Challenges in the Diagnosis of Viral Encephalitis in Children: The Case of Two Siblings
Abstract
:1. Introduction
2. Case Report
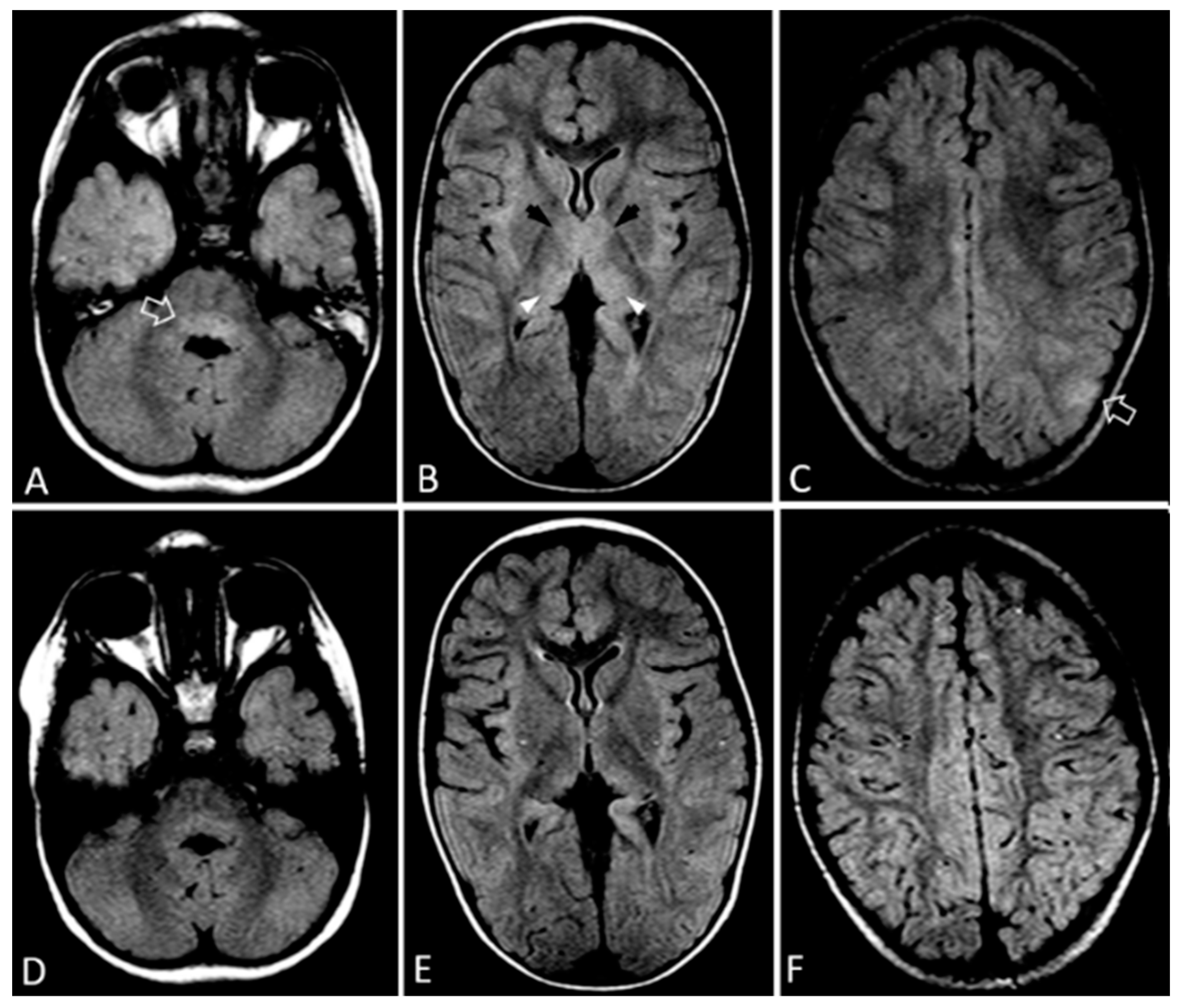
2.1. Case 1
2.2. Case 2
2.3. Final Diagnosis and Outcome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, S.; Patel, B.; Bhatt, G.C. Enteroviral encephalitis in children: Clinical features, pathophysiology, and treatment advances. Pathog. Glob. Health 2014, 108, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messacar, K.; Fischer, M.; Dominguez, S.R.; Tyler, K.L.; Abzug, M.J. Encephalitis in US Children. Infect. Dis. Clin. N. Am. 2018, 32, 145–162. [Google Scholar] [CrossRef]
- Sadarangani, M.; Willis, L.; Kadambari, S.; Gormley, S.; Young, Z.; Beckley, R.; Gantlett, K.; Orf, K.; Blakey, S.; Martin, N.G.; et al. Childhood meningitis in the conjugate vaccine era: A prospective cohort study. Arch. Dis. Child. 2014, 100, 292–294. [Google Scholar] [CrossRef]
- Ekambaram, M.; Nabower, A.; Rajbhandari, P.; Eisenberg, J.; Goodrich, N.; Ampofo, K.; Gollehon, N.S.; Martin, K.C.; Lyden, E.; Snowden, J. Evaluation of Discordant Results Between FilmArray Meningitis/Encephalitis Panel and Conventional Testing in Pediatric Patients: A Multisite Retrospective Cohort Study. J. Pediatric. Infect. Dis. Soc. 2022, 10, piab126. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Richardson, S.E.; Ward, K.N.; Donaldson, C.; MacGregor, D.; Banwell, B.; Mahant, S.; Bitnun, A. Delayed Primary HHV-7 Infection and Neurologic Disease. Pediatrics 2014, 133, e1541–e1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tembo, J.; Kabwe, M.; Chilukutu, L.; Chilufya, M.; Mwaanza, N.; Chabala, C.; Zumla, P.S.A.; Bates, M. Prevalence and Risk Factors for Betaherpesvirus DNAemia in Children >3 Weeks and <2 Years of Age Admitted to a Large Referral Hospital in Sub-Saharan Africa. Clin. Infect. Dis. 2014, 60, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ongrádi, J.; Ablashi, D.V.; Yoshikawa, T.; Stercz, B.; Ogata, M. Roseolovirus-associated encephalitis in immunocompetent and immunocompromised individuals. J. Neurovirol. 2017, 23, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.-S.; Lee, H.-C.; Lee, K.-M.; Gong, Y.-N.; Shih, S.-R. Enterovirus and Encephalitis. Front. Microbiol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.W.; Koo, S.S.; Osman, H.; Wilson, S.; Xerry, J.; Gallimore, C.I.; Allen, D.; Tang, J.W. Predominance of enterovirus B and echovirus 30 as cause of viral meningitis in a UK population. J. Clin. Virol. 2016, 81, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Miao, Z.; Yan, J.; Gong, L.; Chen, Y.; Chen, Y.; Mao, H.; Zhang, Y. Sero-molecular epidemiology of enterovirus-associated encephalitis in Zhejiang Province, China, from 2014 to 2017. Int. J. Infect. Dis. 2019, 79, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho, E.; Sousa, J.I.; Burlandy, F.; Costa, E.; Dias, A.; Serrano, R.; Oliveira, M.; Lopes, R.; Debur, M.; Burger, M.; et al. Identification and Phylogenetic Characterization of Human Enteroviruses Isolated from Cases of Aseptic Meningitis in Brazil, 2013–2017. Viruses 2019, 11, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupila, L.; Vuorinen, T.; Vainionpää, R.; Marttila, R.J.; Kotilainen, P. Diagnosis of enteroviral meningitis by use of polymerase chain reaction of cerebrospinal fluid, stool, and serum specimens. Clin. Infect. Dis. 2005, 40, 982–987. [Google Scholar] [CrossRef]
- Shen, W.C.; Chiu, H.H.; Chow, K.C.; Tsai, C.H. MR imaging findings of enteroviral encephaloymelitis: An outbreak in Taiwan. AJNR Am. J. Neuroradiol. 1999, 20, 1889–1895. [Google Scholar] [PubMed]
- Zeng, H.; Wen, F.; Gan, Y.; Huang, W. MRI and associated clinical characteristics of EV71-induced brainstem encephalitis in children with hand–foot–mouth disease. Neuroradiology 2011, 54, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Abzug, M.J.; Keyserling, H.L.; Lee, M.L.; Levin, M.J.; Rotbart, H.A. Neonatal Enterovirus Infection: Virology, Serology, and Effects of Intravenous Immune Globulin. Clin. Infect. Dis. 1995, 20, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, G.C.; Sankar, J.; Kushwaha, K.P. Use of Intravenous Immunoglobulin Compared with Standard Therapy is Associated with Improved Clinical Outcomes in Children with Acute Encephalitis Syndrome Complicated by Myocarditis. Pediatr. Cardiol. 2012, 33, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, S.B.; Kelly, D.F. Fifteen-minute consultation: Enterovirus meningitis and encephalitis-when can we stop the antibiotics? Arch. Dis. Child. Educ. Pract. Ed. 2017, 102, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, L.E.; Malley, R.; Agrawal, D.; Kuppermann, N. Low Risk of Bacterial Meningitis in Children with a Positive Enteroviral Polymerase Chain Reaction Test Result. Clin. Infect. Dis. 2010, 51, 1221–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Analysis | Pathogens Tested |
|---|---|
| PCR in CSF sample | |
| E. coli, H influenzae, L. monocytogenes, N. meningitidis, S. agalactiae, S. pneumoniae, Cytomegalovirus, Enterovirus, Herpes simplex virus-1,2, Human herpes virus 6 (HHV-6), Human parechovirus, Varicella zoster virus, Cryptococcus neoformans/gattii, Epstein-Barr virus, HHV-7. | |
| PCR in Nasopharyngeal sample | |
| Bordetella pertussis, Chlamydia pneumoniae, Adenovirus, Coronaviruses (HKU1, NL63, 229E, OC43), Human Metapneumovirus, Rhinovirus/Enterovirus, Influenza A/H1, A/H3, A/H1-2009, Influenza B, Parainfluenza Viruses 1–4, Respiratory syncytial virus. | |
| Serological tests | |
| Human immunodeficiency virus (HIV), Measles, EBV, Cytomegalovirus (CMV), Adenovirus, Mycoplasma pneumoniae, Toxoplasma gondii, Coxiella burnetii, Rickettsia spp., Bartonella spp., West Nile virus (CSF). | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergadi, E.; Zacharioudaki, M.; Raissaki, M.; Galanakis, E. Challenges in the Diagnosis of Viral Encephalitis in Children: The Case of Two Siblings. Infect. Dis. Rep. 2022, 14, 106-111. https://doi.org/10.3390/idr14010014
Vergadi E, Zacharioudaki M, Raissaki M, Galanakis E. Challenges in the Diagnosis of Viral Encephalitis in Children: The Case of Two Siblings. Infectious Disease Reports. 2022; 14(1):106-111. https://doi.org/10.3390/idr14010014
Chicago/Turabian StyleVergadi, Eleni, Maria Zacharioudaki, Maria Raissaki, and Emmanouil Galanakis. 2022. "Challenges in the Diagnosis of Viral Encephalitis in Children: The Case of Two Siblings" Infectious Disease Reports 14, no. 1: 106-111. https://doi.org/10.3390/idr14010014







