Enterobius vermicularis Related Acute Appendicitis: A Case Report and Review of the Literature
Abstract
1. Introduction
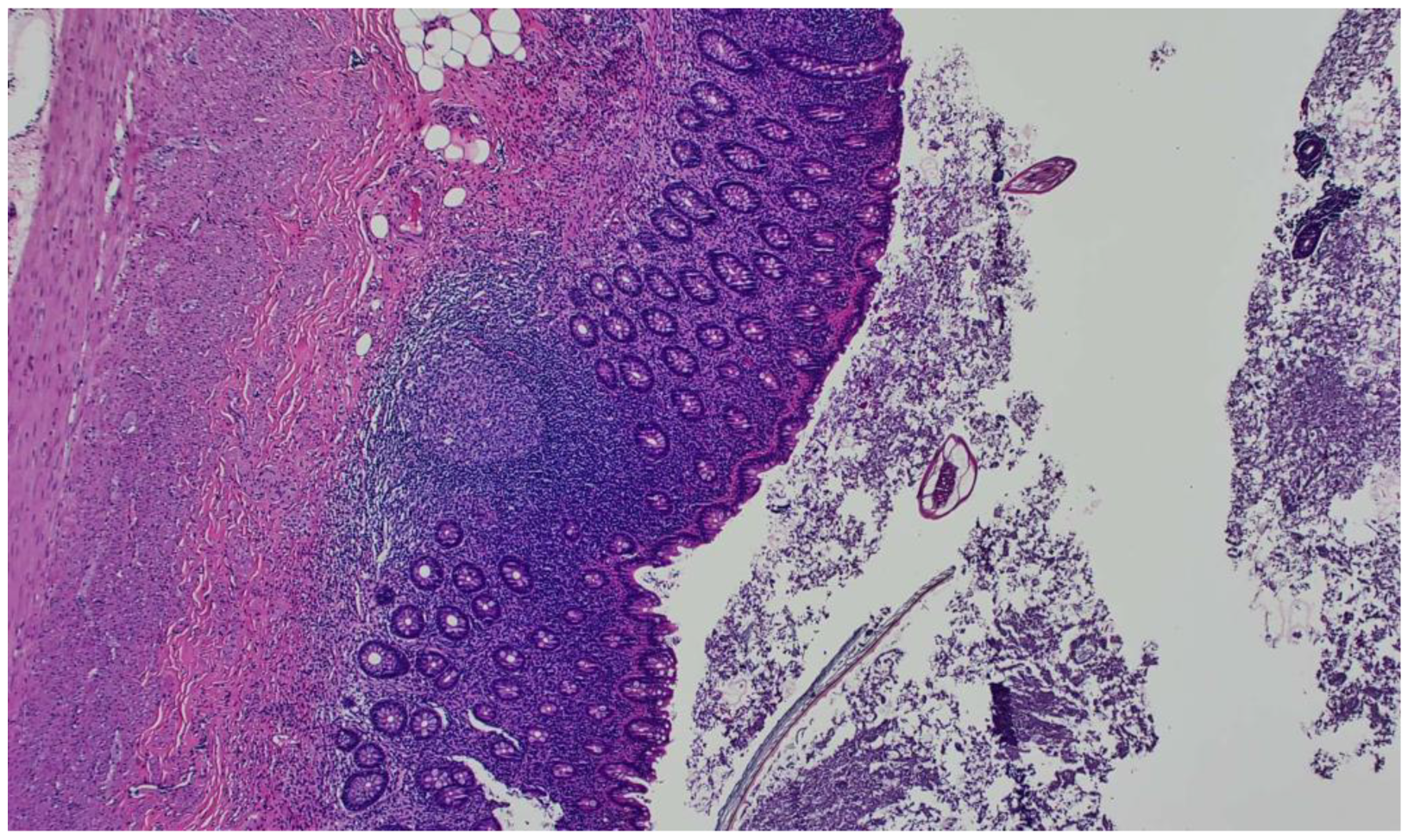
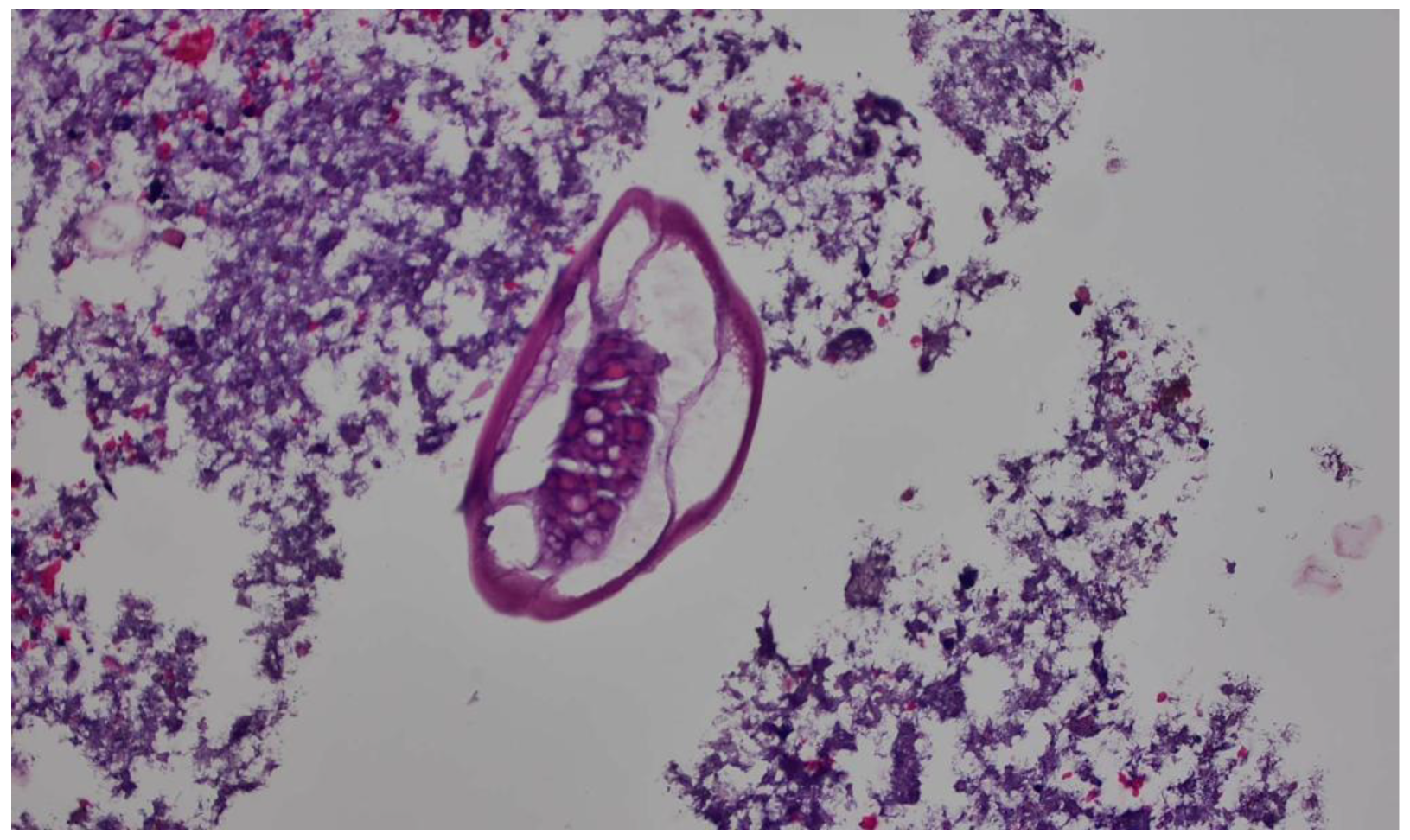
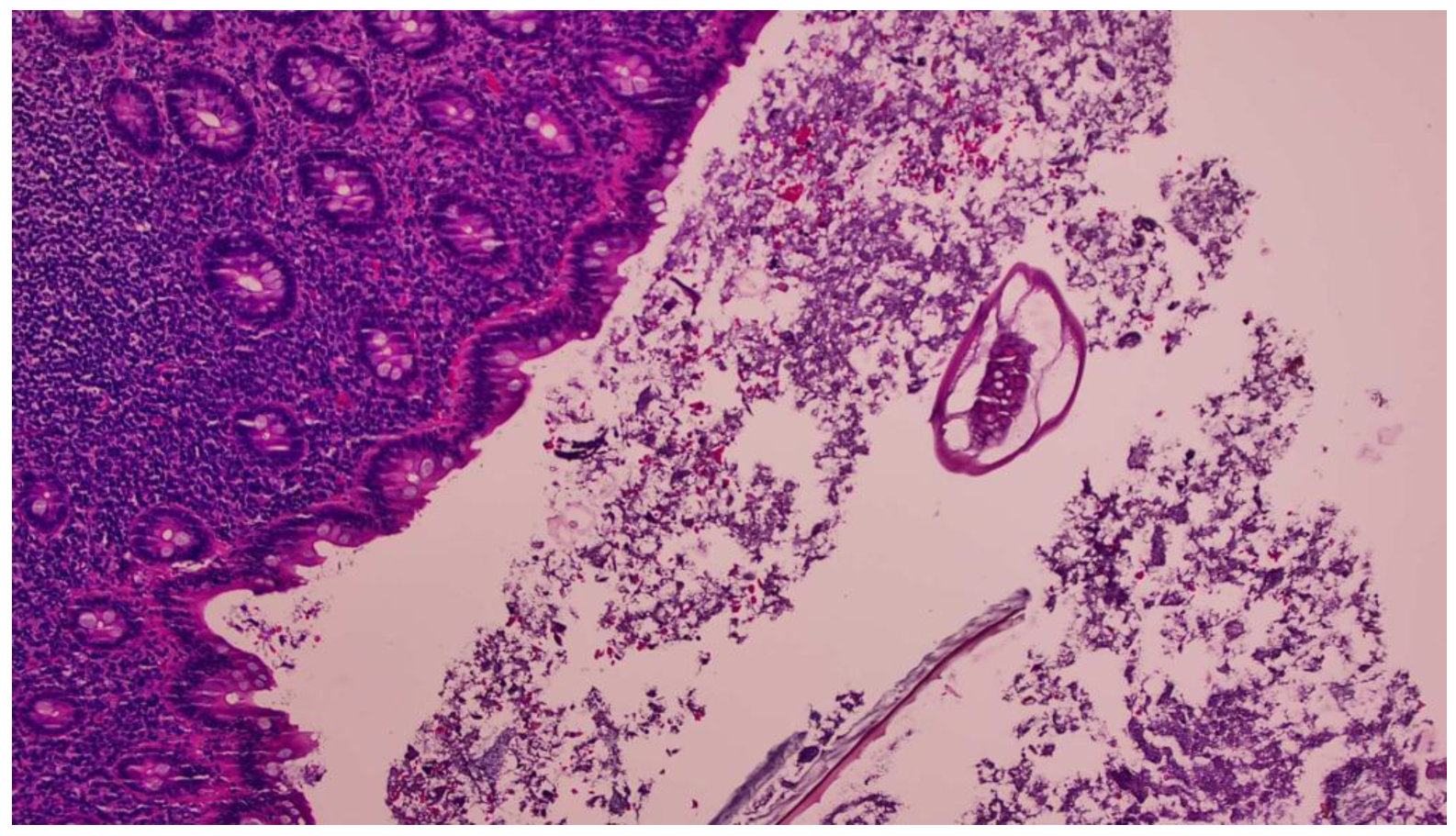
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmadi, M.H.; Seifmanesh, M. Taeniasis caused appendicitis without local tenderness: A rare case. Hosp. Chron. 2011, 6, 207–209. [Google Scholar]
- Moris, D.; Paulson, E.K.; Pappas, T.N. Diagnosis and Management of Acute Appendicitis in Adults: A Review. JAMA 2021, 326, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Kamalı, G.H.; Ulusoy, C.; Nikolovski, A.; Eğin, S.; Kamalı, S. Uncommon causes of acute appendicitis: Retrospective analysis of 6785 histopathological findings in a tertiary center. Turk. J. Trauma Emerg. Surg. 2022, 28, 1708–1715. [Google Scholar]
- Khayyat, R.; Belkebir, S.; Abuseir, S.; Barahmeh, M.; Alsadder, L.; Basha, W. Prevalence of and risk factors for Enterobius vermicularis infestation in preschool children, West Bank, Palestine, 2015. East Medditerr. Health J. 2021, 27, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- CDC. DPDx—Laboratory Identification of Parasites of Public Health Concern: Enterobiasis. Available online: https://www.cdc.gov/dpdx/enterobiasis/index.html (accessed on 28 May 2023).
- CDC. Parasites—Enterobiasis also Known as Pinworm Infection. Available online: https://www.cdc.gov/parasites/pinworm/epi.html (accessed on 28 May 2023).
- Wendt, S.; Trawinski, H.; Schubert, S.; Rodloff, A.C.; Mössner, J.; Lübbert, C. The Diagnosis and Treatment of Pinworm Infection. Dtsch. Arztebl. Int. 2019, 116, 213–219. [Google Scholar] [CrossRef]
- Mohtasebi, S. Parasitological and histopathological features of appendectomy specimens in Fars Province, southern Iran: A retrospective study. Ann. Med. Surg. 2023, 85, 1601–1606. [Google Scholar] [CrossRef]
- Stepek, G.; Buttle, D.J.; Duce, I.R.; Behnke, J.M. Human gastrointestinal nematode infections: Are new control methods required? Int. J. Exp. Pathol. 2006, 87, 325–341. [Google Scholar] [CrossRef]
- Fan, C.K.; Chaung, T.W.; Huang, Y.C.; Yin, A.W.; Chou, C.M.; Hsu, Y.T.; Kios, R.; Hsu, S.L.; Wang, Y.T.; Wu, M.S.; et al. Enterobius vermicularis infection: Prevalence and risk factors among preschool children in kindergarten in the capital area, Republic of the Marshall Islands. BMC Infect. Dis. 2019, 19, 536. [Google Scholar] [CrossRef]
- Romina Rivero, M.R.; De Angelo, C.; Feliziani, C.; Liang, S.; Tiranti, K.; Salas, M.M.; Salomon, O.D. Enterobiasis and its risk factors in urban, rural and indigenous children of subtropical Argentina. Parasitology 2022, 149, 396–406. [Google Scholar] [CrossRef]
- Gunawardena, N.K.; Chandrasena, T.N.; de Silva, N.R. Prevalence of enterobiasis among primary school children in Ragama, Sri Lanka. Ceylon Med. J. 2013, 58, 106–110. [Google Scholar] [CrossRef]
- Chen Kuang-Yao Chen, K.Y.C.; Yen, C.M.; Hwang, K.P.; Wang, L.C. Enterobius vermicularis infection and its risk factors among pre-school children in Taipei, Taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 559–564. [Google Scholar] [CrossRef]
- Bøås, H.; Tapia, G.; Sødahl, J.A.; Rasmussen, T.; Rønningen, K.S. Enterobius vermicularis and risk factors in healthy Norwegian children. Pediatr. Infect. Dis. J. 2012, 31, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Arkoulis, N.; Zerbinis, H.; Simatos, G.; Nisiotis, A. Enterobius vermicularis (pinworm) infection of the liver mimicking malignancy: Presentation of a new case and review of current literature. Int. J. Surg. Case. Rep. 2012, 3, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Pigac, B.; Mašić, S.; Mašić, V. Enterobius vermicularis in the endometrium of the uterus: A case report. Iran. J. Parasitol. 2017, 12, 638–641. [Google Scholar] [PubMed]
- Santos, V.M.; Silva, M.B.; Bernardes, J.M.; Lima, M.A. Nódulo granulomatoso com Enterobius vermicularis em epíploon simulando metástase de câncer de ovário [Granulomatous nodule with Enterobius vermicularis in epiploon simulating metastasis of ovarian cancer]. Rev. Soc. Bras. Med. Trop. 2002, 35, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Tariq, M.U.; Ahmed, Z. ectopic Enterobius vermicularis infestation; an extremely rare cause of mesenteric lymphadenopathy mimicking tuberculous lymphadenitis. J. Ayub Med. Coll. Abbottabad 2018, 30, 124–126. [Google Scholar]
- Prystowsky, J.B.; Pugh, C.M.; Nagle, A.P. Current problems in surgery. Appendicitis. Curr. Probl. Surg. 2005, 42, 688–742. [Google Scholar] [CrossRef]
- Yilmaz, M.; Akbulut, S.; Kutluturk, K.; Sahin, N.; Arabaci, E.; Ara, C.; Yilmaz, S. Unusual histopathological findings in appendectomy specimens from patients with suspected acute appendicitis. World J. Gastroenterol. 2013, 19, 4015–4022. [Google Scholar] [CrossRef]
- Zouari, M.; Louati, H.; Abid, I.; Trabelsi, F.; Ben Dhaou, M.; Jallouli, M.; Mhiri, R. Enterobius vermicularis: A cause of abdominal pain mimicking acute appendicitis in children. A retrospective cohort study. Arch. Iran. Med. 2018, 21, 67–72. [Google Scholar]
- Hooshyar, H.; Jannati Dastgerdi, M.; Kazemi, E. Acute appendicitis: A case report of hyperinfection with Enterobius vermicularis. Gastroenterol. Hepatol. Bed Bench 2021, 14, 286–289. [Google Scholar]
- Hammood, Z.D.; Salih, A.M.; Mohammed, S.H.; Kakamad, F.H.; Salih, K.M.; Omar, D.A.; Hassan, M.N.; Sidiq, S.H.; Mustafa, M.Q.; Habibullah, I.J.; et al. Enterobius vermicularis causing acute appendicitis, a case report with literature review. Int. J. Surg. Case Rep. 2019, 63, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Akkapulu, N.; Abdullazade, S. Is Enterobius vermicularis infestation associated with acute appendicitis? Eur. J. Trauma Emerg. Surg. 2016, 42, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, R.; Eaton, S.; Stanton, M.P.; Pierro, A.; Hall, N.J. Efficacy and safety of nonoperative treatment for acute appendicitis: A meta-analysis. Pediatrics 2017, 139, e2016300. [Google Scholar] [CrossRef] [PubMed]
- Karatepe, O.; Adas, G.; Tukenmez, M.; Battal, M.; Altiok, M.; Karahan, S. Parasitic infestation as cause of acute appendicitis. G. Chir. 2009, 30, 426–428. [Google Scholar] [PubMed]
- Flores Uribe, A.; Pérez Macías, J.P.; González Arévalo, J.A.; Flores Uribe, O.A. Diagnosis and surgical intervention of acute appendicitis secondary to Enterobius vermicularis: Case report. Int. J. Surg. Case Rep. 2022, 99, 107678. [Google Scholar] [CrossRef] [PubMed]
- Alshihmani, S.H.A. Acute appendicitis due to infection with Enterobius vermicularis, A case report. Ann. Med. Surg. 2022, 80, 104094. [Google Scholar] [CrossRef]
- Antilahy, J.A.; Akhoundi, M.; Belaloui, M.; Borovkov, A.; Marteau, A.; Bonte, E.; Izri, A. Acute appendicitis caused by Enterobius vermicularis: Observations from a case report. IDCases 2021, 25, e01227. [Google Scholar] [CrossRef]
- Lala, S.; Upadhyay, V. Enterobius vermicularis and its role in paediatric appendicitis: Protection or predisposition? ANZ J. Surg. 2016, 86, 717–719. [Google Scholar] [CrossRef]
- Fleming, C.A.; Kearney, D.E.; Moriarty, P.; Redmond, H.P.; Andrews, E.J. An evaluation of the relationship between Enterobius vermicularis infestation and acute appendicitis in a paediatric population—A retrospective cohort study. Int. J. Surg. 2015, 18, 154–158. [Google Scholar] [CrossRef]
- Hamdona, S.M.; Lubbad, A.M.; Al-Hindi, A.I. Histopathological study of Enterobius vermicularis among appendicitis patients in Gaza strip, Palestine. J. Parasit. Dis. 2016, 40, 176–183. [Google Scholar] [CrossRef]
- Upadhyaya, P.; Sinha, A.; Agarwal, M.; Paudyal, P.; Shrestha, A. Incidental Enterobius vermicularis infestation in surgically removed appendices with a clinical diagnosis of acute appendicitis: A retrospective analysis. J. Pathol. Nepal. 2015, 5, 720–722. [Google Scholar] [CrossRef]
| Authors/References | Year of Publication | No. of Cases | Gender, Mean Age in Years | Treatment | Country |
|---|---|---|---|---|---|
| Flores [27] | 2022 | 1 | Male, 10 | Laparoscopic appendicectomy/anthelminthic | Mexico |
| Alshihmani [28] | 2022 | 1 | Male, 17 | Open appendicectomy/anti-helminthic | Iraq |
| Hooshyar [22] | 2021 | 1 | Female, 8 | Open appendicectomy | Iran |
| Antilahy [29] | 2021 | 1 | Female, 5 | Laparoscopic appendicectomy/anthelminthic | France |
| Lala [30] | 2014 | 109 | NA, 11.4 | Laparoscopic appendicectomy/anthelminthic | New Zealand |
| Akkapulu [24] | 2015 | 9 | 7 females, 2 males, 31 | Appendectomy | Turkey |
| Fleming [31] | 2013 | 13 | NA, 11 | Appendectomy | Ireland |
| Hamdona [32] | 2013 | 30 | 17 male, 13 female, NA | Appendectomy | Palestine |
| Upadhyaya [33] | 2015 | 7 | 4 females, 3 males, 25 | Appendectomy | Nepal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhetri, S.; Al Mamari, A.H.; Al Awfi, M.M.; Al Khaldi, N.H.N.; Abed, N.M.; Pandak, N.; Khamis, F.; Balushi, Z.A.; Alalawi, R.M.K.; Al Lawati, S.; et al. Enterobius vermicularis Related Acute Appendicitis: A Case Report and Review of the Literature. Infect. Dis. Rep. 2023, 15, 417-424. https://doi.org/10.3390/idr15040042
Chhetri S, Al Mamari AH, Al Awfi MM, Al Khaldi NHN, Abed NM, Pandak N, Khamis F, Balushi ZA, Alalawi RMK, Al Lawati S, et al. Enterobius vermicularis Related Acute Appendicitis: A Case Report and Review of the Literature. Infectious Disease Reports. 2023; 15(4):417-424. https://doi.org/10.3390/idr15040042
Chicago/Turabian StyleChhetri, Shabnam, Ahmed Hamood Al Mamari, Mahmood Mausd Al Awfi, Nasser Humaid Nasser Al Khaldi, Nibras Mejbel Abed, Nenad Pandak, Faryal Khamis, Zakariya Al Balushi, Rashid Mohammed Khamis Alalawi, Sultan Al Lawati, and et al. 2023. "Enterobius vermicularis Related Acute Appendicitis: A Case Report and Review of the Literature" Infectious Disease Reports 15, no. 4: 417-424. https://doi.org/10.3390/idr15040042
APA StyleChhetri, S., Al Mamari, A. H., Al Awfi, M. M., Al Khaldi, N. H. N., Abed, N. M., Pandak, N., Khamis, F., Balushi, Z. A., Alalawi, R. M. K., Al Lawati, S., Ba’Omar, M., Shukaili, N., & Al-Abri, S. (2023). Enterobius vermicularis Related Acute Appendicitis: A Case Report and Review of the Literature. Infectious Disease Reports, 15(4), 417-424. https://doi.org/10.3390/idr15040042







