Streptococcus zooepidemicus Meningitis in an HIV-Positive Horse Breeder Patient: A Case Study and Literature Review
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baracco, G.J. Infections Caused by Group C and G Streptococcus (Streptococcus dysgalactiae subsp. equisimilis and Others): Epidemiological and Clinical Aspects. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Facklam, R. What happened to the streptococci: Overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 2002, 15, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Trell, K.; Nilson, B.; Petersson, A.C.; Rasmussen, M. Clinical and microbiological features of bacteremia with Streptococcus equi. Diagn. Microbiol. Infect. Dis. 2017, 87, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Minces, L.R.; Brown, P.J.; Veldkamp, P.J. Human meningitis from Streptococcus equi subsp. zooepidemicus acquired as zoonoses. Epidemiol. Infect. 2011, 139, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.T.; Roulson, M.; Ironside, M.J. A milk-borne outbreak of serious infection due to Streptococcus zooepidemicus (Lancefield Group C). Epidemiol. Infect. 1988, 101, 43–51. [Google Scholar] [CrossRef]
- Jenkins, E.L.; McGuire, W. Group C streptococcal meningitis in infancy. Acta Paediatr. 2000, 89, 1141–1142. [Google Scholar] [CrossRef]
- Sevilla-Acosta, F.; Ballestero-Pernudi, A.; Jiménez-Cruz, E.; Álvarez-Cabalceta, H.; Naranjo-Zuñiga, G. Streptococcus equi subspecies zooepidemicus Meningitis, Septicemia, and Brain Infarcts in a Costa Rican Infant. Cureus 2021, 13, e17286. [Google Scholar] [CrossRef]
- Zahlanie, Y.; Almatrafi, M.; Filkins, L.; Hsiang, M.S. Possible canine source of Streptococcus equi subspecies zooepidemicus causing meningitis in an infant. IDCases 2019, 31, e00568. [Google Scholar] [CrossRef]
- Shah, S.S.; Matthews, R.P.; Cohen, C. Group C streptococcal meningitis: Case report and review of the literature. Pediatr. Infect. Dis. J. 2001, 20, 445–448. [Google Scholar] [CrossRef]
- Cheeseman, M.; Genain, C.; Smith, C.D. Group C streptococcal meningitis with favorable recovery. A case report. J. Ky. Med. Assoc. 1990, 88, 545–546. [Google Scholar]
- Low, D.E.; Young, M.R.; Harding, G.K. Group C streptococcal meningitis in an adult. Probable Acquistion A Horse. Arch. Intern. Med. 1980, 140, 977–978. [Google Scholar] [CrossRef]
- Rivas, M.T.; Pascual, J.; Sesar, A. Group C Streptococcus meningitis: A very uncommon condition. Neurologia 2008, 23, 604–606. [Google Scholar] [PubMed]
- Patey, O.; Buisson, C.B.; Soussy, C.J. Group C streptococcal meningitis in adults. Rev. Infect. Dis. 1990, 12, 157–158. [Google Scholar] [CrossRef] [PubMed]
- van Samkar, A.; Brouwer, M.C.; van der Ende, A.; van de Beek, D. Streptococcus equi meningitis. Clin. Microbiol. Infect. 2016, 22, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Al-Araji, A.; Orendi, J. Atypical presentation of Streptococcus zooepidemicus bacteraemia and secondary meningitis. Clin. Neurol. Neurosurg. 2007, 109, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Downar, J.; Willey, B.M.; Sutherland, J.W.; Mathew, K.; Low, D.E. Streptococcal meningitis resulting from contact with an infected horse. J. Clin. Microbiol. 2001, 39, 2358–2359. [Google Scholar] [CrossRef] [PubMed]
- Klapa, S.; Grefer, J.; Sobottka, I.; Kurowski, V. A 56-Year-Old Woman with Chronic Hepatitis C Liver Disease and Meningitis due to Streptococcus equi subsp. Zooepidemicus. Case Rep. Crit. Care 2021, 2021, 7227054. [Google Scholar] [CrossRef]
- Pelkonen, S.; Lindahl, S.B.; Suomala, P.; Karhukorpi, J.; Vuorinen, S.; Koivula, I.; Väisänen, T.; Pentikäinen, J.; Autio, T.; Tuuminen, T. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg. Infect. Dis. 2013, 19, 1041–1048. [Google Scholar] [CrossRef]
- Mohr, D.N.; Feist, D.J.; Washington, J.A.; Hermans, P.E. Meningitis due to group C streptococci in an adult. Mayo Clin. Proc. 1978, 53, 529–532. [Google Scholar]
- Poulin, M.F.; Boivin, G. A case of disseminated infection caused by Streptococcus equi subspecies zooepidemicus. Can. J. Infect. Dis. Med. Microbiol. 2009, 20, 59–61. [Google Scholar] [CrossRef]
- Mori, N.; Guevara, J.M.; Tilley, D.H. Streptococcus equi subsp. zooepidemicus meningitis in Peru. J. Med. Microbiol. 2013, 62 Pt 2, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, A.T.; Cooke, E.M. Serious infection caused by group C streptococci. J. Clin. Pathol. 1980, 33, 188–190. [Google Scholar] [CrossRef]
- Latorre, M.; Alvarez, M.; Fernández, J.M.; Berdonces, P.; Llanos, A.; Cisterna, R. A case of meningitis due to “Streptococcus zooepidemicus”. Clin. Infect. Dis. 1993, 17, 932–933. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Stevanović, G.; Tošić, T.; Stošović, B.; Zervos, M.J. Streptococcus equi subsp. zooepidemicus meningitis. J. Med. Microbiol. 2008, 57, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Madžar, D.; Hagge, M.; Möller, S.; Regensburger, M.; Lee, D.H.; Schwab, S.; Jantsch, J. Endogenous endophthalmitis complicating Streptococcus equi subspecies zooepidemicus meningitis: A case report. BMC Res. Notes 2015, 8, 184. [Google Scholar] [CrossRef]
- Aida, Z.; Lamia, A.; Souheil, Z.; Badreddine, K.; Monika, B.; Rim, A.; Aida, B.; Hajer, H.; Hanen, T.B. Meningitis due to Streptococcus equi in a 73 year old woman with an osteodural defect. ID Cases 2020, 21, e00779. [Google Scholar] [CrossRef]
- Ferrandière, M.; Cattier, B.; Dequin, P.F.; Hazouard, E.; Legras, A.; Perrotin, D. Septicemia and meningitis due to Streptococcus zooepidemicus. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 290–291. [Google Scholar] [CrossRef]
- Ural, O.; Tuncer, I.; Dikici, N.; Aridogan, B. Streptococcus zooepidemicus meningitis and bacteraemia. Scand. J. Infect. Dis. 2003, 35, 206–207. [Google Scholar] [CrossRef]
- Eyre, D.W.; Kenkre, J.S.; Bowler, I.C.; McBride, S.J. Streptococcus equi subspecies zooepidemicus meningitis--a case report and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1459–1463. [Google Scholar] [CrossRef]
- Bordes-Benítez, A.; Sánchez-Oñoro, M.; Suárez-Bordón, P.; García-Rojas, A.J.; Saéz-Nieto, J.A.; González-García, A.; Alamo-Antúnez, I.; Sánchez-Maroto, A.; Bolaños-Rivero, M. Outbreak of Streptococcus equi subsp. zooepidemicus infections on the island of Gran Canaria associated with the consumption of inadequately pasteurized cheese. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 242–246. [Google Scholar] [CrossRef]
- van de Beek, D.; Cabellos, C.; Dzupova, O.; Esposito, S.; Klein, M.; Kloek, A.T.; Leib, S.L.; Mourvillier, B.; Ostergaard, C.; Pagliano, P.; et al. ESCMID Study Group for Infections of the Brain (ESGIB). ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clin. Microbiol. Infect. 2016, 22 (Suppl. S3), S37–S62. [Google Scholar] [CrossRef] [PubMed]
- Bosica, S.; Chiaverini, A.; De Angelis, M.E.; Petrini, A.; Averaimo, D.; Martino, M.; Rulli, M.; Saletti, M.A.; Cantelmi, M.C.; Ruggeri, F.; et al. Severe Streptococcus equi Subspecies zooepidemicus Outbreak from Unpasteurized Dairy Product Consumption, Italy. Emerg. Infect. Dis. 2023, 29, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
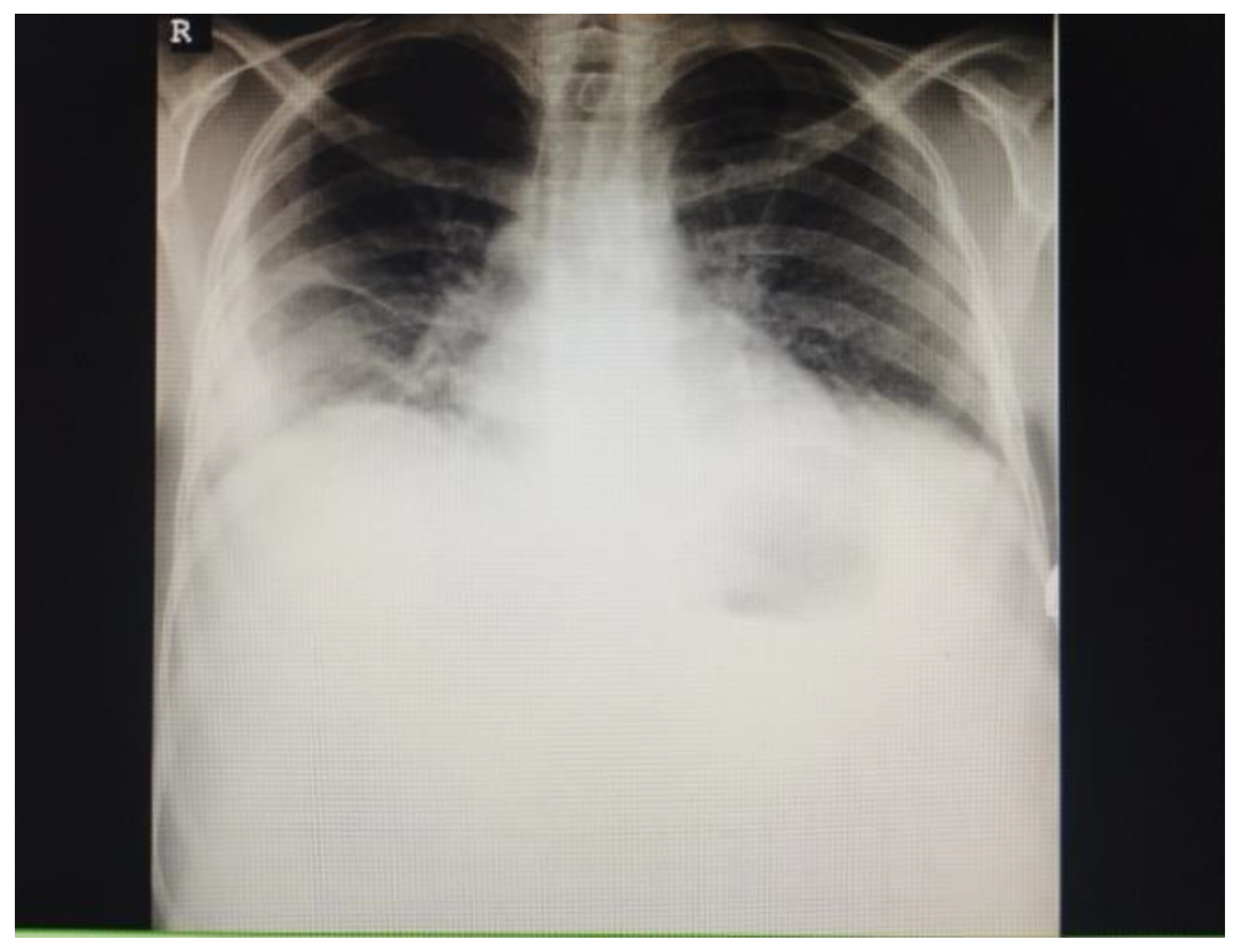
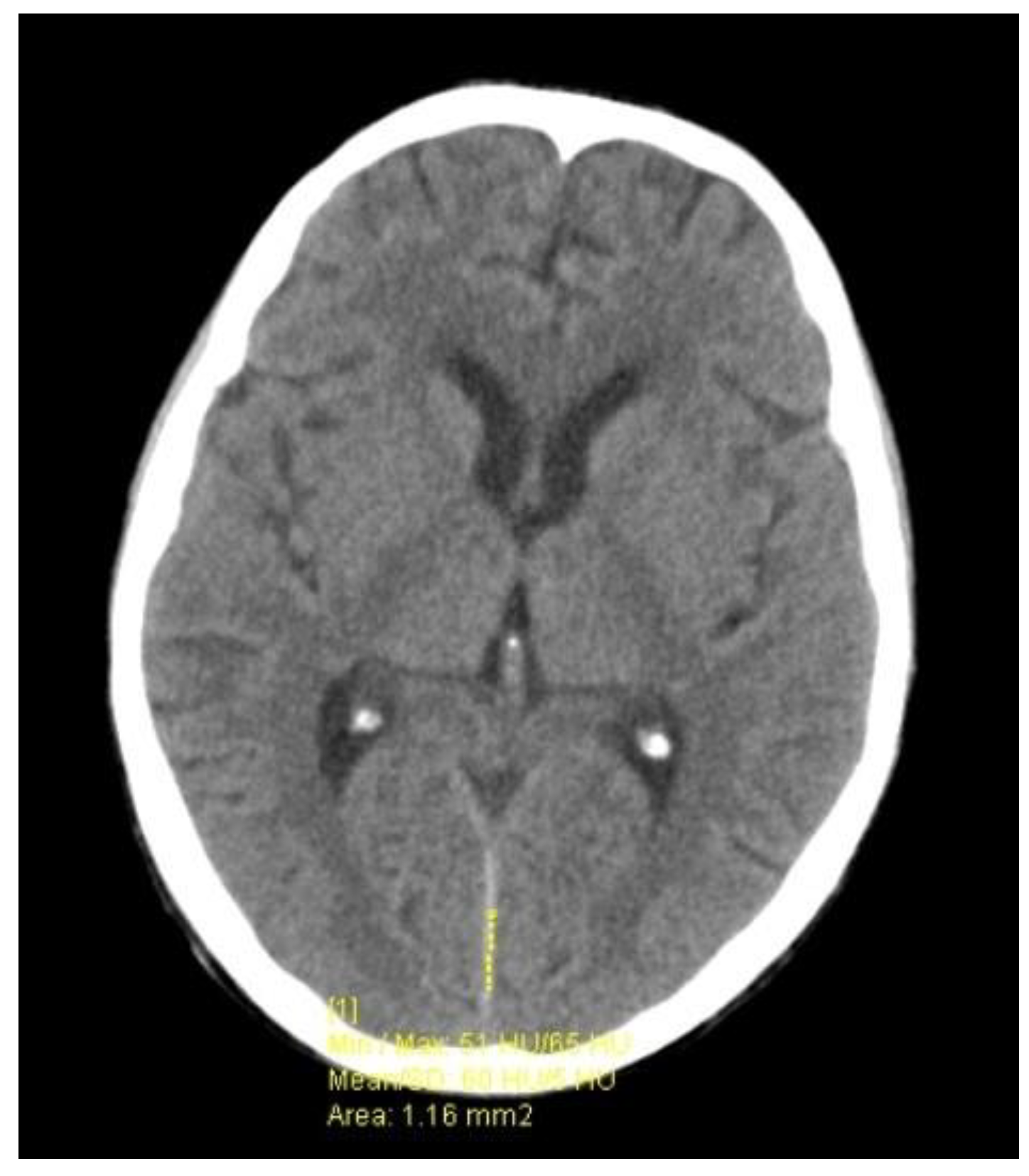
| Parameters | 1st LP (Day 1) | 2nd LP (Day 8) | 3rd LP (Day 16) |
|---|---|---|---|
| WBC, ×106/L | 8 | 6826 | 271 |
| Protein, g/L | 0.19 | 1.22 | 0.55 |
| Glucose, mmol/L | 1.50 | 1.30 | 3.4 |
| Serum glucose, mmol/L | 1.52 | 5.2 | 5.6 |
| Ratio * | 0.99 | 0.25 | 0.61 |
| Age | Sex | Concomitant Conditions | Exposure | Comorbidities | Outcome | Ref. |
|---|---|---|---|---|---|---|
| 1 day | M | Bacteremia, pneumonia | Unpasteurized milk | Prematurity | Died | [5] |
| 3 mo | M | Bacteremia | Unknown | nd | Recovered | [6] |
| 5 mo | M | Bacteremia, cerebral infarction | Unknown | None | Deafness, quadriplegia | [7] |
| 6 mo | F | Bacteremia | Ill dogs | None | Deafness | [8] |
| 13 yr | F | Bacteremia | Horse | Asthma/allergy | Deafness | [9] |
| 24 yr | M | None | Horses | None | Recovered | [10] |
| 24 yr | F | Bacteremia | Horse | None | Recovered | [11] |
| 30 yr | F | nd | Horses | Cocaine use | Recovered | [12] |
| 33 yr | M | Bacteremia | Unknown | nd | Deafness | [13] |
| 37 yr | F | Brain abscesses | Horse bite | None | Hemiparesis, aphasia | [14] |
| 41 yr | M | Bacteremia, sinusitis | Horse manure | None | Recovered | [15] |
| 41 yr | F | Cardiac and respiratory failure | Horses | None | Died | [14] |
| 49 yr | F | Bacteremia | Horse | None | Diplopia | [16] |
| 51 yr | F | Bacteremia, sinusitis, mastoiditis | Horse | Diabetes | Recovered | [4] |
| 56 yr | F | None | Horses | Hepatitis C | Recovered | [17] |
| 57 yr | M | Bacteremia, sepsis, endocarditis | Horses | AoVI | Prolonged recovery | [18] |
| 59 yr | F | Bacteremia | Unpasteurized milk | None | Slow recovery | [19] |
| 59 yr | F | Bacteremia, endophtalmitis, endocarditis | Sporadic contact with horses | Polymorbid * | Blindness, deafness | [20] |
| 59 yr | M | Otitis, mastoiditis, pneumonia | Unpasteurized cheese | Hypertension, stroke | Recovered | [21] |
| 66 yr | M | Bacteremia | Ill dogs | None | Recovered | [22] |
| 67 yr | F | Otitis | Cattle farmer | None | Recovered | [23] |
| 71 yr | M | Bacteremia | Unpasteurized milk | VT | Recovered | [5] |
| 72 yr | F | None | Horse | MI | Prolonged recovery | [24] |
| 73 yr | M | Bacteremia | Unpasteurized milk | nd | Died | [5] |
| 73 yr | M | Bacteremia, endocarditis | Unpasteurized milk | Aortic aneurysm | Died | [5] |
| 73 yr | M | Bacteremia, endophtalmitis, pneumonia | Horse | MI | Impaired visual acuity | [25] |
| 73 yr | F | Otitis | Unpasteurized milk | Polymorbid ** | Recovered | [26] |
| 74 yr | M | Bacteremia | Horses | None | Recovered | [27] |
| 75 yr | M | Bacteremia | Horse | None | Died | [28] |
| 79 yr | M | Wound infection | Horses | nd | nd | [29] |
| 80 yr | F | Bacteremia | Unpasteurized milk | nd | Died | [5] |
| 83 yr | F | Pneumonia | Unpasteurized cheese | Hypertension | Died | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argirova, P.; Kalchev, Y.; Baltadzhiev, I.; Stoycheva, M.; Murdjeva, M. Streptococcus zooepidemicus Meningitis in an HIV-Positive Horse Breeder Patient: A Case Study and Literature Review. Infect. Dis. Rep. 2023, 15, 527-534. https://doi.org/10.3390/idr15050052
Argirova P, Kalchev Y, Baltadzhiev I, Stoycheva M, Murdjeva M. Streptococcus zooepidemicus Meningitis in an HIV-Positive Horse Breeder Patient: A Case Study and Literature Review. Infectious Disease Reports. 2023; 15(5):527-534. https://doi.org/10.3390/idr15050052
Chicago/Turabian StyleArgirova, Petya, Yordan Kalchev, Ivan Baltadzhiev, Mariyana Stoycheva, and Marianna Murdjeva. 2023. "Streptococcus zooepidemicus Meningitis in an HIV-Positive Horse Breeder Patient: A Case Study and Literature Review" Infectious Disease Reports 15, no. 5: 527-534. https://doi.org/10.3390/idr15050052
APA StyleArgirova, P., Kalchev, Y., Baltadzhiev, I., Stoycheva, M., & Murdjeva, M. (2023). Streptococcus zooepidemicus Meningitis in an HIV-Positive Horse Breeder Patient: A Case Study and Literature Review. Infectious Disease Reports, 15(5), 527-534. https://doi.org/10.3390/idr15050052






