.9) | 253 | 32.4 | 1.1 (0.9, 1.4) | 76 | 9.7 | 1.3 (0.9, 1.8) |
| Female | 178 | 19.3 | Referent | 272 | 29.5 | Referent | 70 | 7.6 | Referent |
| Age Group | |||||||||
| <18 | 58 | 34.5 | 1.7 (1.2, 2.5) | 44 | 26.2 | 0.8 (0.6, 1.2) | 14 | 8.3 | 0.9 (0.5, 1.7) |
| 18–44 | 134 | 23.3 | Referent | 172 | 30.0 | Referent | 51 | 8.9 | Referent |
| 45–64 | 98 | 20.0 | 0.8 (0.6, 1.1) | 149 | 30.3 | 1.0 (0.8, 1.3) | 42 | 8.6 | 1.0 (0.6, 1.5) |
| ≥65 | 93 | 19.6 | 0.8 (0.6, 1.1) | 161 | 34.0 | 1.2 (0.9, 1.6) | 40 | 8.4 | 0.9 (0.6, 1.5) |
| Region | |||||||||
| Northeast | 113 | 18.1 | 0.6 (0.4, 1.0) | 137 | 21.9 | 0.4 (0.3, 0.6) | 29 | 4.6 | 0.3 (0.2, 0.7) |
| Midwest | 23 | 26.1 | Referent | 36 | 40.9 | Referent | 11 | 12.5 | Referent |
| South | 180 | 25.2 | 1.0 (0.6, 1.6) | 288 | 40.4 | 1.0 (0.6, 1.5) | 86 | 12.1 | 1.0 (0.5, 1.9) |
| West | 61 | 23.4 | 0.9 (0.5, 1.5) | 61 | 23.4 | 0.4 (0.3, 0.7) | 19 | 7.3 | 0.5 (0.3, 1.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.K.; Wang, T.; Lincoln, S.A.; Foreman, H.-C.; Bakotic, W.L. Molecular Identification of Etiological Agents in Fungal and Bacterial Skin Infections: United States, 2020–2024. Infect. Dis. Rep. 2024, 16, 1075-1083. https://doi.org/10.3390/idr16060087
Gupta AK, Wang T, Lincoln SA, Foreman H-C, Bakotic WL. Molecular Identification of Etiological Agents in Fungal and Bacterial Skin Infections: United States, 2020–2024. Infectious Disease Reports. 2024; 16(6):1075-1083. https://doi.org/10.3390/idr16060087
Chicago/Turabian StyleGupta, Aditya K., Tong Wang, Sara A. Lincoln, Hui-Chen Foreman, and Wayne L. Bakotic. 2024. "Molecular Identification of Etiological Agents in Fungal and Bacterial Skin Infections: United States, 2020–2024" Infectious Disease Reports 16, no. 6: 1075-1083. https://doi.org/10.3390/idr16060087
APA StyleGupta, A. K., Wang, T., Lincoln, S. A., Foreman, H.-C., & Bakotic, W. L. (2024). Molecular Identification of Etiological Agents in Fungal and Bacterial Skin Infections: United States, 2020–2024. Infectious Disease Reports, 16(6), 1075-1083. https://doi.org/10.3390/idr16060087







