Antimicrobial Susceptibility Pattern of Salmonella spp. Isolated from Enteric Fever Patients in Nepal
Abstract
1. Background
2. Material and Methods
2.1. Study Design and Sample Population
2.2. Sample Collection and Transport
2.3. Laboratory Processing and Identification of the Isolates
2.4. Antimicrobial Susceptibility Testing (AST)
Determination of Minimum Inhibitory Concentrations (MICs)
2.5. Data Analysis
3. Results
3.1. Distribution of Salmonella Positive Cases by Months
3.2. Sample Distribution According to the Gender and Age Group
3.3. Antibiotic Susceptibility Pattern of Salmonella Isolates
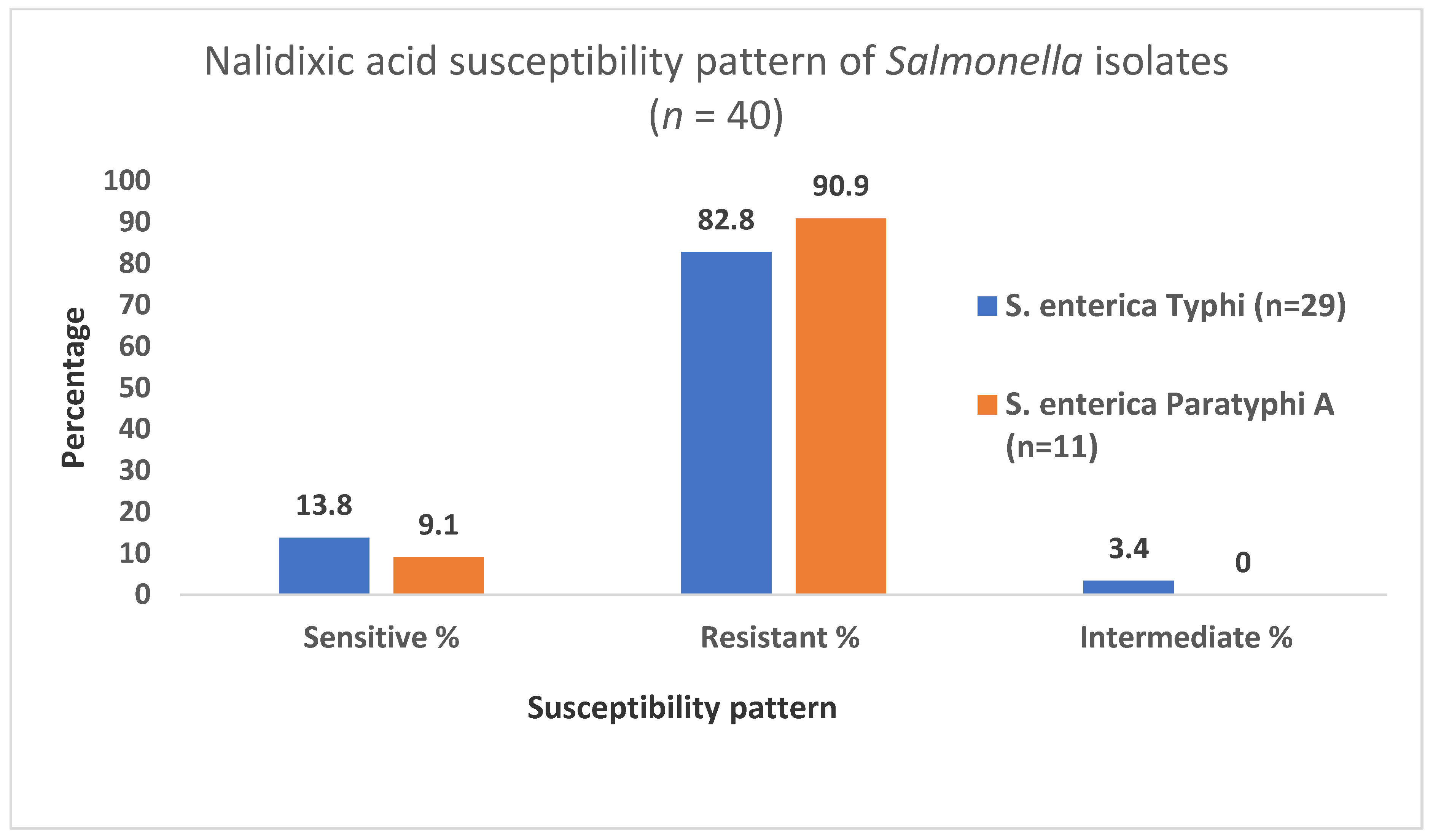
3.4. Nalidixic Acid Susceptibility Pattern of the Isolates
3.5. Determination of Minimum Inhibitory Concentrations (MIC) of Ciprofloxacin
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Crump, J.A.; Mintz, E.D. Global trends in typhoid and paratyphoid Fever. Clin. Infect. Dis. 2010, 50, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Cheng, S.; Liu, G.; Tan, Z.; Dong, C.; Bao, J.; Hong, J.; Jin, D.; Bao, C.; Gu, B. Discovery of seven novel mutations of gyrB, parC and parE in Salmonella Typhi and Paratyphi strains from Jiangsu Province of China. Sci. Rep. 2020, 10, 7359. [Google Scholar] [CrossRef]
- Salerno-Goncalves, R.; Kayastha, D.; Fasano, A.; Levine, M.M.; Sztein, M.B. Crosstalk between leukocytes triggers differential immune responses against Salmonella enterica serovars Typhi and Paratyphi. PLoS Negl. Trop. Dis. 2019, 13, e0007650. [Google Scholar] [CrossRef]
- Karkey, A.; Arjyal, A.; Anders, K.L.; Boni, M.F.; Dongol, S.; Koirala, S.; My, P.V.; Nga, T.V.; Clements, A.C.; Holt, K.E.; et al. The burden and characteristics of enteric fever at a healthcare facility in a densely populated area of Kathmandu. PLoS ONE 2010, 5, e13988. [Google Scholar] [CrossRef]
- Environment and Public Health Organization Typhoid: The Neglected Urgent in Nepal. 2019. Available online: http://enpho.org/featured/typhoid-the-neglected-urgent-in-nepal/ (accessed on 27 August 2020).
- Petersiel, N.; Shrestha, S.; Tamrakar, R.; Koju, R.; Madhup, S.; Shrestha, A.; Bedi, T.; Zmora, N.; Paran, Y.; Schwartz, E.; et al. The epidemiology of typhoid fever in the Dhulikhel area, Nepal: A prospective cohort study. PLoS ONE 2018, 13, e0204479. [Google Scholar] [CrossRef] [PubMed]
- Ugboko, H.; De, N. Mechanisms of Antibiotic resistance in Salmonella Typhi. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 461–476. [Google Scholar]
- Kunwar, D.; Bhatta, S.; Chaudhary, R.; Rijal, K.R. Antibiotic susceptibility pattern of nalidixic acid resistant Salmonella isolates in shree Birendra hospital chhauni. TUJM 2017, 4, 11–14. [Google Scholar] [CrossRef]
- Rowe, B.; Ward, L.R.; Threlfall, E.J. Multidrug-resistant Salmonella Typhi: A worldwide epidemic. Clin. Infect. Dis. 1997, 24 (Suppl. 1), S106–S109. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis Part 2: Management strategies and new agents. Pharm. Ther. 2015, 40, 344–352. [Google Scholar]
- Hooper, D.C. Bacterial topoisomerases, anti-topoisomerases, and anti-topoisomerase resistance. Clin. Infect. Dis. 1998, 27 (Suppl. 1), S54–S63. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 2005, 56, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, J.; Yang, C.; Liang, B.; Ma, Q.; Yi, S.; Li, H.; Liu, H.; Li, P.; Wu, Z.; et al. Prevalence and antimicrobial resistance of Shigella flexneri serotype 2 variants in China. Front. Microbiol. 2015, 6, 435. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef] [PubMed]
- Khadka, P.; Thapaliya, J.; Thapa, S. Susceptibility pattern of Salmonella enterica against commonly prescribed antibiotics, to febrile-pediatric cases, in low-income countries. BMC Pediatrics 2021, 21, 38. [Google Scholar] [CrossRef]
- Pokharel, B.M.; Koirala, J.; Dahal, R.K.; Mishra, S.K.; Khadga, P.K.; Tuladhar, N.R. Multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing Salmonella enterica (serotypes Typhi and Paratyphi A) from blood isolates in Nepal: Surveillance of resistance and a search for newer alternatives. Int. J. Infect. Dis. 2006, 10, 434–438. [Google Scholar] [CrossRef]
- Shrestha, K.L.; Pant, N.D.; Bhandari, R.; Khatri, S.; Shrestha, B.; Lekhak, B. Re-emergence of the susceptibility of the Salmonella spp. isolated from blood samples to conventional first line antibiotics. Antimicrob. Resist. Infect. Control. 2016, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Chand, H.J.; Rijal, K.R.; Neupane, B.; Sharma, V.K.; Jha, B. Re-emergence of susceptibility to conventional first line drugs in Salmonella isolates from enteric fever patients in Nepal. J. Infect. Dev. Ctries. 2014, 8, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Acharya, B.; Kinoshita, S.; Kumagai, S.; Gotoh, A.; Kawabata, M. Decreased susceptibility to fluoroquinolones and gyrA gene mutation in the Salmonella enterica serovar Typhi and Paratyphi A isolated in Katmandu, Nepal, in 2003. Diagn. Microbiol. Infect. Dis. 2006, 54, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, H.; Bo, Y.; Li, Y.; Zhang, Y.; Liu, Y.; Zhang, J.; Jiang, L.; Chen, G.; Zhang, X. Prevalence and antimicrobial resistance of Salmonella enterica subspecies enterica serovar Enteritidis isolated from broiler chickens in Shandong Province, China, 2013–2018. Poult. Sci. 2021, 100, 1016–1023. [Google Scholar] [CrossRef]
- Khanal, P.R.; Satyal, D.; Bhetwal, A.; Maharjan, A.; Shyakaya, S.; Tandukar, S.; Parajuli, N.P. Renaissance of conventional first-line antibiotics in Salmonella enterica clinical isolates: Assessment of MICs for therapeutic antimicrobials in enteric fever cases from Nepal. Biomed. Res. Int. 2017, 2017, 2868143. [Google Scholar] [CrossRef]
- Kayastha, K.; Dhungel, B.; Karki, S.; Adhikari, B.; Banjara, M.R.; Rijal, K.R.; Ghimire, P. Extended-spectrum β-lactamase producing Escherichia coli and Klebsiella species in pediatric patients visiting international friendship children’s hospital, Kathmandu, Nepal. Infect. Dis. 2020, 13, 1178633720909798. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.S.P.; Dhungel, B.; Yadav, B.K.; Adhikari, N.; Thapa Shrestha, U.; Lekhak, B.; Banjara, M.R.; Adhikari, B.; Ghimire, P.; Rijal, K.R. Detection of TEM and CTX-M Genes in Escherichia coli isolated from clinical specimens at tertiary care heart hospital, Kathmandu, Nepal. Diseases 2021, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Basnyat, B.; Shrestha, P.; Prajapati, K.G.; Dongol, S.; Sharma, P.K.; Koirala, S.; Darton, T.C.; Dolecek, C.; Thompson, C.N.; et al. A 23-year retrospective investigation of Salmonella Typhi and Salmonella Paratyphi isolated in a tertiary Kathmandu hospital. PLoS Negl. Trop. Dis. 2017, 11, e0006051. [Google Scholar] [CrossRef]
- Isenberg, H.D. Clinical Microbiology Procedures Handbook, 2nd ed.; ASM Press: Washington, DC, USA, 2004. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA; Volume 38, p. M100.
- Sivakumar, T.; Avinans, S.N.; Prabhu, D.; Shankar, T.; Vijayabaskar, P. Characterization of multidrug resistant patterns of Salmonella spp. World. J. Med. 2012, 7, 64–67. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Antillon, M.; Warren, J.L.; Crawford, F.W.; Weinberger, D.M.; Kurum, E.; Pak, G.D.; Marks, F.; Pitzer, V.E. The burden of typhoid fever in low- and middle-income countries: A meta-regression approach. PLoS Negl. Trop. Dis. 2017, 11, e0005376. [Google Scholar] [CrossRef]
- Prajapati, B.; Rai, G.K.; Rai, S.K.; Upreti, H.C.; Thapa, M.; Singh, G.; Shrestha, R.M. Prevalence of Salmonella Typhi and Paratyphi infection in children: A hospital-based study. Nepal. Med. Coll. J. 2008, 10, 238–241. [Google Scholar]
- Parry, C.M.; Hoa, N.T.; Diep, T.S.; Wain, J.; Chinh, N.T.; Vinh, H.; Hien, T.T.; White, N.J.; Farrar, J.J. Value of a single-tube widal test in diagnosis of typhoid fever in Vietnam. J. Clin. Microbiol. 1999, 37, 2882–2886. [Google Scholar] [CrossRef]
- Malla, S.; Kansakar, P.; Serichantalergs, O.; Rahman, M.; Basnet, S. Epidemiology of typhoid and paratyphoid fever in Kathmandu: Two years study and trends of antimicrobial resistance. J. Nepal. Med. Assoc. 2005, 44, 18–22. [Google Scholar] [CrossRef]
- Maskey, A.P.; Basnyat, B.; Thwaites, G.E.; Campbell, J.I.; Farrar, J.J.; Zimmerman, M.D. Emerging trends in enteric fever in Nepal: 9124 cases confirmed by blood culture 1993–2003. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 91–95. [Google Scholar] [CrossRef]
- Adhikari, D.; Shrestha, P.; Amatya, R. Ciprofloxacin susceptibility of Salmonella enterica serovar Typhi and Paratyphi A from blood Samples of suspected enteric fever patients. Int. J. Infect. Microbiol. 2012, 1, 9–13. [Google Scholar] [CrossRef]
- Acharya, D.; B, D.; Malla, S.; Dumre, S.P.; Adhikari, N.; Kandel, B.P. Salmonella enterica serovar Paratyphi A: An emerging cause of febrile illness in Nepal. NMCJ 2011, 13, 69–73. [Google Scholar]
- Bhattarai, P.M.; Bista, K.P.; Dhakwa, J.R.; Rai, G.K.; Shrestha, R.M.; Thapa, P.B.; Upadhyaya, U.R. A clinical profile of enteric fever at Kanti Children’s Hospital. J. Nepal Paediatr. Soc. 2003, 21, 50–53. [Google Scholar]
- Ansari, I.; Adhikari, N.; Pandey, R.; Dangal, M.M.; Karanjit, R.; Acharya, A. Enteric fever: Is ciprofloxacin failing? J. Nepal. Paed. Soc. 2002, 20, 6–16. [Google Scholar]
- Joshi, R.D.; Khadka, S.; Joshi, D.M.; Shrestha, B.; Dangal, G.; Acharya, K.P.; Shrestha, S.; Dongol, Y. Antimicrobial sensitivity trend in blood culture positive enteric fever. J. Nepal. Health Res. Counc. 2018, 16, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.T.; Campbell, J.I.; Galindo, C.M.; Van, M.; Hoang, N.; Diep, T.S.; Nga, T.T.; Van, V.; Chau, N.; Tuan, P.Q.; et al. Antimicrobial drug resistance of Salmonella enterica serovar Typhi in Asia and molecular mechanism of reduced susceptibility to the fluoroquinolones. Antimicrob. Agents Chemother. 2007, 51, 4315–4323. [Google Scholar] [CrossRef]
- Shrestha, S.K.; Basnet, S. Antibiotic sensitivity pattern in culture positive typhoid fever cases isolated from Patan hospital. J. Pathol. Nepal. 2019, 9, 1450–1452. [Google Scholar] [CrossRef]
- Paniker, C.K.; Vimala, K.N. Transferable chloramphenicol resistance in Salmonella Typhi. Nature 1972, 239, 109–110. [Google Scholar] [CrossRef]
- Butler, T.; Arnold, K.; Linh, N.; Pollack, M. Chloramphenicol resistant typhoid fever in Vietnam associated with R factor. Lancet 1973, 302, 983–985. [Google Scholar] [CrossRef]
- Goldstein, F.W.; Guevara, J.M.; Papadopoulou, B.; Acar, J.F.; Vieu, J.F. Plasmid mediated resistant to multiple antibiotics in Salmonella Typhi. J. Infect. Dis. 1986, 153, 261–266. [Google Scholar] [CrossRef]
- Threlfall, E.J.; Skinner, J.A.; Smith, H.R.; Locky, S. Ciprofloxacin resistant Salmonella Typhi and treatment failure. Lancet 1999, 353, 1590–1591. [Google Scholar] [CrossRef]
- Bhetwal, A.; Maharjan, A.; Khanal, P.R.; Parajuli, N.P. Enteric fever caused by Salmonella enterica serovars with reduced susceptibility of fluoroquinolones at a community-based teaching hospital of Nepal. Int. J. Microbiol. 2017, 2017, 2869458. [Google Scholar] [CrossRef]
- Thaver, D.; Critchley, J.A.; Azmatullah, A.; Madni, S.A.; Bhutta, Z.A. Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever). Cochrae Database Syst. Rev. 2008, 8, CD004530. [Google Scholar]
- Al Naiemi, N.; Rijnsburger, M.C.; Roosendaal, R.; Debets-Ossenkopp, Y.J.; Mulder, J.A.; Fijen, C.A.; Maten, W.; Vandenbroucke-Grauls, C.M.; Savelkoul, P.H. Extended-spectrum-beta-lactamase production in a Salmonella enterica serotype Typhi strain from the Philippines. J. Clin. Microbiol. 2008, 46, 2794–2795. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, Y.; Matten, J.; Rabsch, W. Salmonella enterica serovar Typhi with CTX-M β-lactamase, Germany. Emerg. Infect. Dis. 2009, 15, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Gokul, B.N.; Menezes, G.A.; Harish, B.N. ACC-1 β-lactamase-producing Salmonella enterica serovar Typhi, India. Emerg. Infect. Dis. 2010, 16, 1170–1171. [Google Scholar] [CrossRef]
- Ahmed, D.; Mazumder, R.; Nahar, K.; Islam, N.; Gazi, S.A.; Hossain, M.A. Salmonella enterica serovar Typhi strain producing extended-spectrum beta-lactamases in Dhaka, Bangladesh. J. Med. Microbiol. 2012, 61, 1032–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Britto, C.D.; Wong, V.K.; Dougan, G.; Pollard, A.J. A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Negl. Trop. Dis. 2018, 12, e0006779. [Google Scholar] [CrossRef]
- Effa, E.E.; Bukirwa, H. Azithromycin for treating uncomplicated typhoid and paratyphoid fever (enteric fever). Cochrane. Database Syst. Rev. 2008, 4, CD006083. [Google Scholar]
- Jain, S.; Das, C.T. Antimicrobial resistance among blood culture isolates of Salmonella enterica in New Delhi. J. Infect. Dev. Ctries. 2013, 7, 788–795. [Google Scholar] [CrossRef]
- Geetha, V.K.; Srinivasan, R.; Harish, B.N. Plasmid-mediated quinolone resistance in typhoidal salmonellae: A preliminary report from South India. Indian J. Med. Microbiol. 2014, 32, 31–34. [Google Scholar]
- Jacoby, G.A.; Strahilevitz, J.; Hooper, D.C. Plasmid-mediated quinolone resistance. Microbiol. Spectr. 2014, 2, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Robicsek, A.; Jacoby, G.A.; Hooper, D.C. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 2006, 6, 629–640. [Google Scholar] [CrossRef]
- Sjolund-Karlsson, M.; Folster, J.P.; Pecic, G.; Joyce, K.; Medalla, F.; Rickert, R.; Whichard, J.M. Emergence of plasmid-mediated quinolone resistance among non-Typhi Salmonella enterica isolates from humans in the United States. Antimicrob. Agents Chemother. 2009, 53, 2142–2144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strahilevitz, J.; Jacoby, G.A.; Hooper, D.C.; Robicsek, A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin. Microbiol. Rev. 2009, 22, 664–689. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.P.; Steele, D.; Chaignat, C.L.; Kieny, M.P. A review of vaccine research and development: Human enteric infections. Vaccine 2006, 24, 2732–2750. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, B.; Pragasam, A.K.; Bakthavatchalam, Y.D.; Ralph, R. Typhoid fever: Issues in laboratory detection, treatment options & concerns in management in developing countries. Future Sci. OA 2018, 4, FSO312. [Google Scholar]
- Ahmadi, S.; Rabiee, N.; Fatahi, Y.; Hooshmand, S.E.; Bagherzadeh, M.; Rabiee, M.; Jajarmi, V.; Dinarvand, R.; Habibzadeh, S.; Saeb, M.R.; et al. Green chemistry and coronavirus. Sustain. Chem. Pharm. 2021, 21, 100415. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Ghasemi, A.; Zare, H.; Ahmadi, S.; Fatahi, Y.; Dinarvand, R.; Rabiee, M.; Ramakrishna, S.; Shokouhimehr, M.; et al. Point-of-use rapid detection of SARS-CoV-2: Nanotechnology-enabled solutions for the COVID-19 pandemic. Int. J. Mol. Sci. 2020, 21, 5126. [Google Scholar] [CrossRef]


| Character | Total Blood Culture Subjected | Salmonella spp. Isolated | |
|---|---|---|---|
| Gender | n (%) | n (%) | p-Value |
| Male | 772 (59.5) | 26 (65) | 0.4 |
| Female | 526 (40.5) | 14(35) | |
| Total | 1298 | 40 | |
| Age group in (years) | |||
| 0–10 | 43 (3.3) | 2 (5) | 0.002 |
| 11–20 | 238 (18.3) | 13 (32.5) | |
| 21–30 | 391 (30.1) | 19 (47.5) | |
| 31–40 | 267 (20.6) | 5 (12.5) | |
| 41–50 | 159 (12.3) | 1 (2.5) | |
| >51 | 200 (15.4) | 0 | |
| Antibiotics | Total Isolates | Antibiotic Susceptibility Pattern of Salmonella Isolates | |||||
|---|---|---|---|---|---|---|---|
| Sensitive | Resistant | Intermediate | |||||
| n | % | n | % | n | % | ||
| Amoxicillin | 40 | 40 | 100 | 0 | 0.0 | 0 | 0.0 |
| Chloramphenicol | 40 | 100 | 0 | 0.0 | 0 | 0.0 | |
| Cotrimoxazole | 40 | 100 | 0 | 0.0 | 0 | 0.0 | |
| Nalidixic acid | 5 | 12.5 | 34 | 85.0 | 1 | 2.5 | |
| Ciprofloxacin | 0 | 0.0 | 14 | 35.0 | 26 | 65.0 | |
| Ofloxacin | 6 | 15.0 | 1 | 2.5 | 33 | 82.5 | |
| Levofloxacin | 8 | 20.0 | 1 | 2.5 | 31 | 77.5 | |
| Cefixime | 40 | 100 | 0 | 0.0 | 0 | 0.0 | |
| Cefotaxime | 29 | 72.5 | 2 | 5.0 | 9 | 22.5 | |
| Ceftriaxone | 38 | 95.0 | 1 | 2.5 | 1 | 2.5 | |
| Azithromycin | 39 | 97.5 | 1 | 2.5 | 0 | 0.0 | |
| Organism | Total n (%) | MIC Breakpoint of Ciprofloxacin n (%) | ||
|---|---|---|---|---|
| Sensitive ≤0.0625 | Intermediate 0.125–0.5 | Resistant ≥1 | ||
| Salmonella spp. | 40 (100) | 2 (5.0%) | 17 (42.5%) | 21 (52.5%) |
| Test Performed | Total n (%) | Sensitivity Pattern of Ciprofloxacin n (%) | |||
|---|---|---|---|---|---|
| Sensitive | Resistant | Intermediate | p-Value | ||
| Disc diffusion method | 40 (100) | 0 | 14 (35) | 26 (65) | 0.07 |
| MIC | 40 (100) | 2 (5) | 21 (52.5) | 17 (42.5) | |
| MIC (µg/mL) | S. enterica Typhi (n = 29) | Sensitivity Pattern for Ciprofloxacin | S. enterica Paratyphi A (n = 11) | Sensitivity Pattern for Ciprofloxacin | ||||
|---|---|---|---|---|---|---|---|---|
| NAS | NAI | NAR | NAS | NAI | NAR | |||
| 0.06 | 2 | Sensitive (n = 2) 6.9% | ||||||
| 0.12 | Intermediate (n = 16) 55.2% | 1 | Intermediate (n = 1) 9.1% | |||||
| 0.25 | 2 | |||||||
| 0.5 | 1 | 13 | ||||||
| 1 | 7 | Resistant (n = 11) 37.9% | 7 | Resistant (n = 10) 99.9% | ||||
| 2 | 2 | 3 | ||||||
| 4 | ||||||||
| 8 | 1 | |||||||
| 16 | 1 | |||||||
| Total | 4 | 1 | 24 | 1 | 10 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, A.; Dhungel, B.; Bastola, A.; Thapa Shrestha, U.; Adhikari, N.; Banjara, M.R.; Lekhak, B.; Ghimire, P.; Rijal, K.R. Antimicrobial Susceptibility Pattern of Salmonella spp. Isolated from Enteric Fever Patients in Nepal. Infect. Dis. Rep. 2021, 13, 388-400. https://doi.org/10.3390/idr13020037
Maharjan A, Dhungel B, Bastola A, Thapa Shrestha U, Adhikari N, Banjara MR, Lekhak B, Ghimire P, Rijal KR. Antimicrobial Susceptibility Pattern of Salmonella spp. Isolated from Enteric Fever Patients in Nepal. Infectious Disease Reports. 2021; 13(2):388-400. https://doi.org/10.3390/idr13020037
Chicago/Turabian StyleMaharjan, Anu, Binod Dhungel, Anup Bastola, Upendra Thapa Shrestha, Nabaraj Adhikari, Megha Raj Banjara, Binod Lekhak, Prakash Ghimire, and Komal Raj Rijal. 2021. "Antimicrobial Susceptibility Pattern of Salmonella spp. Isolated from Enteric Fever Patients in Nepal" Infectious Disease Reports 13, no. 2: 388-400. https://doi.org/10.3390/idr13020037
APA StyleMaharjan, A., Dhungel, B., Bastola, A., Thapa Shrestha, U., Adhikari, N., Banjara, M. R., Lekhak, B., Ghimire, P., & Rijal, K. R. (2021). Antimicrobial Susceptibility Pattern of Salmonella spp. Isolated from Enteric Fever Patients in Nepal. Infectious Disease Reports, 13(2), 388-400. https://doi.org/10.3390/idr13020037







