SARS–CoV-2 Immuno-Pathogenesis and Potential for Diverse Vaccines and Therapies: Opportunities and Challenges
Abstract
:1. Introduction
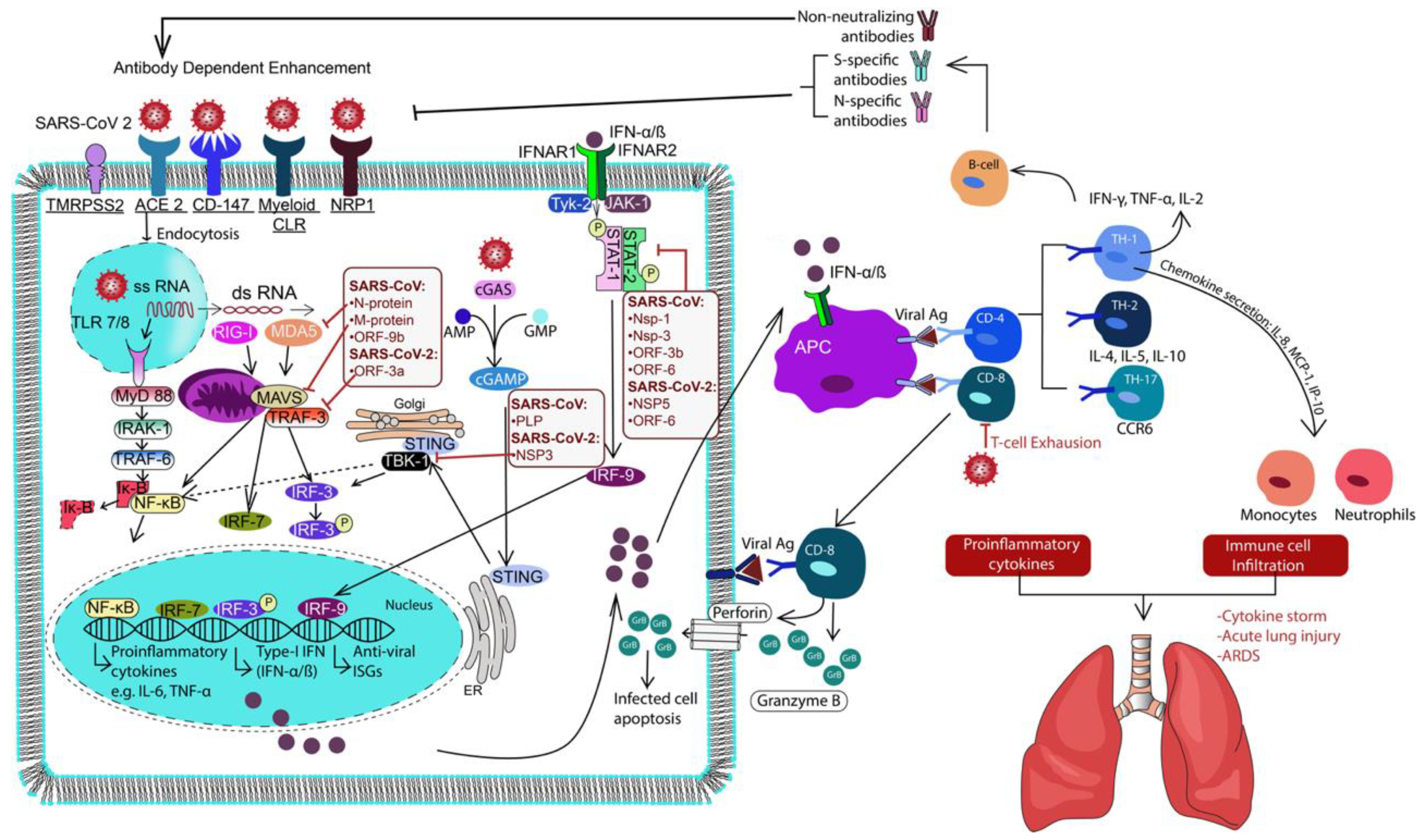
2. Biology of SARS-CoV-2 Infection
2.1. Virus–Host Interaction and Viral Entry
2.2. Potential Role of CD147
2.3. Role of NRP1
3. Immune Response to SARS-CoV-2
3.1. Immune Subversion by NsPs of SARS-CoV-2
3.2. Innate Immune Activation
3.3. Adaptive Immune Responses
4. Vaccine Development for SARS-CoV-2
4.1. Whole Virus Vaccine
4.2. Recombinant Protein Subunit Vaccines
4.3. Nucleic Acid Vaccines
5. Immunotherapies for COVID-19
5.1. Cytokine Therapies
5.2. Therapies for SARS-CoV-2/Coronavirus Infection
6. Variants of SARS-CoV-2
6.1. United Kingdom Variant B.1.1.7
6.2. South Africa Variant B.1.351
6.3. Brazil Variant P.1
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.E. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (sars-cov-2; coronavirus disease-19). Clin. Exp. Pediatr. 2020. [Google Scholar] [CrossRef]
- Morassi, M.; Bagatto, D.; Cobelli, M.; D’Agostini, S.; Gigli, G.L.; Bna, C.; Vogrig, A. Stroke in patients with sars-cov-2 infection: Case series. J. Neurol. 2020. [Google Scholar] [CrossRef]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. Covid-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; Prevention, C.F.D.C.A., Ed.; NCHS Data Brief, no 360; National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Peters, S.A.E.; MacMahon, S.; Woodward, M. Obesity as a risk factor for covid-19 mortality in women and men in the uk biobank: Comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes. Metab. 2021, 23, 258–262. [Google Scholar] [CrossRef]
- Li, J.; Guo, T.; Dong, D.; Zhang, X.; Chen, X.; Feng, Y.; Wei, B.; Zhang, W.; Zhao, M.; Wan, J. Defining heart disease risk for death in covid-19 infection. QJM 2020, 113, 876–882. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Y.; Chen, M.; Wan, Q.; Chen, X. Clinical analysis of risk factors for severe covid-19 patients with type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107666. [Google Scholar] [CrossRef]
- Lai, C.C.; Liu, Y.H.; Wang, C.Y.; Wang, Y.H.; Hsueh, S.C.; Yen, M.Y.; Ko, W.C.; Hsueh, P.R. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (sars-cov-2): Facts and myths. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef]
- Wells, P.M.; Doores, K.J.; Couvreur, S.; Nunez, R.M.; Seow, J.; Graham, C.; Acors, S.; Kouphou, N.; Neil, S.J.D.; Tedder, R.S.; et al. Estimates of the rate of infection and asymptomatic covid-19 disease in a population sample from se england. J. Infect. 2020, 81, 931–936. [Google Scholar] [CrossRef]
- McCullough, P.A.; Alexander, P.E.; Armstrong, R.; Arvinte, C.; Bain, A.F.; Bartlett, R.P.; Berkowitz, R.L.; Berry, A.C.; Borody, T.J.; Brewer, J.H.; et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk sars-cov-2 infection (covid-19). Rev. Cardiovasc. Med. 2020, 21, 517–530. [Google Scholar]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for sars-cov-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of sars-cov-2 viral rna in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of sars-cov2 may play a role in the respiratory failure of covid-19 patients. J. Med. Virol. 2020. [Google Scholar]
- Zhang, H.; Li, H.B.; Lyu, J.R.; Lei, X.M.; Li, W.; Wu, G.; Lyu, J.; Dai, Z.M. Specific ace2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-ncov infection. Int. J. Infect. Dis. 2020, 96, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ace2) converts angiotensin i to angiotensin 1-9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, C.W.; Park, D.I.; Woo, H.Y.; Cheong, H.S.; Shin, H.C.; Ahn, K.; Kwon, M.J.; Joo, E.J. Detection of sars-cov-2 in fecal samples from patients with asymptomatic and mild covid-19 in korea. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Gandhi, S.; Srivastava, A.K.; Ray, U.; Tripathi, P.P. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in covid-19 patients? ACS Chem. Neurosci. 2020, 11, 1379–1381. [Google Scholar] [CrossRef]
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ace2 and its downregulation in sars-cov-2 infection leading to macrophage activation syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ace2 deficiency and sars-cov-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Bungau, S.; Kumar, A.; Uddin, M.S.; Kumar, C.; Pal, G.; Sahil; Shrivastava, K.; Zengin, G.; et al. The dual impact of ace2 in covid-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020, 257, 118075. [Google Scholar] [CrossRef]
- Ulrich, H.; Pillat, M.M. Cd147 as a target for covid-19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 2020, 16, 434–440. [Google Scholar] [CrossRef]
- Helal, M.A.; Shouman, S.; Abdelwaly, A.; Elmehrath, A.O.; Essawy, M.; Sayed, S.M.; Saleh, A.H.; El-Badri, N. Molecular basis of the potential interaction of sars-cov-2 spike protein to cd147 in covid-19 associated-lymphopenia. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef]
- Chen, Z.; Mi, L.; Xu, J.; Yu, J.; Wang, X.; Jiang, J.; Xing, J.; Shang, P.; Qian, A.; Li, Y.; et al. Function of hab18g/cd147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005, 191, 755–760. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. Sars-cov-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280 e278. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. Tmprss2 and tmprss4 promote sars-cov-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Xia, S.; Lan, Q.; Su, S.; Wang, X.; Xu, W.; Liu, Z.; Zhu, Y.; Wang, Q.; Lu, L.; Jiang, S. The role of furin cleavage site in sars-cov-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduct. Target. Ther. 2020, 5, 92. [Google Scholar] [CrossRef]
- Claas, E.C.; Osterhaus, A.D.; van Beek, R.; De Jong, J.C.; Rimmelzwaan, G.F.; Senne, D.A.; Krauss, S.; Shortridge, K.F.; Webster, R.G. Human influenza a h5n1 virus related to a highly pathogenic avian influenza virus. Lancet 1998, 351, 472–477. [Google Scholar] [CrossRef]
- Liu, C.; von Brunn, A.; Zhu, D. Cyclophilin a and cd147: Novel therapeutic targets for the treatment of covid-19. Med. Drug Discov. 2020, 7, 100056. [Google Scholar] [CrossRef] [PubMed]
- Ke Wang, W.C.; Zhou, Y.-S.; Lian, J.-Q.; Zhang, Z.; Du, P.; Gong, L.; Zhang, Y.; Cui, H.-Y.; Geng, J.-J.; Wang, B.; et al. Sars-cov-2 invades host cells via a novel route: Cd147-spike protein. biorxiv 2020. [Google Scholar]
- Bian, H.; Zheng, Z.-H.; Wei, D.; Zhang, Z.; Kang, W.-Z.; Hao, C.-Q.; Dong, K.; Kang, W.; Xia, J.-L.; Miao, J.-L.; et al. Meplazumab treats covid-19 pneumonia: An open-labelled, concurrent controlled add-on clinical trial. medrxiv 2020. [Google Scholar]
- Kumar, D. Vetrivel, Umashankar, Parameswaran, Sowmya, Subramanian, Krishna Kumar. Structural insights on druggable hotspots in cd147: A bull’s eye view. Life Sci. 2019, 224, 76–87. [Google Scholar] [CrossRef]
- Pushkarsky, T.; Zybarth, G.; Dubrovsky, L.; Yurchenko, V.; Tang, H.; Guo, H.; Toole, B.; Sherry, B.; Bukrinsky, M. Cd147 facilitates hiv-1 infection by interacting with virus-associated cyclophilin a. Proc. Natl. Acad. Sci. USA 2001, 98, 6360–6365. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Scala, C.D.; Chahinian, H.; Yahi, N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against sars-cov-2 infection. Int. J. Antimicrob. Agents 2020, 105960. [Google Scholar] [CrossRef]
- Chaudhary, B.; Khaled, Y.S.; Ammori, B.J.; Elkord, E. Neuropilin 1: Function and therapeutic potential in cancer. Cancer Immunol. Immunother. 2014, 63, 81–99. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Kolodkin, A.L.; Matthes, D.J.; Goodman, C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1993, 75, 1389–1399. [Google Scholar] [CrossRef]
- Leclerc, M.; Voilin, E.; Gros, G.; Corgnac, S.; de Montpreville, V.; Validire, P.; Bismuth, G.; Mami-Chouaib, F. Regulation of antitumour cd8 t-cell immunity and checkpoint blockade immunotherapy by neuropilin-1. Nat. Commun. 2019, 10, 3345. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Sun, Y.; Carroll, C.R.; Usherwood, E.J. Neuropilin-1 regulates the secondary cd8 t cell response to virus infection. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hanif, M.; Ali, M.J.; Haider, M.A.; Kherani, D.; Memon, G.M.; Karim, A.H.; Sattar, A. Neurological manifestations of covid-19 (sars-cov-2): A review. Front. Neurol. 2020, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for sars-cov-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates sars-cov-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Braciale, T.J.; Hahn, Y.S. Immunity to viruses. Immunol. Rev. 2013, 255, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef]
- Belz, G.; Mount, A.; Masson, F. Dendritic cells in viral infections. Handb. Exp. Pharmacol. 2009, 51–77. [Google Scholar]
- Freer, G.; Matteucci, D. Influence of dendritic cells on viral pathogenicity. PLoS Pathog. 2009, 5, e1000384. [Google Scholar] [CrossRef]
- Alcami, A.; Koszinowski, U.H. Viral mechanisms of immune evasion. Immunol. Today 2000, 21, 447–455. [Google Scholar] [CrossRef]
- Beachboard, D.C.; Horner, S.M. Innate immune evasion strategies of DNA and rna viruses. Curr. Opin. Microbiol. 2016, 32, 113–119. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the rna-dependent rna polymerase from covid-19 virus. Science 2020. [Google Scholar] [CrossRef]
- Kamitani, W.; Narayanan, K.; Huang, C.; Lokugamage, K.; Ikegami, T.; Ito, N.; Kubo, H.; Makino, S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mrna degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 12885–12890. [Google Scholar] [CrossRef]
- Tohya, Y.; Narayanan, K.; Kamitani, W.; Huang, C.; Lokugamage, K.; Makino, S. Suppression of host gene expression by nsp1 proteins of group 2 bat coronaviruses. J. Virol. 2009, 83, 5282–5288. [Google Scholar] [CrossRef]
- Angelini, M.M.; Akhlaghpour, M.; Neuman, B.W.; Buchmeier, M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio 2013, 4. [Google Scholar] [CrossRef]
- Hurst, K.R.; Koetzner, C.A.; Masters, P.S. Characterization of a critical interaction between the coronavirus nucleocapsid protein and nonstructural protein 3 of the viral replicase-transcriptase complex. J. Virol. 2013, 87, 9159–9172. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Chen, Z.J. Sting specifies irf3 phosphorylation by tbk1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012, 5, ra20. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, Z.; Song, S.; Wang, S.; Tian, C.; Xing, G.; Chen, X.; Xiao, Z.X.; He, F.; Zhang, L. P53 degradation by a coronavirus papain-like protease suppresses type i interferon signaling. J. Biol. Chem. 2015, 290, 3172–3182. [Google Scholar] [CrossRef]
- Fehr, A.R.; Channappanavar, R.; Jankevicius, G.; Fett, C.; Zhao, J.; Athmer, J.; Meyerholz, D.K.; Ahel, I.; Perlman, S. The conserved coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome coronavirus infection. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tian, J.; Li, Z.; Kang, H.; Zhang, J.; Huang, J.; Yin, H.; Hu, X.; Qu, L. Feline infectious peritonitis virus nsp5 inhibits type i interferon production by cleaving nemo at multiple sites. Viruses 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Kusov, Y.; Nian, Y.; Ma, Q.; Wang, J.; von Brunn, A.; Leyssen, P.; Lanko, K.; Neyts, J.; et al. Alpha-ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: Structure-based design, synthesis, and activity assessment. J. Med. Chem. 2020, 63, 4562–4578. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Zhuang, Z.; Cai, S.; Zhao, Z.; Zhou, L.; Zhang, J.; Wang, P.-H.; Zhao, J.; Cui, J. Main protease of sars-cov-2 serves as a bifunctional molecule in restricting type i interferon antiviral signaling. Signal Transduct. Target. Ther. 2020, 5, 221. [Google Scholar] [CrossRef]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.-C.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.-Y. Evasion of type i interferon by sars-cov-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef]
- Lei, X.; Dong, X.; Ma, R.; Wang, W.; Xiao, X.; Tian, Z.; Wang, C.; Wang, Y.; Li, L.; Ren, L.; et al. Activation and evasion of type i interferon responses by sars-cov-2. Nat. Commun. 2020, 11, 3810. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.K.Y.; Moriyama, M.; Iwasaki, A. Inflammasomes and pyroptosis as therapeutic targets for covid-19. J. Immunol. 2020. [Google Scholar] [CrossRef]
- Sun, L.; Xing, Y.; Chen, X.; Zheng, Y.; Yang, Y.; Nichols, D.B.; Clementz, M.A.; Banach, B.S.; Li, K.; Baker, S.C.; et al. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of sting-mediated signaling. PLoS ONE 2012, 7, e30802. [Google Scholar] [CrossRef] [PubMed]
- Sallenave, J.M.; Guillot, L. Innate immune signaling and proteolytic pathways in the resolution or exacerbation of sars-cov-2 in covid-19: Key therapeutic targets? Front. Immunol. 2020, 11, 1229. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.; Morrison, T.E.; Funkhouser, W.; Uematsu, S.; Akira, S.; Baric, R.S.; Heise, M.T. Myd88 is required for protection from lethal infection with a mouse-adapted sars-cov. PLoS Pathog. 2008, 4, e1000240. [Google Scholar] [CrossRef]
- Totura, A.L.; Whitmore, A.; Agnihothram, S.; Schafer, A.; Katze, M.G.; Heise, M.T.; Baric, R.S. Toll-like receptor 3 signaling via trif contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio 2015, 6, e00638-15. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D.; Morgan, A.P.; Totura, A.L.; Beall, A.; Kocher, J.; Plante, J.; Harrison-Shostak, D.C.; Schäfer, A.; Pardo-Manuel de Villena, F.; et al. Allelic variation in the toll-like receptor adaptor protein ticam2 contributes to sars-coronavirus pathogenesis in mice. G3 (Bethesda) 2017, 7, 1653–1663. [Google Scholar] [CrossRef]
- Li, S.W.; Wang, C.Y.; Jou, Y.J.; Huang, S.H.; Hsiao, L.H.; Wan, L.; Lin, Y.J.; Kung, S.H.; Lin, C.W. Sars coronavirus papain-like protease inhibits the tlr7 signaling pathway through removing lys63-linked polyubiquitination of traf3 and traf6. Int. J. Mol. Sci. 2016, 17, 678. [Google Scholar] [CrossRef]
- Cao, L.; Ge, X.; Gao, Y.; Ren, Y.; Ren, X.; Li, G. Porcine epidemic diarrhea virus infection induces nf-kappab activation through the tlr2, tlr3 and tlr9 pathways in porcine intestinal epithelial cells. J. Gen. Virol. 2015, 96, 1757–1767. [Google Scholar] [CrossRef]
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeno, J.M.; Fernandez-Delgado, R.; Fett, C.; Castano-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of nf-kappab-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014, 88, 913–924. [Google Scholar] [CrossRef]
- Mu, J.; Fang, Y.; Yang, Q.; Shu, T.; Wang, A.; Huang, M.; Jin, L.; Deng, F.; Qiu, Y.; Zhou, X. Sars-cov-2 n protein antagonizes type i interferon signaling by suppressing phosphorylation and nuclear translocation of stat1 and stat2. Cell Discov. 2020, 6, 65. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhuang, M.-W.; Han, L.; Zhang, J.; Nan, M.-L.; Zhan, P.; Kang, D.; Liu, X.; Gao, C.; Wang, P.-H. Severe acute respiratory syndrome coronavirus 2 (sars-cov-2) membrane (m) protein inhibits type i and iii interferon production by targeting rig-i/mda-5 signaling. Signal Transduct. Target. Ther. 2020, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-S.; Qi, H.-Y.; Boularan, C.; Huang, N.-N.; Abu-Asab, M.; Shelhamer, J.; Kehrl, J. Sars-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the mavs/traf3/traf6 signalosome. J. Immunol. (Baltim. MD 1950) 2014, 193, 3080–3089. [Google Scholar] [CrossRef]
- Jiang, H.-W.; Zhang, H.-N.; Meng, Q.-F.; Xie, J.; Li, Y.; Chen, H.; Zheng, Y.-X.; Wang, X.-N.; Qi, H.; Zhang, J.; et al. Sars-cov-2 orf9b suppresses type i interferon responses by targeting tom70. Cell. Mol. Immunol. 2020, 17, 998–1000. [Google Scholar] [CrossRef]
- Ni, G.; Ma, Z.; Damania, B. Cgas and sting: At the intersection of DNA and rna virus-sensing networks. PLoS Pathog. 2018, 14, e1007148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-Y.; He, X.-B.; Jia, H.-J.; Chen, G.-H.; Jin, Q.-W.; Long, Z.-L.; Jing, Z.-Z. The cgas–sting signaling pathway is required for the innate immune response against ectromelia virus. Front. Immunol. 2018, 9, 1297. [Google Scholar] [CrossRef]
- McGuckin Wuertz, K.; Treuting, P.M.; Hemann, E.A.; Esser-Nobis, K.; Snyder, A.G.; Graham, J.B.; Daniels, B.P.; Wilkins, C.; Snyder, J.M.; Voss, K.M.; et al. Sting is required for host defense against neuropathological west nile virus infection. PLoS Pathog. 2019, 15, e1007899. [Google Scholar] [CrossRef]
- Maringer, K.; Fernández-Sesma, A. Message in a bottle: Lessons learned from antagonism of sting signalling during rna virus infection. Cytokine Growth Factor Rev. 2014, 25, 669–679. [Google Scholar] [CrossRef]
- Holm, C.K.; Rahbek, S.H.; Gad, H.H.; Bak, R.O.; Jakobsen, M.R.; Jiang, Z.; Hansen, A.L.; Jensen, S.K.; Sun, C.; Thomsen, M.K.; et al. Influenza a virus targets a cgas-independent sting pathway that controls enveloped rna viruses. Nat. Commun. 2016, 7, 10680. [Google Scholar] [CrossRef] [PubMed]
- Franz, K.M.; Neidermyer, W.J.; Tan, Y.-J.; Whelan, S.P.J.; Kagan, J.C. Sting-dependent translation inhibition restricts rna virus replication. Proc. Nat. Acad. Sci. USA 2018, 115, E2058–E2067. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like protease regulates sars-cov-2 viral spread and innate immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef]
- Bermejo-Jambrina, M.; Eder, J.; Helgers, L.C.; Hertoghs, N.; Nijmeijer, B.M.; Stunnenberg, M.; Geijtenbeek, T.B.H. C-type lectin receptors in antiviral immunity and viral escape. Front. Immunol. 2018, 9, 590. [Google Scholar] [CrossRef]
- del Fresno, C.; Iborra, S.; Saz-Leal, P.; Martínez-López, M.; Sancho, D. Flexible signaling of myeloid c-type lectin receptors in immunity and inflammation. Front. Immunol. 2018, 9, 804. [Google Scholar] [CrossRef]
- Jeffers, S.A.; Tusell, S.M.; Gillim-Ross, L.; Hemmila, E.M.; Achenbach, J.E.; Babcock, G.J.; Thomas, W.D.; Thackray, L.B.; Young, M.D.; Mason, R.J.; et al. Cd209l (l-sign) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Nat. Acad. Sci. USA 2004, 101, 15748. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Huang, Y.; Ganesh, L.; Leung, K.; Kong, W.P.; Schwartz, O.; Subbarao, K.; Nabel, G.J. Ph-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through dc-sign. J. Virol. 2004, 78, 5642–5650. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, K.; Pfefferle, S.; Bertram, S.; Glowacka, I.; Drosten, C.; Pöhlmann, S.; Simmons, G. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010, 84, 8753–8764. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Cui, X.; Zhu, X.; Zhou, J. A Hint on the Covid-19 Risk: Population Disparities in Gene Expression of Three Receptors of SARS-CoV. Preprints 2020. [Google Scholar]
- Zhao, X.; Chu, H.; Wong, B.H.; Chiu, M.C.; Wang, D.; Li, C.; Liu, X.; Yang, D.; Poon, V.K.; Cai, J.; et al. Activation of c-type lectin receptor and (rig)-i-like receptors contributes to proinflammatory response in middle east respiratory syndrome coronavirus-infected macrophages. J. Infect. Dis. 2020, 221, 647–659. [Google Scholar] [CrossRef]
- Brutkiewicz, R.R. Cell signaling pathways that regulate antigen presentation. J. Immunol. 2016, 197, 2971. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Young, J.W. Human dendritic cells: Potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005, 175, 1373. [Google Scholar] [CrossRef] [PubMed]
- Law, H.K.W.; Cheung, C.Y.; Ng, H.Y.; Sia, S.F.; Chan, Y.O.; Luk, W.; Nicholls, J.M.; Peiris, J.S.M.; Lau, Y.L. Chemokine up-regulation in sars-coronavirus-infected, monocyte-derived human dendritic cells. Blood 2005, 106, 2366–2374. [Google Scholar] [CrossRef]
- Magro, G. Sars-cov-2 and covid-19: Is interleukin-6 (il-6) the ‘culprit lesion’ of ards onset? What is there besides tocilizumab? Sgp130fc. Cytokine X 2020, 100029. [Google Scholar] [CrossRef]
- Atal, S.; Fatima, Z. Il-6 inhibitors in the treatment of serious covid-19: A promising therapy? Pharmaceut. Med. 2020, 34, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Hill, T.; Li, K.; Peters, C.J.; Tseng, C.-T.K. Severe acute respiratory syndrome (sars) coronavirus-induced lung epithelial cytokines exacerbate sars pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 2009, 83, 3039. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Z.; Diao, B.; Wang, R.; Wang, G.; Wang, C.; Tan, Y.; Liu, L.; Wang, C.; Liu, Y.; et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv 2020. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts covid-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Otsuka, R.; Seino, K.I. Macrophage activation syndrome and covid-19. Inflamm. Regen. 2020, 40, 19. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; Klug, Z.M.; et al. Alveolitis in severe sars-cov-2 pneumonia is driven by self-sustaining circuits between infected alveolar macrophages and t cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wu, F.; Wang, A.; Liu, M.; Wang, Q.; Chen, J.; Xia, S.; Ling, Y.; Zhang, Y.; Xun, J.; Lu, L.; et al. Neutralizing antibody responses to sars-cov-2 in a covid-19 recovered patient cohort and their implications. medRxiv 2020. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Beziat, V.; et al. Autoantibodies against type i ifns in patients with life-threatening covid-19. Science 2020, 370. [Google Scholar] [CrossRef]
- Yeh, K.-M.; Chiueh, T.-S.; Siu, L.K.; Lin, J.-C.; Chan, P.K.S.; Peng, M.-Y.; Wan, H.-L.; Chen, J.-H.; Hu, B.-S.; Perng, C.-L.; et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a taiwan hospital. J. Antimicrob. Chemother. 2005, 56, 919–922. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe covid-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.-L.; Feng, Y.; Deng, Y.-Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of sars-cov-2-specific humoral and cellular immunity in covid-19 convalescent individuals. Immunity 2020, 52, 971–977.e3. [Google Scholar] [CrossRef] [PubMed]
- Hoepel, W.; Chen, H.-J.; Allahverdiyeva, S.; Manz, X.; Aman, J.; Bonta, P.; Brouwer, P.; de Taeye, S.; Caniels, T.; van der Straten, K.; et al. Anti-sars-cov-2 igg from severely ill covid-19 patients promotes macrophage hyper-inflammatory responses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Rosendahl Huber, S.; van Beek, J.; de Jonge, J.; Luytjes, W.; van Baarle, D. T cell responses to viral infections—Opportunities for peptide vaccination. Front. Immunol. 2014, 5, 171. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for cd4⁺ t cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Li, C.K.-f.; Wu, H.; Yan, H.; Ma, S.; Wang, L.; Zhang, M.; Tang, X.; Temperton, N.J.; Weiss, R.A.; Brenchley, J.M.; et al. T cell responses to whole sars coronavirus in humans. J. Immunol. (Baltim. MD 1950) 2008, 181, 5490–5500. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Lam, C.W.K.; Wu, A.K.L.; Ip, W.K.; Lee, N.L.S.; Chan, I.H.S.; Lit, L.C.W.; Hui, D.S.C.; Chan, M.H.M.; Chung, S.S.C.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic t cells and inflammatory monocytes incite inflammatory storm in severe covid-19 patients. Natl. Sci. Rev. 2020, nwaa041. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. Presence of sars-cov-2 reactive t cells in covid-19 patients and healthy donors. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Qiao, L.; Wang, W.; Chen, D. Inflammatory response cells during acute respiratory distress syndrome in patients with coronavirus disease 2019 (covid-19). Ann. Intern. Med. 2020, 173, 402–404. [Google Scholar] [CrossRef]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. Covid-19 and neutrophils: The relationship between hyperinflammation and neutrophil extracellular traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with coronavirus 2019 (covid-19) in wuhan, china. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetite, D.; Tavares, L.A.; Paiva, I.M.; et al. Sars-cov-2-triggered neutrophil extracellular traps mediate covid-19 pathology. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. Fda-approved disulfiram inhibits pyroptosis by blocking gasdermin d pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Schwertz, H.; Cody, M.J.; Wallace, J.A.; Campbell, R.A.; Vieira-de-Abreu, A.; Araujo, C.V.; Schubert, S.; Harris, E.S.; Rowley, J.W.; et al. Neonatal net-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J. Clin. Investig. 2016, 126, 3783–3798. [Google Scholar] [CrossRef] [PubMed]
- Davanzo, G.G.; Codo, A.C.; Brunetti, N.S.; Boldrini, V.O.; Knittel, T.L.; Monterio, L.B.; de Moraes, D.; Ferrari, A.J.R.; de Souza, G.F.; Muraro, S.P.; et al. Sars-cov-2 uses cd4 to infect t helper lymphocytes. medRxiv 2020. [Google Scholar] [CrossRef]
- Habel, J.R.; Nguyen, T.H.O.; van de Sandt, C.E.; Juno, J.A.; Chaurasia, P.; Wragg, K.; Koutsakos, M.; Hensen, L.; Jia, X.; Chua, B.; et al. Suboptimal SARS-Cov-2−specific CD8+ T cell response associated with the prominent HLA-A*02:01 phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 24384. [Google Scholar] [PubMed]
- Tillett, R.L.; Sevinsky, J.R.; Hartley, P.D.; Kerwin, H.; Crawford, N.; Gorzalski, A.; Laverdure, C.; Verma, S.C.; Rossetto, C.C.; Jackson, D.; et al. Genomic evidence for reinfection with sars-cov-2: A case study. Lancet Infect. Dis. 2021, 21, 52–58. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. Sars-cov-2-specific t cell immunity in cases of covid-19 and sars, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Chen, W.H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The sars-cov-2 vaccine pipeline: An overview. Curr. Trop. Med. Rep. 2020, 1–4. [Google Scholar] [CrossRef]
- Biospace. Codagenix and Serum Institute of India Initiate Co-Development of a Scalable, Live-Attenuated Vaccine against the Novel Coronavirus, Covid-19. Available online: https://www.biospace.com/article/releases/codagenix-and-serum-institute-of-indiainitiate-co-development-of-a-scalable-live-attenuated-vaccineagainst-the-2019-novel-coronavirus-covid-19/ (accessed on 3 February 2021).
- AstraZeneca. Azd1222 Vaccine Met Primary Efficacy Endpoint in Preventing Covid-19. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html (accessed on 3 February 2021).
- ClinicalTrials.gov. Phase iii Double-Blind, Placebo-Controlled Study of azd1222 for the Prevention of Covid-19 in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04516746 (accessed on 3 February 2021).
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two rna-based covid-19 vaccine candidates. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pious, N.; Ingole, S.D. Race for covid-19 vaccine. Trends Biomater. Artif. Organs 2020, 34, 62–65. [Google Scholar]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.P.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase 1/2 study to describe the safety and immunogenicity of a covid-19 rna vaccine candidate (bnt162b1) in adults 18 to 55 years of age: Interim report. medRxiv 2020. [Google Scholar] [CrossRef]
- Pfizer. Pfpfizer and Biontech Announce Vaccine Candidate against Covid-19 Achieved Success in First Interim Analysis from Phase 3 Study. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against (accessed on 3 February 2021).
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for covid-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based covid-19 vaccine encoding a prefusion-stabilized sars-cov-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Galili, U. Amplifying immunogenicity of prospective covid-19 vaccines by glycoengineering the coronavirus glycan-shield to present alpha-gal epitopes. Vaccine 2020, 38, 6487–6499. [Google Scholar] [CrossRef]
- Reiter, P.L.; Pennell, M.L.; Katz, M.L. Acceptability of a covid-19 vaccine among adults in the united states: How many people would get vaccinated? Vaccine 2020, 38, 6500–6507. [Google Scholar] [CrossRef]
- Kindler, E.; Thiel, V.; Weber, F. Chapter seven—Interaction of sars and mers coronaviruses with the antiviral interferon response. In Advances in Virus Research; Ziebuhr, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 96, pp. 219–243. [Google Scholar]
- Ye, Q.; Wang, B.; Mao, J. Cytokine storm in covid-19 and treatment. J. Infect. 2020. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated type i interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in sars-cov-infected mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Rose, K.M.; Elliott, R.; Martínez-Sobrido, L.; García-Sastre, A.; Weiss, S.R. Murine coronavirus delays expression of a subset of interferon-stimulated genes. J. Virol. 2010, 84, 5656. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Zheng, J.; Wohlford-Lenane, C.; Abrahante, J.E.; Mack, M.; Sompallae, R.; McCray, P.B., Jr.; Meyerholz, D.K.; Perlman, S. Ifn-i response timing relative to virus replication determines mers coronavirus infection outcomes. J. Clin. Investig. 2019, 129, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Haagmans, B.L.; Kuiken, T.; Martina, B.E.; Fouchier, R.A.; Rimmelzwaan, G.F.; van Amerongen, G.; van Riel, D.; de Jong, T.; Itamura, S.; Chan, K.H.; et al. Pegylated interferon-alpha protects type 1 pneumocytes against sars coronavirus infection in macaques. Nat. Med. 2004, 10, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Zorzitto, J.; Galligan, C.L.; Ueng, J.J.M.; Fish, E.N. Characterization of the antiviral effects of interferon-alpha against a sars-like coronoavirus infection in vitro. Cell Res. 2006, 16, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Lescure, F.-X.; Yazdanpanah, Y.; Mentre, F.; Peiffer-Smadja, N.; Committee, C.D.F.S. Type 1 interferons as a potential treatment against covid-19. Antiviral. Res. 2020, 178, 104791. [Google Scholar] [CrossRef] [PubMed]
- Broggi, A.; Ghosh, S.; Sposito, B.; Spreafico, R.; Balzarini, F.; Lo Cascio, A.; Clementi, N.; De Santis, M.; Mancini, N.; Granucci, F.; et al. Type iii interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020, 369, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of covid-19: Current state of the science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. Cytokine release syndrome in severe covid-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in covid-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-ncov). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef]
- Xia, S.; Yan, L.; Xu, W.; Agrawal, A.S.; Algaissi, A.; Tseng, C.K.; Wang, Q.; Du, L.; Tan, W.; Wilson, I.A.; et al. A pan-coronavirus fusion inhibitor targeting the hr1 domain of human coronavirus spike. Sci. Adv. 2019, 5, eaav4580. [Google Scholar] [CrossRef]
- Elsawah, H.K.; Elsokary, M.A.; Abdallah, M.S.; ElShafie, A.H. Efficacy and safety of remdesivir in hospitalized covid-19 patients: Systematic review and meta-analysis including network meta-analysis. Rev. Med. Virol. 2020, e2187. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.; Salgo, M.; Greenberg, M.; Chung, J.; DeMasi, R.; Bolognesi, D. Enfuvirtide: The first therapy to inhibit the entry of hiv-1 into host cd4 lymphocytes. Nat. Rev. Drug Discov. 2004, 3, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Figueira, T.N.; Mendonca, D.A.; Gaspar, D.; Melo, M.N.; Moscona, A.; Porotto, M.; Castanho, M.; Veiga, A.S. Structure-stability-function mechanistic links in the anti-measles virus action of tocopherol-derivatized peptide nanoparticles. ACS Nano 2018, 12, 9855–9865. [Google Scholar] [CrossRef]
- Shu, C.; Huang, X.; Huang, T.; Chen, L.; Yao, B.; Zhou, J.; Deng, C. Potential inhibitors for targeting mpro and spike of sars-cov-2 based on sequence and structural pharmacology analysis. STEMedicine 2020, 1, e41. [Google Scholar] [CrossRef]
- Sainz, B., Jr.; Mossel, E.C.; Gallaher, W.R.; Wimley, W.C.; Peters, C.J.; Wilson, R.B.; Garry, R.F. Inhibition of severe acute respiratory syndrome-associated coronavirus (sars-cov) infectivity by peptides analogous to the viral spike protein. Virus Res. 2006, 120, 146–155. [Google Scholar] [CrossRef]
- Badani, H.; Garry, R.F.; Wimley, W.C. Peptide entry inhibitors of enveloped viruses: The importance of interfacial hydrophobicity. Biochim. Biophys. Acta 2014, 1838, 2180–2197. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of sars-cov-2 (previously 2019-ncov) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, J.; Zhang, K.; Chu, H.; Liu, D.; Poon, V.K.; Chan, C.C.; Leung, H.C.; Fai, N.; Lin, Y.P.; et al. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci. Rep. 2016, 6, 22008. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Chiba, S.; Halfmann, P.; Ehre, C.; Kuroda, M.; Dinnon, K.H.; Leist, S.R.; Schäfer, A.; Nakajima, N.; Takahashi, K.; et al. Sars-cov-2 d614g variant exhibits efficient replication ex vivo and transmission in vivo. Science 2020, 370, 1464. [Google Scholar]
- Chen, J.; Wang, R.; Wang, M.; Wei, G.W. Mutations strengthened sars-cov-2 infectivity. J. Mol. Biol. 2020, 432, 5212–5226. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: New coronavirus variant is identified in uk. BMJ 2020, 371, m4857. [Google Scholar] [CrossRef]
- World Health Organization. Sars-Cov-2 Variants. Available online: https://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed on 3 February 2021).
- Leung, K.; Shum, M.H.; Leung, G.M.; Lam, T.T.; Wu, J.T. Early transmissibility assessment of the n501y mutant strains of sars-cov-2 in the united kingdom, october to november 2020. Euro Surveill 2021, 26. [Google Scholar] [CrossRef]
- Muth, D.; Corman, V.M.; Roth, H.; Binger, T.; Dijkman, R.; Gottula, L.T.; Gloza-Rausch, F.; Balboni, A.; Battilani, M.; Rihtaric, D.; et al. Attenuation of replication by a 29 nucleotide deletion in sars-coronavirus acquired during the early stages of human-to-human transmission. Sci. Rep. 2018, 8, 15177. [Google Scholar] [CrossRef] [PubMed]
- Flower, T.G.; Buffalo, C.Z.; Hooy, R.M.; Allaire, M.; Ren, X.; Hurley, J.H. Structure of sars-cov-2 orf8, a rapidly evolving immune evasion protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2021785118. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.; Catherine, H.; Nick, D.; John, E.; Neil, F.; Graham, M.; Andrew, H.; Muge, C.; Calum, S. Nervtag Note on b.1.1.7 Severity. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/955239/NERVTAG_paper_on_variant_of_concern__VOC__B.1.1.7.pdf (accessed on 3 February 2021).
- England, P.H. Variant of concern 202012/01: Technical Briefing 2. Investigation of Novel SARS-CoV-2 Variant. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/949639/Technical_Briefing_VOC202012-2_Briefing_2_FINAL.pdf (accessed on 3 February 2021).
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (sars-cov-2) lineage with multiple spike mutations in south africa. medRxiv 2020. [Google Scholar] [CrossRef]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Lambson, B.E.; Vermeulen, M.; van den Berg, K.; Rossouw, T.; Boswell, M.; et al. Sars-cov-2 501y.V2 escapes neutralization by south african covid-19 donor plasma. bioRxiv 2021. [Google Scholar] [CrossRef]
- Naveca, F. Sars-cov-2 reinfection by the new variant of concern (voc) p.1 in amazonas, brazil. Virological 2020. [Google Scholar]
- Ari, A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with covid-19. Respir. Med. 2020, 167, 105987. [Google Scholar] [CrossRef]
- Safdar, A.; Shelburne, S.A.; Evans, S.E.; Dickey, B.F. Inhaled therapeutics for prevention and treatment of pneumonia. Expert. Opin. Drug Saf. 2009, 8, 435–449. [Google Scholar] [CrossRef]
- Russell, B.; Moss, C.; Rigg, A.; Van Hemelrijck, M. Covid-19 and treatment with nsaids and corticosteroids: Should we be limiting their use in the clinical setting? Ecancermedicalscience 2020, 14, 1023. [Google Scholar] [CrossRef]
- Ledford, H. Coronavirus breakthrough: Dexamethasone is first drug shown to save lives. Nature 2020, 582, 469. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.H.D.; Dingens, A.S.; Eguia, R.; Wolf, C.R.; Wilcox, N.; Logue, J.K.; Shuey, K.; Casto, A.M.; Fiala, B.; Wrenn, S.; et al. Dynamics of neutralizing antibody titers in the months after sars-cov-2 infection. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to sars-cov-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic sars-cov-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
| Name of Vaccine | Organization |
|---|---|
| Nucleic Acid Vaccines | |
| Fusogenix | Entos Pharmaceuticals |
| self-amplifying RNA (saRNA | Imperial College London |
| modified mRNA (modRNA): uRNA (uridine-mRNA) Spike, RBD | Pfizer |
| modified mRNA (modRNA): saRNA: spike, RBD | BioNTech |
| mRNA | Moderna |
| DNA vaccine | Inovio Pharm. |
| viral proteins in cell culture | Queensland in Australia |
| TB vaccines | Netherlands and Australia |
| Live-Attenuated/Whole Virus Vaccines | |
| Codegenix & dia | Serum Institute of In |
| AdVac adenoviral vector | Janssen |
| PER.C6 technology | Johnson & Johnson |
| AZD 1222 adenoviral vector | AstraZeneca |
| Recombinant Protein Subunit Vaccines | |
| S-trimer recombinant protein Trimer-Tag | Clover Biopharmaceuticals |
| Recombinant nanoparticle | Novavax |
| Molecular clamp technology | University of Queensland |
| Phase I clinical trial of ChAdOx1, a nonreplicating chimpanzee adenovirus vector, engineered to encode the spike protein | Oxford University and Jenner Institute |
| RBD formulated with alum | University of Texas Medical Branch and New York Blood Center |
| Oral recombinant vaccine using VAASTTM platform | Vaxart, Inc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGill, A.R.; Khalil, R.; Dutta, R.; Green, R.; Howell, M.; Mohapatra, S.; Mohapatra, S.S. SARS–CoV-2 Immuno-Pathogenesis and Potential for Diverse Vaccines and Therapies: Opportunities and Challenges. Infect. Dis. Rep. 2021, 13, 102-125. https://doi.org/10.3390/idr13010013
McGill AR, Khalil R, Dutta R, Green R, Howell M, Mohapatra S, Mohapatra SS. SARS–CoV-2 Immuno-Pathogenesis and Potential for Diverse Vaccines and Therapies: Opportunities and Challenges. Infectious Disease Reports. 2021; 13(1):102-125. https://doi.org/10.3390/idr13010013
Chicago/Turabian StyleMcGill, Andrew R., Roukiah Khalil, Rinku Dutta, Ryan Green, Mark Howell, Subhra Mohapatra, and Shyam S. Mohapatra. 2021. "SARS–CoV-2 Immuno-Pathogenesis and Potential for Diverse Vaccines and Therapies: Opportunities and Challenges" Infectious Disease Reports 13, no. 1: 102-125. https://doi.org/10.3390/idr13010013
APA StyleMcGill, A. R., Khalil, R., Dutta, R., Green, R., Howell, M., Mohapatra, S., & Mohapatra, S. S. (2021). SARS–CoV-2 Immuno-Pathogenesis and Potential for Diverse Vaccines and Therapies: Opportunities and Challenges. Infectious Disease Reports, 13(1), 102-125. https://doi.org/10.3390/idr13010013









