Modulation of the Gut Microbiota to Control Antimicrobial Resistance (AMR)—A Narrative Review with a Focus on Faecal Microbiota Transplantation (FMT)
Abstract
1. Introduction
1.1. Antimicrobial Resistance (AMR)
1.2. The Gastrointestinal Tract as a Reservoir of AMR
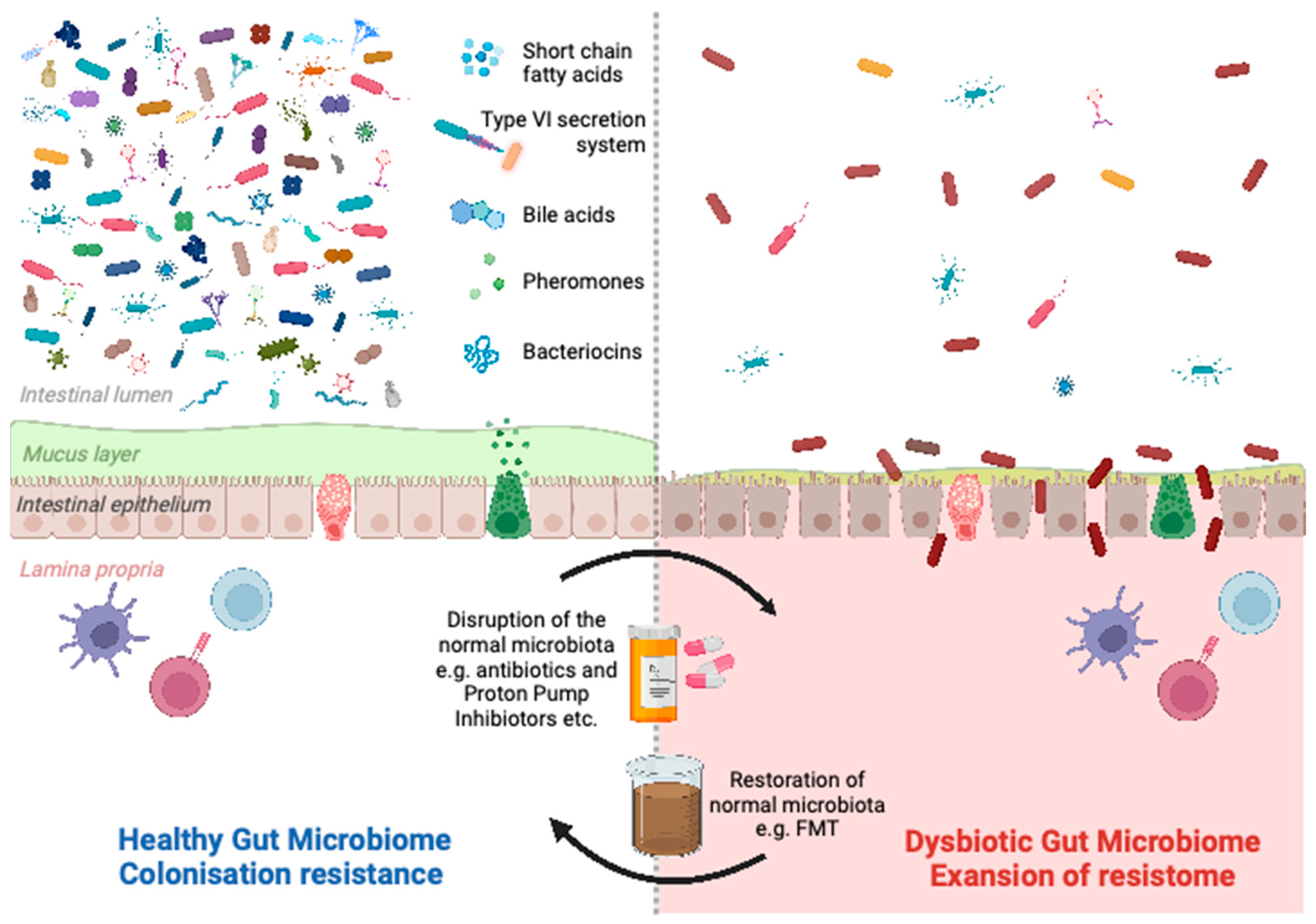
1.3. Colonisation Resistance
1.4. Modulation of the Gut Microbiota
2. Strategies to Reduce Expansion of the Gut Resistome by Modulating the Gut Microbiota
2.1. Diet/Prebiotics
2.1.1. Live Biotherapeutics
2.1.2. Bacteriophages
2.1.3. Faecal Microbiota Transplantation (FMT)
2.2. Randomised Controlled Trials (RCTs)
2.3. Non-Randomised Trials
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleming, A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Abraham, E.; Chain, E.; Fletcher, C.; Gardner, A.; Heatley, N.; Jennings, M.; Florey, H. Further observations on penicillin. Lancet 1941, 238, 177–189. [Google Scholar] [CrossRef]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, A.E.P.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Ikhimiukor, O.O.; Odih, E.E.; Donado-Godoy, P.; Okeke, I.N. A bottom-up view of antimicrobial resistance transmission in developing countries. Nat. Microbiol. 2022, 7, 757–765. [Google Scholar] [CrossRef]
- Esteve-Palau, E.; Solande, G.; Sánchez, F.; Sorlí, L.; Montero, M.; Güerri, R.; Villar, J.; Grau, S.; Horcajada, J. Clinical and economic impact of urinary tract infections caused by ESBL-producing Escherichia coli requiring hospitalization: A matched cohort study. J. Infect. 2015, 71, 667–674. [Google Scholar] [CrossRef]
- Tamma, P.D.; Goodman, K.E.; Harris, A.D.; Tekle, T.; Roberts, A.; Taiwo, A.; Simner, P.J. Comparing the Outcomes of Patients with Carbapenemase-Producing and Non-Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Bacteremia. Clin. Infect. Dis. 2017, 64, 257–264. [Google Scholar] [CrossRef]
- Collaborators Antimicrobial Resistance. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- World Health Organization. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. 2019. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 28 November 2022).
- Willems, R.P.J.; van Dijk, K.; Vehreschild, M.J.G.T.; Biehl, L.M.; Ket, J.C.F.; Remmelzwaal, S.; E Vandenbroucke-Grauls, C.M.J. Incidence of infection with multidrug-resistant Gram-negative bacteria and vancomycin-resistant enterococci in carriers: A systematic review and meta-regression analysis. Lancet Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Tacconelli, E.; Mazzaferri, F.; de Smet, A.M.; Bragantini, D.; Eggimann, P.; Huttner, B.; Kuijper, E.J.; Lucet, J.-C.; Mutters, N.T.; Sanguinetti, M.; et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 2019, 25, 807–817. [Google Scholar] [CrossRef]
- Leo, S.; Lazarevic, V.; Girard, M.; Gaïa, N.; Schrenzel, J.; de Lastours, V.; Fantin, B.; Bonten, M.; Carmeli, Y.; Rondinaud, E.; et al. Metagenomic Characterization of Gut Microbiota of Carriers of Extended-Spectrum Beta-Lactamase or Carbapenemase-Producing Enterobacteriaceae Following Treatment with Oral Antibiotics and Fecal Microbiota Transplantation: Results from a Multicenter Randomized Trial. Microorganisms 2020, 8, 941. [Google Scholar]
- Anderson, E.S. Transferable drug resistance. Br. Med. J. 1969, 2, 397–398. [Google Scholar]
- Karami, N.; Martner, A.; Enne, V.I.; Swerkersson, S.; Adlerberth, I.; Wold, A.E. Transfer of an ampicillin resistance gene between two Escherichia coli strains in the bowel microbiota of an infant treated with antibiotics. J. Antimicrob. Chemother. 2007, 60, 1142–1145. [Google Scholar] [CrossRef]
- Lanza, V.F.; Tedim, A.P.; Martinez, J.L.; Baquero, F.; Coque, T.M. The Plasmidome of Firmicutes: Impact on the Emergence and the Spread of Resistance to Antimicrobials. Microbiol. Spectr. 2015, 3, PLAS-0039-2014. [Google Scholar] [CrossRef]
- Carlet, J. The gut is the epicentre of antibiotic resistance. Antimicrob. Resist. Infect. Control 2012, 1, 39. [Google Scholar] [CrossRef]
- Miller, C.P.; Bohnhoff, M.; Rifkind, D. The effect of an antibiotic on the susceptibility of the mouse’s intestinal tract to Salmonella infection. Trans. Am. Clin. Climatol. Assoc. 1956, 68, 51–55, discussion 55–58. [Google Scholar]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: Mechanisms and regulation. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Teng, C.; Reveles, K.R.; Obodozie-Ofoegbu, O.O.; Frei, C.R. Clostridium difficile Infection Risk with Important Antibiotic Classes: An Analysis of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2019, 16, 630–635. [Google Scholar] [CrossRef]
- Huttner, B.; de Lastours, V.; Wassenberg, M.; Maharshak, N.; Mauris, A.; Galperine, T.; Zanichelli, V.; Kapel, N.; Bellanger, A.; Olearo, F.; et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A randomized clinical trial. Clin. Microbiol. Infect. 2019, 25, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Shamsaddini, A.; Fagan, A.; Sterling, R.K.; Gavis, E.; Khoruts, A.; Fuchs, M.; Lee, H.; Sikaroodi, M.; Gillevet, P.M. Fecal Microbiota Transplant in Cirrhosis Reduces Gut Microbial Antibiotic Resistance Genes: Analysis of Two Trials. Hepatol. Commun. 2021, 5, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Langdon, A.; Program, F.T.C.P.E.; Schwartz, D.J.; Bulow, C.; Sun, X.; Hink, T.; Reske, K.A.; Jones, C.; Burnham, C.-A.D.; Dubberke, E.R.; et al. Microbiota restoration reduces antibiotic-resistant bacteria gut colonization in patients with recurrent Clostridioides difficile infection from the open-label PUNCH CD study. Genome Med. 2021, 13, 28. [Google Scholar] [CrossRef]
- Ghani, R.; Mullish, B.H.; McDonald, J.A.K.; Ghazy, A.; Williams, H.R.T.; Brannigan, E.T.; Mookerjee, S.; Satta, G.; Gilchrist, M.; Duncan, N.; et al. Disease Prevention Not Decolonization: A Model for Fecal Microbiota Transplantation in Patients Colonized with Multidrug-resistant Organisms. Clin. Infect. Dis. 2021, 72, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yoseph, H.; Carasso, S.; Shklar, S.; Korytny, A.; Dar, R.E.; Daoud, H.; Nassar, R.; Maharshak, N.; Hussein, K.; Geffen, Y.; et al. Oral Capsulized Fecal Microbiota Transplantation for Eradication of Carbapenemase-producing Enterobacteriaceae Colonization with a Metagenomic Perspective. Clin. Infect. Dis. 2021, 73, e166–e175. [Google Scholar] [CrossRef]
- Bilsen, M.P.; Lambregts, M.M.; van Prehn, J.; Kuijper, E.J. Faecal microbiota replacement to eradicate antimicrobial resistant bacteria in the intestinal tract—A systematic review. Curr. Opin. Gastroenterol. 2022, 38, 15–25. [Google Scholar] [CrossRef]
- ISAPP. Prebiotics. 2020. Available online: https://isappscience.org/for-scientists/resources/prebiotics/ (accessed on 7 March 2023).
- Oliver, A.; Xue, Z.; Villanueva, Y.T.; Durbin-Johnson, B.; Alkan, Z.; Taft, D.H.; Liu, J.; Korf, I.; Laugero, K.D.; Stephensen, C.B.; et al. Association of Diet and Antimicrobial Resistance in Healthy U.S. Adults. mBio 2022, 13, e00101–e00122. [Google Scholar] [CrossRef]
- Mulder, M.; Jong, J.K.-D.; Goessens, W.; de Visser, H.; Ikram, M.A.; Verbon, A.; Stricker, B. Diet as a risk factor for antimicrobial resistance in community-acquired urinary tract infections in a middle-aged and elderly population: A case–control study. Clin. Microbiol. Infect. 2019, 25, 613–619. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Moens, F.; Abbeele, P.V.D.; Basit, A.W.; Dodoo, C.; Chatterjee, R.; Smith, B.; Gaisford, S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int. J. Pharm. 2019, 555, 1–10. [Google Scholar] [CrossRef]
- Yakult UK, Ltd. 2021. Available online: https://www.yakult.co.uk/products/yakult-original/ (accessed on 23 January 2023).
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F.; Pintado, T.; de Menezes, C.R.; Fries, L.L.M. Effect of different strategies of Lactobacillus plantarum incorporation in chorizo sausages. J. Sci. Food Agric. 2019, 99, 6706–6712. [Google Scholar] [CrossRef]
- Weng, Y.-J.; Jiang, D.-X.; Liang, J.; Ye, S.-C.; Tan, W.-K.; Yu, C.-Y.; Zhou, Y. Effects of Pretreatment with Bifidobacterium bifidum Using 16S Ribosomal RNA Gene Sequencing in a Mouse Model of Acute Colitis Induced by Dextran Sulfate Sodium. Med. Sci. Monit. 2021, 27, e928478. [Google Scholar] [CrossRef]
- Sasaki, K.; Sasaki, D.; Inoue, J.; Hoshi, N.; Maeda, T.; Yamada, R.; Kondo, A. Bacillus coagulans SANK 70258 suppresses Enterobacteriaceae in the microbiota of ulcerative colitis in vitro and enhances butyrogenesis in healthy microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 3859–3867. [Google Scholar] [CrossRef]
- Saliu, E.-M.; Ren, H.; Boroojeni, F.G.; Zentek, J.; Vahjen, W. The Impact of Direct-Fed Microbials and Phytogenic Feed Additives on Prevalence and Transfer of Extended-Spectrum Beta-Lactamase Genes in Broiler Chicken. Microorganisms 2020, 8, 322. [Google Scholar] [CrossRef]
- Karbalaei, M.; Keikha, M. Probiotics and intestinal decolonization of antibiotic-resistant microorganisms; A reality or fantasy? Ann. Med. Surg. 2022, 80, 104269. [Google Scholar] [CrossRef]
- Ljungquist, O.; Kampmann, C.; Resman, F.; Riesbeck, K.; Tham, J. Probiotics for intestinal decolonization of ESBL-producing Enterobacteriaceae: A randomized, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2020, 26, 456–462. [Google Scholar] [CrossRef]
- Rutter, J.W.; Dekker, L.; Owen, K.A.; Barnes, C.P. Microbiome engineering: Engineered live biotherapeutic products for treating human disease. Front. Bioeng. Biotechnol. 2022, 10, 1000873. [Google Scholar] [CrossRef]
- Chanishvili, N. Phage Therapy—History from Twort and d’Herelle through soviet experience to current approaches. Adv. Virus Res. 2012, 83, 3–40. [Google Scholar]
- Baral, B. Phages against killer superbugs: An enticing strategy against antibiotics-resistant pathogens. Front. Pharmacol. 2023, 14, 1036051. [Google Scholar] [CrossRef]
- Bernasconi, O.J.; Campos-Madueno, E.I.; Donà, V.; Perreten, V.; Carattoli, A.; Endimiani, A. Investigating the use of bacteriophages as a new decolonization strategy for intestinal carriage of CTX-M-15-producing ST131 Escherichia coli: An in vitro continuous culture system model. J. Glob. Antimicrob. Resist. 2020, 22, 664–671. [Google Scholar] [CrossRef]
- Fang, Q.; Feng, Y.; McNally, A.; Zong, Z. Characterization of phage resistance and phages capable of intestinal decolonization of carbapenem-resistant Klebsiella pneumoniae in mice. Commun. Biol. 2022, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Galtier, M.; De Sordi, L.; Maura, D.; Arachchi, H.; Volant, S.; Dillies, M.; Debarbieux, L. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol. 2016, 18, 2237–2245. [Google Scholar] [CrossRef]
- Javaudin, F.; Bémer, P.; Batard, E.; Montassier, E. Impact of Phage Therapy on Multidrug-Resistant Escherichia coli Intestinal Carriage in a Murine Model. Microorganisms 2021, 9, 2580. [Google Scholar] [CrossRef] [PubMed]
- Corbellino, M.; Kieffer, N.; Kutateladze, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Tsertsvadze, G.; Rimoldi, S.G.; Nizharadze, D.; Hoyle, N.; et al. Eradication of a Multidrug-Resistant, Carbapenemase-Producing Klebsiella pneumoniae Isolate Following Oral and Intra-rectal Therapy with a Custom Made, Lytic Bacteriophage Preparation. Clin. Infect. Dis. 2019, 70, 1998–2001. [Google Scholar] [CrossRef]
- Kuipers, S.; Ruth, M.M.; Mientjes, M.; de Sévaux, R.G.L.; van Ingen, J. A Dutch Case Report of Successful Treatment of Chronic Relapsing Urinary Tract Infection with Bacteriophages in a Renal Transplant Patient. Antimicrob. Agents Chemother. 2019, 64, e01281-19. [Google Scholar] [CrossRef] [PubMed]
- Brives, C.; Pourraz, J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J. Hosp. Infect. 2018, 100 (Suppl. 1), S1–S31. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.-H.; Park, S.-H.; Cha, B.; Kwon, K.S.; Kim, H.; Shin, Y.W. Efficacy and Safety of Fecal Microbiota Transplantation for Clearance of Multidrug-Resistant Organisms under Multiple Comorbidities: A Prospective Comparative Trial. Biomedicines 2022, 10, 2404. [Google Scholar] [CrossRef]
- Goldenberg, S.D.; Merrick, B. The role of faecal microbiota transplantation: Looking beyond Clostridioides difficile infection. Ther. Adv. Infect. Dis. 2021, 8, 2049936120981526. [Google Scholar]
- Millan, B.; Park, H.; Hotte, N.; Mathieu, O.; Burguiere, P.; Tompkins, T.A.; Kao, D.; Madsen, K.L. Fecal Microbial Transplants Reduce Antibiotic-resistant Genes in Patients with Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2016, 62, 1479–1486. [Google Scholar] [CrossRef]
- Lamberte, L.E.; van Schaik, W. Antibiotic resistance in the commensal human gut microbiota. Curr. Opin. Microbiol. 2022, 68, 102150. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. REBYOTA. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/rebyota (accessed on 23 January 2023).
- Huttner, B.; Haustein, T.; Uckay, I.; Renzi, G.; Stewardson, A.; Schaerrer, D.; Agostinho, A.; Andremont, A.; Schrenzel, J.; Pittet, D.; et al. Decolonization of intestinal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: A randomized, double-blind, placebo-controlled trial. J. Antimicrob. Chemother. 2013, 68, 2375–2382. [Google Scholar]
- Zipursky, J.S.; Sidorsky, T.I.; Freedman, C.A.; Kirkland, K.B. Patient Attitudes Toward the Use of Fecal Microbiota Transplantation in the Treatment of Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2012, 55, 1652–1658. [Google Scholar] [CrossRef]
- Guilfoyle, J.; Considine, J.; Bouchoucha, S.L. Faecal microbiota transplantation and the patient experience: A systematic review. J. Clin. Nurs. 2021, 30, 1236–1252. [Google Scholar] [CrossRef]
- Zain, N.M.M.; ter Linden, D.; Lilley, A.K.; Royall, P.G.; Tsoka, S.; Bruce, K.D.; Mason, A.J.; Hatton, G.B.; Allen, E.; Goldenberg, S.D.; et al. Design and manufacture of a lyophilised faecal microbiota capsule formulation to GMP standards. J. Control. Release 2022, 350, 324–331. [Google Scholar] [CrossRef]
- Hyde, M.K.; Masser, B.M. Determinants of community members’ willingness to donate stool for faecal microbiota transplantation. PLoS ONE 2020, 15, e0243751. [Google Scholar] [CrossRef]
- Ianiro, G.; Porcari, S.; Bibbò, S.; Giambò, F.; Quaranta, G.; Masucci, L.; Sanguinetti, M.; Gasbarrini, A.; Cammarota, G. Donor program for fecal microbiota transplantation: A 3-year experience of a large-volume Italian stool bank. Dig. Liver Dis. 2021, 53, 1428–1432. [Google Scholar] [CrossRef]
- Bénard, M.V.; de Bruijn, C.M.A.; Fenneman, A.C.; Wortelboer, K.; Zeevenhoven, J.; Rethans, B.; Herrema, H.J.; van Gool, T.; Nieuwdorp, M.; Benninga, M.A.; et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS ONE 2022, 17, e0276323. [Google Scholar] [CrossRef]
- Quaranta, G.; Ianiro, G.; De Maio, F.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Bibbo, S.; Amedei, A.; Sanguinetti, M.; et al. “Bacterial Consortium”: A Potential Evolution of Fecal Microbiota Transplantation for the Treatment of Clostridioides difficile Infection. BioMed Res. Int. 2022, 2022, 5787373. [Google Scholar] [CrossRef]
- De Oliveira, C.P.; da Silva, J.A.; de Siqueira-Junior, J.P. Nature of the antimicrobial activity of Lactobacillus casei, Bifidobacterium bifidum and Bifidobacterium animalis against foodborne pathogenic and spoilage microorganisms. Nat. Prod. Res. 2015, 29, 2133–2136. [Google Scholar] [CrossRef]
- Battipaglia, G.; Malard, F.; Rubio, M.T.; Ruggeri, A.; Mamez, A.C.; Brissot, E.; Giannotti, F.; Dulery, R.; Joly, A.C.; Baylatry, M.T.; et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 2019, 104, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, L.; Allegretti, J.R.; Aroniadis, O.; Brandt, L.; Donovan, D.; Fischer, M.; Grinspan, A.; Kassam, Z.; Kelly, C.R.; Kim, C.; et al. Clearance of Vancomycin-Resistant Enterococcus Colonization with Fecal Microbiota Transplantation Among Patients with Recurrent Clostridium difficile Infection. Open Forum Infect. Dis. 2016, 3 (Suppl. 1), S599. [Google Scholar] [CrossRef]
- Davido, B.; Batista, R.; Fessi, H.; Michelon, H.; Escaut, L.; Lawrence, C.; Denis, M.; Perronne, C.; Salomon, J.; Dinh, A. Fecal microbiota transplantation to eradicate vancomycin-resistant enterococci colonization in case of an outbreak. Med. Mal. Infect. 2018, 49, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Fessi, H.; Duran, C.; Batista, R.; Michelon, H.; Bouchand, F.; Lepeule, R.; Vittecoq, D.; Escaut, L.; Sobhani, I.; et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: A prospective comparative study. J. Hosp. Infect. 2018, 99, 481–486. [Google Scholar] [CrossRef]
- Saïdani, N.; Lagier, J.-C.; Cassir, N.; Million, M.; Baron, S.; Dubourg, G.; Eldin, C.; Kerbaj, J.; Valles, C.; Raoult, D.; et al. Faecal microbiota transplantation shortens the colonisation period and allows re-entry of patients carrying carbapenamase-producing bacteria into medical care facilities. Int. J. Antimicrob. Agents 2019, 53, 355–361. [Google Scholar] [CrossRef]
- Lee, J.-J.; Yong, D.; Suk, K.T.; Kim, D.J.; Woo, H.-J.; Lee, S.S.; Kim, B.-S. Alteration of Gut Microbiota in Carbapenem-Resistant Enterobacteriaceae Carriers during Fecal Microbiota Transplantation According to Decolonization Periods. Microorganisms 2021, 9, 352. [Google Scholar] [CrossRef]
- Seong, H.; Kil Lee, S.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Jeong, W.Y.; Lee, W.J.; Sohn, Y.; Cho, Y.; et al. Fecal Microbiota Transplantation for multidrug-resistant organism: Efficacy and Response prediction. J. Infect. 2020, 81, 719–725. [Google Scholar] [CrossRef]
- Bilinski, J.; Grzesiowski, P.; Sorensen, N.; Madry, K.; Muszynski, J.; Robak, K.; Wroblewska, M.; Dzieciatkowski, T.; Dulny, G.; Dwilewicz-Trojaczek, J.; et al. Fecal Microbiota Transplantation in Patients with Blood Disorders Inhibits Gut Colonization with Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin. Infect. Dis. 2017, 65, 364–370. [Google Scholar] [CrossRef]
| NCT/EUCTR Number | Study Design and Location | Enrolment (n) | Start Date | Estimated Completion (and Preliminary Results If Posted) | Inclusion Criteria | Arms and Interventions | Primary Outcome (Secondary Outcome Is Mentioned If Relevant) |
|---|---|---|---|---|---|---|---|
| NCT05632315 | Randomised, open-label, controlled trial USA | 150 estimated | January 2023 | January 2026 | Adults with MDRO infection receiving appropriate antimicrobial therapy for at least 5 days, with at least 2, but no more than 7 days of treatment remaining | Group 1: FMT via enema or suspension Group 2: standard of care | Decolonisation rate at 1 month. Frequency of adverse events in 6 months |
| NCT03802461 | Randomised, open-label, controlled trial Canada | 40 estimated | March 2019 | March 2024 | Adults with ≥1 rectal swab, groin, stool, or urine specimen positive for CRE within the past month | Group 1: bowel lavage followed by FMT (50 g healthy donor stool)administered by enema, given on 3 occasions Group 2: no intervention | Decolonisation rate (undefined) after 3 months |
| NCT04188743 | Randomised, double-blind, controlled trial Belgium | 150 estimated | December 2019 | December 2023 | Adults with at least two consecutive confirmations of MDRO colonisation in faeces | Group 1: allogenic FMT: 50 g of healthy donor stool, frozen, administered by nasoduodenal tube Group 2: autologous FMT: 50 g of own stool, frozen, administered by nasoduodenal tube Group 3: no intervention | Decolonisation rate, defined as three consecutive negative stool cultures in minimal time span of 2 weeks, after 1 month after treatment |
| NCT04181112 | Randomised, open-label, controlled trial Canada | 90 estimated | November 2019 | November 2023 | Adult renal transplant recipients colonised with a multidrug-resistant organism (undefined), confirmed by rectal swab or stool culture | Group 1: FMT using retention enema Group 2: antibiotic pre-treatment (undefined) followed by FMT using retention enema Group 3: no intervention | Decolonisation rate, defined by negative culture/PCR at 14 and 30 days post-FMT |
| NCT04746222 | Randomised, double-blind, controlled trial Singapore | 108 estimated | July 2021 | July 2023 | Adults (age ≥21) colonisation with CRE, confirmed with at least one positive rectal swab (PCR) taken ≤7 days before randomisation. Antibiotics ceased for at least 48 h pre-randomisation evaluation. | Group 1: single dose of 30 oral capsules containing healthy donor stool Group 2: single dose of 30 placebo capsules | Decolonisation rate, defined by negative rectal swab (PCR/culture), at 12 weeks |
| NCT04759001 | Randomised, double-blind, controlled trial Italy | 52 estimated | February 2021 | February 2023 | Adults with CRE colonisation, confirmed by a rectal swab | Group 1: FMT by colonoscopy with healthy donor stool Group 2: placebo (water) administered through colonoscopy | Decolonisation rate, defined by negative rectal swab at 4 weeks |
| NCT04431934 | Randomised, open-label, controlled trial Spain | 437 estimated | November 2020 | December 2022 (still recruiting January 2023) | Adults with documented rectal colonisation with multidrug-resistant Gram-negative bacteria, eligible for routine digestive decolonisation | 7 days of non-absorbable antibiotics followed by: Group 1: FMT 2 doses, once a week, 14–17 capsules per dose (dose is equivalent to 50 g of healthy donor stool) Group 2: 2 sachets of probiotics every 12 h for 14 days Group 3: no intervention | Decolonisation rate, defined as negative rectal swab after 60 days |
| NCT04760665 | Randomised, double-blind, controlled trial Spain | 120 estimated | April 2021 | July 2022 (still recruiting January 2023) | Adult patients colonised with KPC-producing Klebsiella pneumoniae (undefined) without an active infection in the month prior to inclusion | Group 1: four oral capsules containing healthy donor faeces Group 2: four oral placebo capsules | Decolonisation rate (undefined) at 30 days |
| NCT04146337 | Randomised, open-label, controlled trial Israel | 3/60 actual | October 2020 | June 2022 (marked as completed, no results published yet) | Adult inpatients positive for CRE of any strain and resistance mechanism in rectal surveillance stool samples, with or without CRE clinical samples. A positive rectal swab within one week before randomization is mandatory. | Group 1: FMT, 15 capsules a day for two consecutive days after an eight-hour fast Group 2: no intervention | Decolonisation rate, defined as three consecutive negative rectal cultures, at 28 days |
| EUCTR2019-001618-41 | Randomised, participant-blinded, controlled, feasibility trial UK | 44/80 actual | September 2019 | March 2022 (follow-up to complete June 2023) | Adults with documented gastrointestinal carriage of ESBL-E or CRE (stool sample) in the 21 days prior to consent and symptomatic infection with the target organism in the preceding 6 months | Group 1: FMT capsules (80 g of healthy donor faeces per 5 capsules) on three consecutive days. Pre-treatment with a proton-pump inhibitor Group 2: placebo capsules | To determine the feasibility and acceptability of administering encapsulated FMT to participants colonised with ESBL-E/CPE. A secondary objective is to provide early evidence of efficacy (decolonisation rate by culture/PCR at days 10, 40, 100 and 190). |
| NCT03063437 | Randomised, double-blind, controlled trial USA | 9/? actual | August 2017 | February 2019 Preliminary results: VRE decolonisation at day 10: 1 out of 4 participants in the FMT group and 1 out of 5 participants in the placebo group | Adults colonised with VRE (by stool culture) in the last 14 days | Group 1: single dose of FMT (30 capsules per dose) Group 2: placebo capsules | VRE decolonisation rate (absence of VRE on stool culture) at day 10 |
| NCT03061097 | Randomised, double-blind, controlled trial USA | 4/20 actual | July 2017 | June 2019 Preliminary results: 0 out of 4 patients were decolonised 28 days after autologous FMT | Long-term care residents with a history of an infection requiring antimicrobial treatment at the discretion of the treating physician | Group 1: Autologous 125 mL FMT (biobanked stool from the same patient collected before infection requiring antibiotics) via enema Group 2: Placebo | Safety (short-term) at Day 7 is defined as NIH Grade ≥2 adverse events. Secondary objective: among patients with MDRO colonisation at day 0: decolonisation rate at day 3, day 7 and day 28 |
| EUCTR2019-004402-10-FR | Randomised, double-blind, controlled trial France | 214 estimated | Not mentioned | Not mentioned (marked as ongoing) | Adult patients colonised with ESBL-E or CRE, assessed with stool culture and having suffered from infection with ESBL-E in the previous 12 months | Group 1: FMT capsules (n = 25) for two days in a row Group 2: placebo | Decolonisation rate at 30 days, determined by (undefined) culture methods |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merrick, B.; Sergaki, C.; Edwards, L.; Moyes, D.L.; Kertanegara, M.; Prossomariti, D.; Shawcross, D.L.; Goldenberg, S.D. Modulation of the Gut Microbiota to Control Antimicrobial Resistance (AMR)—A Narrative Review with a Focus on Faecal Microbiota Transplantation (FMT). Infect. Dis. Rep. 2023, 15, 238-254. https://doi.org/10.3390/idr15030025
Merrick B, Sergaki C, Edwards L, Moyes DL, Kertanegara M, Prossomariti D, Shawcross DL, Goldenberg SD. Modulation of the Gut Microbiota to Control Antimicrobial Resistance (AMR)—A Narrative Review with a Focus on Faecal Microbiota Transplantation (FMT). Infectious Disease Reports. 2023; 15(3):238-254. https://doi.org/10.3390/idr15030025
Chicago/Turabian StyleMerrick, Blair, Chrysi Sergaki, Lindsey Edwards, David L. Moyes, Michael Kertanegara, Désirée Prossomariti, Debbie L. Shawcross, and Simon D. Goldenberg. 2023. "Modulation of the Gut Microbiota to Control Antimicrobial Resistance (AMR)—A Narrative Review with a Focus on Faecal Microbiota Transplantation (FMT)" Infectious Disease Reports 15, no. 3: 238-254. https://doi.org/10.3390/idr15030025
APA StyleMerrick, B., Sergaki, C., Edwards, L., Moyes, D. L., Kertanegara, M., Prossomariti, D., Shawcross, D. L., & Goldenberg, S. D. (2023). Modulation of the Gut Microbiota to Control Antimicrobial Resistance (AMR)—A Narrative Review with a Focus on Faecal Microbiota Transplantation (FMT). Infectious Disease Reports, 15(3), 238-254. https://doi.org/10.3390/idr15030025







