Idiopathic CD4 T Cell Lymphocytopenia: A Case of Overexpression of PD-1/PDL-1 and CTLA-4
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient
2.2. Sample Processing and Cell Culture
2.3. Cell Stimulation with CD3/CD28
2.4. Antibodies
2.5. Multiparameter Flow Cytometry
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Primary immunodeficiency diseases. Report of a WHO scientific group. Clin. Exp. Immunol. 1997, 109, 1–28.
- Update: CD4+ T-lymphocytopenia in persons without evident HIV infection—United States. MMWR 1992, 41, 578–579.
- Regent, A.; Autran, B.; Carcelain, G.; Cheynier, R.; Terrier, B.; Charmeteau-De Muylder, B.; Krivitzky, A.; Oksenhendler, E.; Costedoat-Chalumeau, N.; Hubert, P.; et al. Idiopathic CD4 lymphocytopenia: Clinical and immunologic characteristics and follow-up of 40 patients. Medicine 2014, 93, 61–72. [Google Scholar] [CrossRef]
- Yarmohammadi, H.; Cunningham-Rundles, C. Idiopathic CD4 lymphocytopenia: Pathogenesis, etiologies, clinical presentations and treatment strategies. Ann. Allergy Asthma Immunol. 2017, 119, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Zonios, D.I.; Falloon, J.; Bennett, J.E.; Shaw, P.A.; Chaitt, D.; Baseler, M.W.; Adelsberger, J.W.; Metcalf, J.A.; Polis, M.A.; Kovacs, S.J.; et al. Idiopathic CD4+ lymphocytopenia: Natural history and prognostic factors. Blood 2008, 112, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.A.; Von Reyn, C.F.; Alliegro, G.M.; Toossi, Z.; Sugar, A.M.; Levitz, S.M. Idiopathic CD4+ T-lymphocytopenia—four patients with opportunistic infections and no evidence of HIV infection. N. Engl. J. Med. 1993, 328, 393–398. [Google Scholar] [CrossRef]
- Laurence, J.; Siegal, F.P.; Schattner, E.; Gelman, I.H.; Morse, S. Acquired immunodeficiency without evidence of infection with human immunodeficiency virus types 1 and 2. Lancet 1992, 340, 273–274. [Google Scholar] [CrossRef]
- Bignon, A.; Regent, A.; Klipfel, L.; Desnoyer, A.; De La Grange, P.; Martinez, V.; Lortholary, O.; Dalloul, A.; Mouthon, L.; Balabanian, K. DUSP4-mediated accelerated T-cell senescence in idiopathic CD4 lymphopenia. Blood 2015, 125, 2507–2518. [Google Scholar] [CrossRef]
- Bugault, F.; Benati, D.; Mouthon, L.; Landires, I.; Rohrlich, P.; Pestre, V.; Thèze, J.; Lortholary, O.; Chakrabarti, L.A. Altered responses to homeostatic cytokines in patients with idiopathic CD4 lymphocytopenia. PLoS ONE 2013, 8, e55570. [Google Scholar] [CrossRef]
- Isgro, A.; Sirianni, M.C.; Gramiccioni, C.; Mezzaroma, I.; Fantauzzi, A.; Aiuti, F. Idiopathic CD4+ lymphocytopenia may be due to decreased bone marrow clonogenic capability. Int. Arch. Allergy Immunol. 2005, 136, 379–384. [Google Scholar] [CrossRef]
- Netea, M.G.; Brouwer, A.E.; Hoogendoorn, E.H.; Van der Meer, J.W.; Koolen, M.; Verweij, P.E.; Kullberg, B.J. Two patients with cryptococcal meningitis and idiopathic CD4 lymphopenia: Defective cytokine production and reversal by recombinant interferon-gamma therapy. Clin. Infect. Dis. 2004, 39, e83–e87. [Google Scholar] [CrossRef] [PubMed]
- Laurence, J.; Mitra, D.; Steiner, M.; Lynch, D.H.; Siegal, F.P.; Staiano-Coico, L. Apoptotic depletion of CD4+ T cells in idiopathic CD4+ T lymphocytopenia. J. Clin. Investig. 1996, 97, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Bergeron, F.; Ferreira, V.; Seligmann, M.; Oksenhendler, E.; Debre, P.; Autran, B. Defective p56Lck activity in T cells from an adult patient with idiopathic CD4+ lymphocytopenia. Int. Immunol. 2000, 12, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Roger, P.M.; Bernard-Pomier, G.; Counillon, E.; Breittmayer, J.P.; Bernard, A.; Dellamonica, P. Overexpression of Fas/CD95 and Fas-induced apoptosis in a patient with idiopathic CD4+ T lymphocytopenia. Clin. Infect. Dis. 1999, 28, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, H.; Turka, L.A. A closer look at homeostatic proliferation of CD4+ T cells: Costimulatory requirements and role in memory formation. J. Immunol. 2001, 167, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Prlic, M.; Blazar, B.R.; Khoruts, A.; Zell, T.; Jameson, S.C. Homeostatic expansion occurs independently of costimulatory signals. J. Immunol. 2001, 167, 5664–5668. [Google Scholar] [CrossRef]
- Chan, A.C.; Shaw, A.S. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Curr. Opin. Immunol. 1996, 8, 394–401. [Google Scholar] [CrossRef]
- Walker, U.A.; Warnatz, K. Idiopathic CD4 lymphocytopenia. Curr. Opin. Rheumatol. 2006, 18, 389–395. [Google Scholar] [CrossRef]
- Kumar, G.; Roger, P.M.; Ticchioni, M.; Trojani, C.; Bernard de Dompsur, R.; Bronsard, N.; Carles, M.; Bernard, E. T cells from chronic bone infection show reduced proliferation and a high proportion of CD28(-) CD4 T cells. Clin. Exp. Immunol. 2014, 176, 49–57. [Google Scholar] [CrossRef]
- Roger, P.M.; Hyvernat, H.; Ticchioni, M.; Kumar, G.; Dellamonica, J.; Bernardin, G. The early phase of human sepsis is characterized by a combination of apoptosis and proliferation of T cells. J. Crit. Care 2012, 27, 384–393. [Google Scholar] [CrossRef]
- Risso, K.; Kumar, G.; Ticchioni, M.; Sanfiorenzo, C.; Dellamonica, J.; Guillouet-de Salvador, F.; Bernardin, G.; Marquette, C.-H.; Roger, P.-M. Early infectious acute respiratory distress syndrome is characterized by activation and proliferation of alveolar T-cells. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1111–1118. [Google Scholar] [CrossRef]
- Chemnitz, J.M.; Parry, R.V.; Nichols, K.E.; June, C.H.; Riley, J.L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004, 173, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Walunas, T.L.; Lenschow, D.J.; Bakker, C.Y.; Linsley, P.S.; Freeman, G.J.; Green, J.M.; Thompson, C.B.; Bluestone, J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994, 1, 405–413. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.Y.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001, 193, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Cell. Mol. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Francisco, L.M.; Sharpe, A.H. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 2007, 19, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- LaGier, A.J.; Pober, J.S. Immune accessory functions of human endothelial cells are modulated by overexpression of B7-H1 (PDL1). Hum. Immunol. 2006, 67, 568–578. [Google Scholar] [CrossRef]
- Campbell, D.J.; Koch, M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011, 11, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Bluestone, J.A. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat. Immunol. 2008, 9, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Muller, Y.D.; Golshayan, D.; Ehirchiou, D.; Wyss, J.C.; Giovannoni, L.; Meier, R.; Serre-Beinier, V.; Yung, G.P.; Morel, P.; Bühler, L.H.; et al. Immunosuppressive effects of streptozotocin-induced diabetes result in absolute lymphopenia and a relative increase of T regulatory cells. Diabetes 2011, 60, 2331–2340. [Google Scholar] [CrossRef]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef]
- Fife, B.T.; Guleria, I.; Bupp, M.G.; Eagar, T.N.; Tang, Q.; Bour-Jordan, H.; Yagita, H.; Azuma, M.; Sayegh, M.H.; Bluestone, J.A. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 2006, 203, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Dorfman, D.M.; Ma, F.R.; Sullivan, E.L.; Munoz, O.; Wood, C.R.; Greenfield, E.A.; Freeman, G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003, 170, 1257–1266. [Google Scholar] [CrossRef]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.M.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Transl. Med. 2011, 3, 111ra20. [Google Scholar] [CrossRef]
- Nishimura, H.; Agata, Y.; Kawasaki, A.; Sato, M.; Imamura, S.; Minato, N.; Yagita, H.; Nakano, T.; Honjo, T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int. Immunol. 1996, 8, 773–780. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Lazzeri, E.; Manetti, R.; Vanini, V.; Romagnani, P.; Maggi, E.; Romagnani, S. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J. Exp. Med. 2002, 196, 379–387. [Google Scholar] [CrossRef]
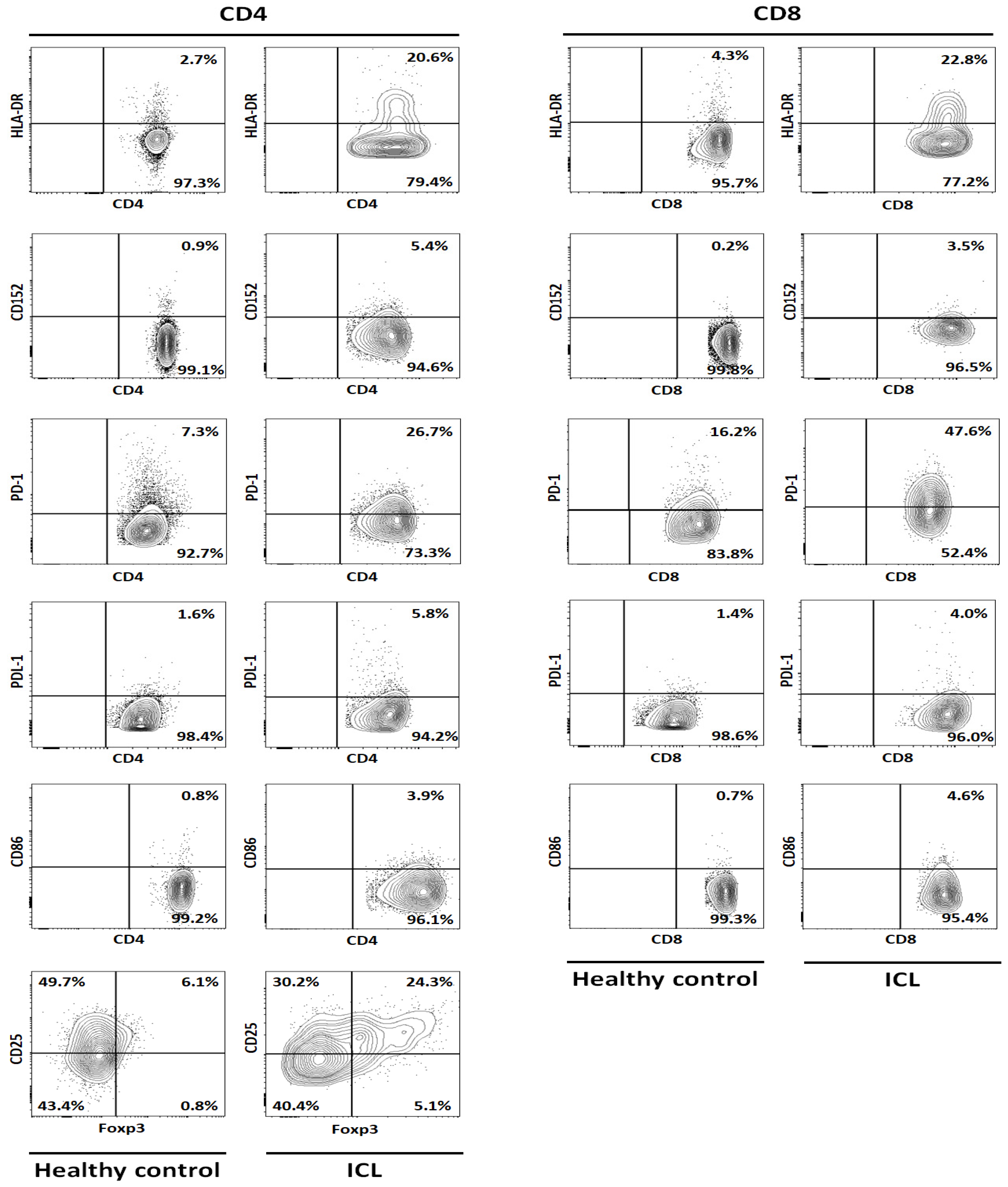
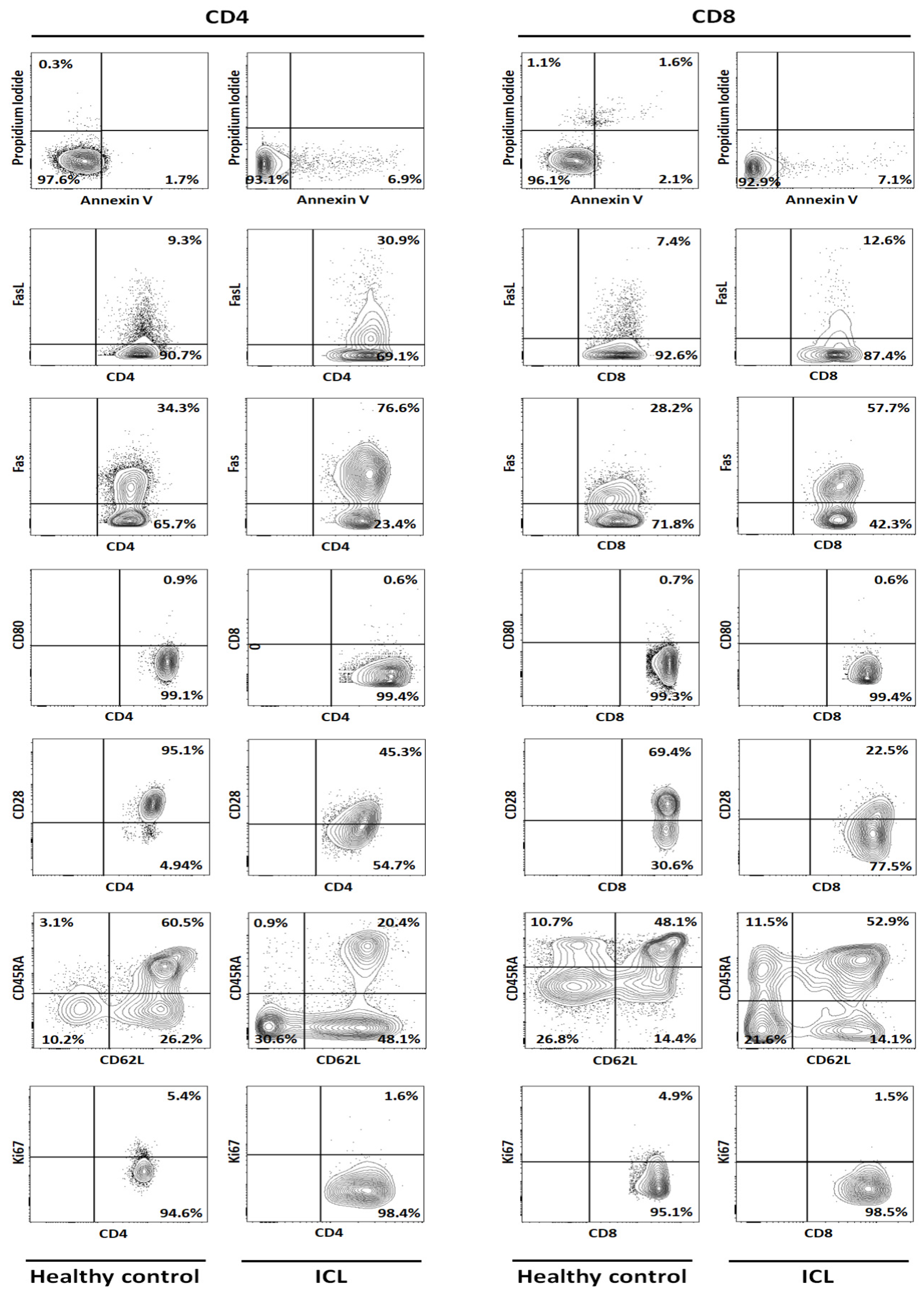
| Marker | CD4 | CD8 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC 1 | HC 2 | HC 3 | HC 4 | HC 5 | Mean HC | ICL | % Var | HC 1 | HC 2 | HC 3 | HC 4 | HC 5 | Mean HC | ICL | % Var | |
| HLA-DR | 5.21 | 2.72 | 1.10 | 1.40 | 4.13 | 2.91 | 20.60 | 607.42 | 3.18 | 6.26 | 3.46 | 7.76 | 7.34 | 5.60 | 22.80 | 307.14 |
| CD152 | 1.25 | 0.57 | 0.55 | 0.11 | 1.87 | 0.87 | 5.40 | 520.69 | 0.93 | 0.20 | 0.65 | 0.86 | 0.86 | 0.70 | 2.50 | 257.14 |
| PD-1 | 8.39 | 10.80 | 16.20 | 19.00 | 6.12 | 12.10 | 64.00 | 428.84 | 8.19 | 13.10 | 22.10 | 16.60 | 13.70 | 14.74 | 47.60 | 222.97 |
| PDL-1 | 0.80 | 2.20 | 0.60 | 1.79 | 0.61 | 1.20 | 5.80 | 383.33 | 1.87 | 2.20 | 2.90 | 0.84 | 2.04 | 1.97 | 4.02 | 104.06 |
| CD86 | 1.07 | 0.91 | 0.53 | 0.72 | 1.10 | 0.87 | 3.90 | 350.35 | 0.61 | 0.48 | 0.45 | 0.46 | 3.50 | 1.10 | 4.60 | 318.18 |
| T-reg | 6.57 | 6.64 | 6.51 | 6.63 | 2.85 | 5.84 | 24.30 | 316.10 | ||||||||
| Annexin-V | 1.16 | 2.85 | 0.24 | 3.63 | 0.51 | 1.68 | 6.90 | 311.20 | 3.42 | 2.50 | 0.10 | 2.93 | 1.54 | 2.10 | 7.00 | 233.65 |
| FasL | 16.50 | 9.31 | 8.52 | 6.12 | 8.33 | 9.76 | 30.90 | 216.73 | 13.40 | 5.53 | 7.44 | 3.33 | 8.72 | 7.68 | 12.60 | 63.98 |
| Fas | 38.40 | 34.70 | 48.00 | 34.30 | 41.60 | 39.40 | 76.60 | 94.42 | 22.50 | 10.70 | 24.00 | 28.20 | 41.10 | 25.30 | 57.70 | 128.06 |
| CD80 | 1.21 | 1.41 | 0.28 | 0.54 | 0.94 | 0.88 | 0.60 | −31.51 | 3.22 | 0.19 | 1.02 | 1.46 | 1.19 | 1.42 | 4.00 | 182.49 |
| CD28 | 97.50 | 97.30 | 97.90 | 95.10 | 92.80 | 96.12 | 45.30 | −52.87 | 58.90 | 73.80 | 69.40 | 70.10 | 67.40 | 67.92 | 22.50 | −66.87 |
| Naïve | 63.50 | 63.80 | 77.80 | 63.20 | 38.50 | 61.36 | 20.40 | −66.75 | 67.00 | 44.20 | 53.80 | 47.00 | 39.30 | 50.26 | 52.90 | 5.25 |
| Ki67 | 2.92 | 7.50 | 5.80 | 3.99 | 9.95 | 6.03 | 1.60 | −73.47 | 1.01 | 9.59 | 5.34 | 0.56 | 5.03 | 4.31 | 1.60 | −62.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, G.; Schmid-Antomarchi, H.; Schmid-Alliana, A.; Ticchioni, M.; Roger, P.-M. Idiopathic CD4 T Cell Lymphocytopenia: A Case of Overexpression of PD-1/PDL-1 and CTLA-4. Infect. Dis. Rep. 2021, 13, 72-81. https://doi.org/10.3390/idr13010009
Kumar G, Schmid-Antomarchi H, Schmid-Alliana A, Ticchioni M, Roger P-M. Idiopathic CD4 T Cell Lymphocytopenia: A Case of Overexpression of PD-1/PDL-1 and CTLA-4. Infectious Disease Reports. 2021; 13(1):72-81. https://doi.org/10.3390/idr13010009
Chicago/Turabian StyleKumar, Gaurav, Heidy Schmid-Antomarchi, Annie Schmid-Alliana, Michel Ticchioni, and Pierre-Marie Roger. 2021. "Idiopathic CD4 T Cell Lymphocytopenia: A Case of Overexpression of PD-1/PDL-1 and CTLA-4" Infectious Disease Reports 13, no. 1: 72-81. https://doi.org/10.3390/idr13010009
APA StyleKumar, G., Schmid-Antomarchi, H., Schmid-Alliana, A., Ticchioni, M., & Roger, P.-M. (2021). Idiopathic CD4 T Cell Lymphocytopenia: A Case of Overexpression of PD-1/PDL-1 and CTLA-4. Infectious Disease Reports, 13(1), 72-81. https://doi.org/10.3390/idr13010009





