Ileal Bile Acid Transporter Inhibitors for Adult Patients with Autoimmune Cholestatic Liver Diseases: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Eligibility Criteria
2.3. Search Strategy and Data Extraction
2.4. Endpoints
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
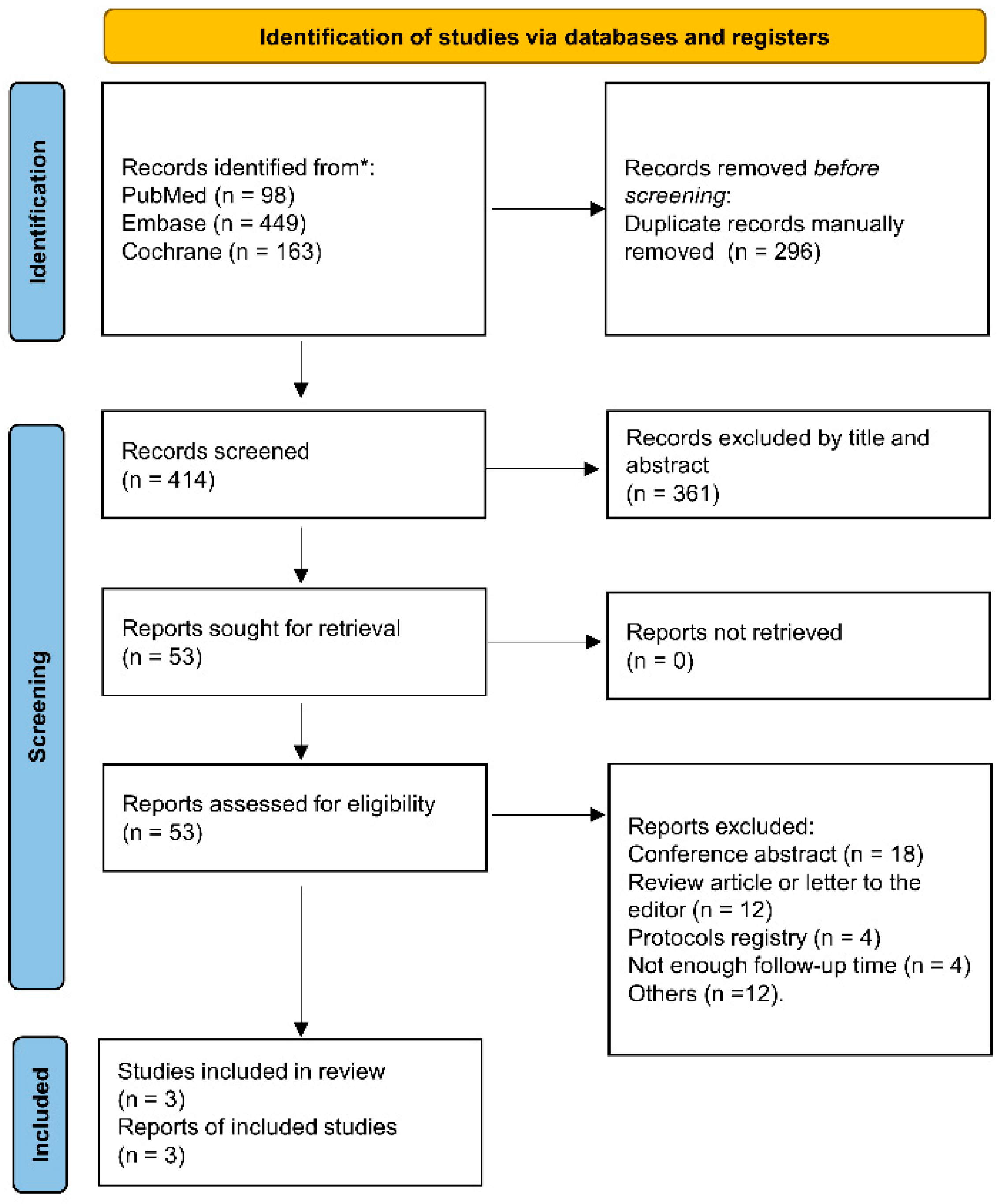
3.1. Study Selection and Baseline Characteristics
3.2. Design and Inclusion Criteria
3.3. Quality Assessment
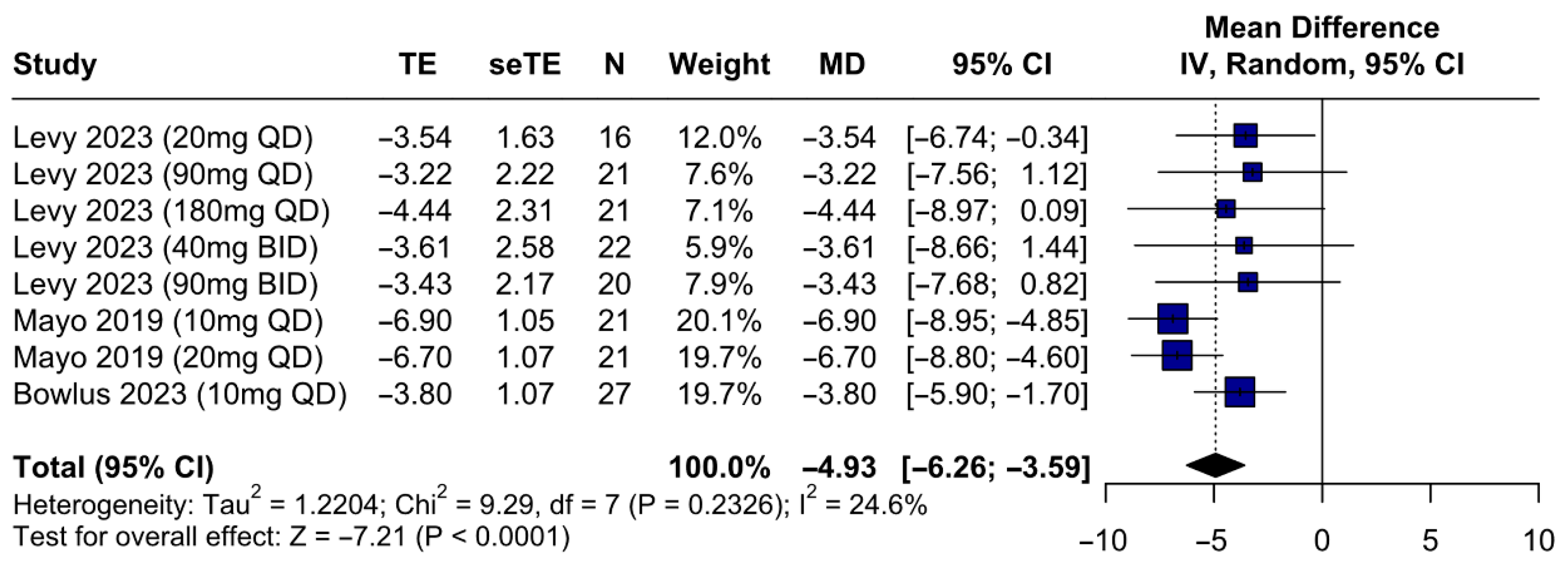
3.4. Primary Outcome
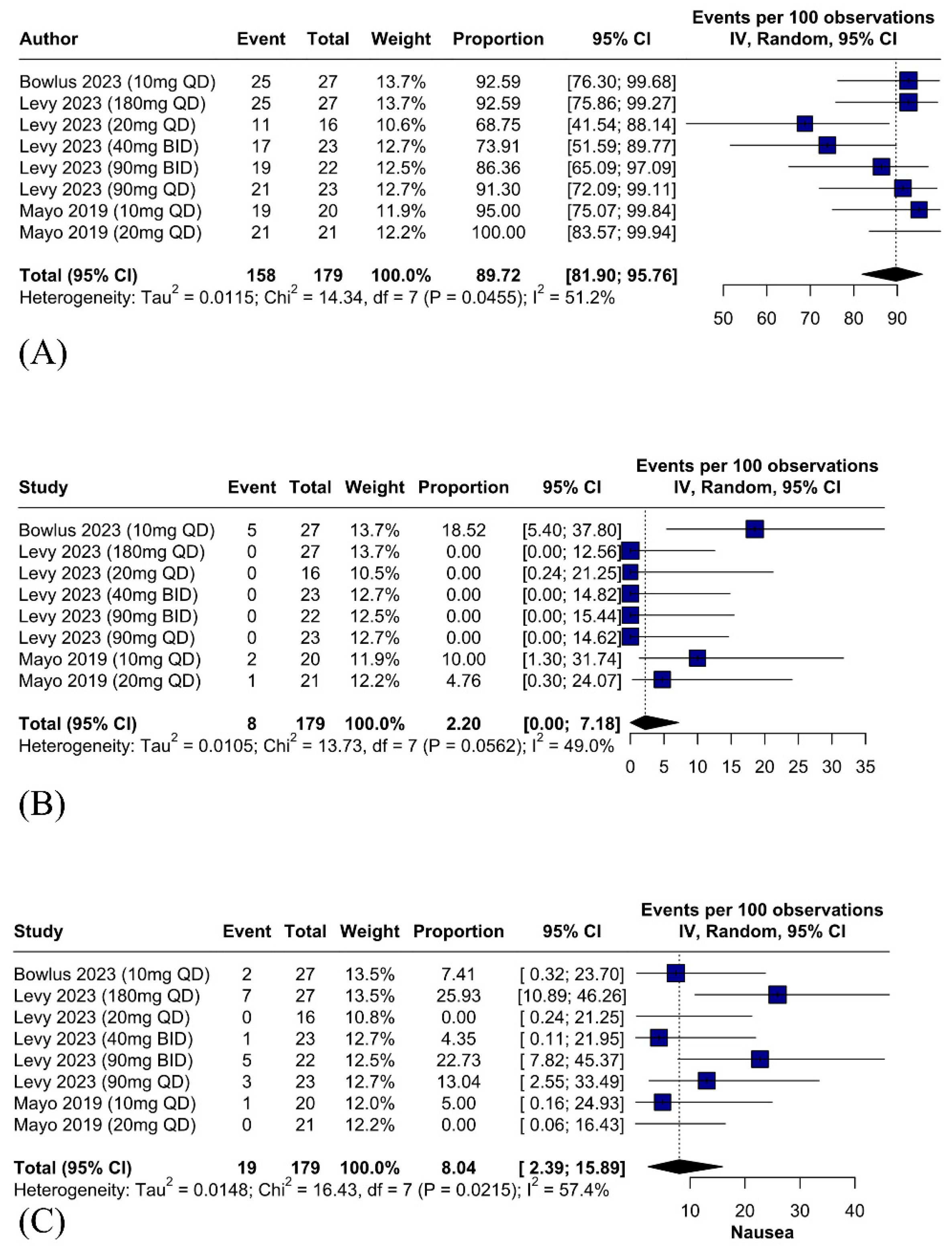
IBAT Inhibitors Are Associated with a Significant Improvement of Pruritus
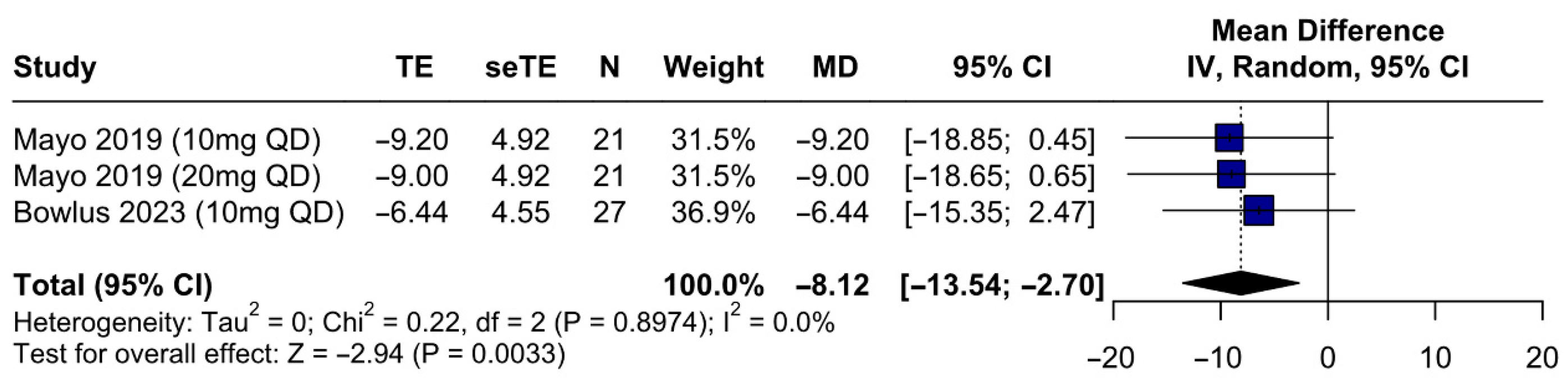
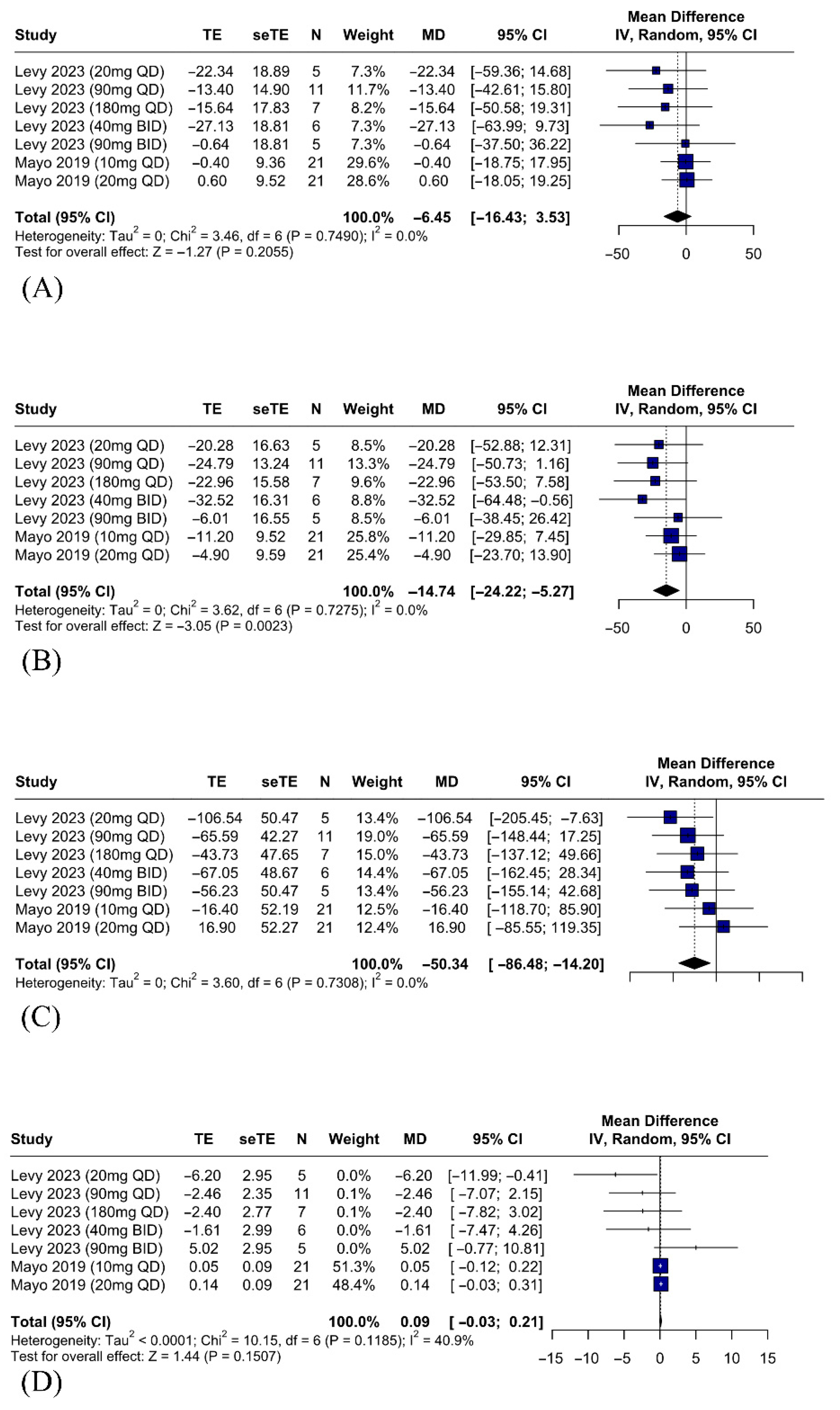
3.5. Secondary Outcomes
3.5.1. Efficacy Outcomes—IBAT Inhibitors Improve Sleep Quality and Reduce Serum Bile Acids, FGF19, and Autotaxin
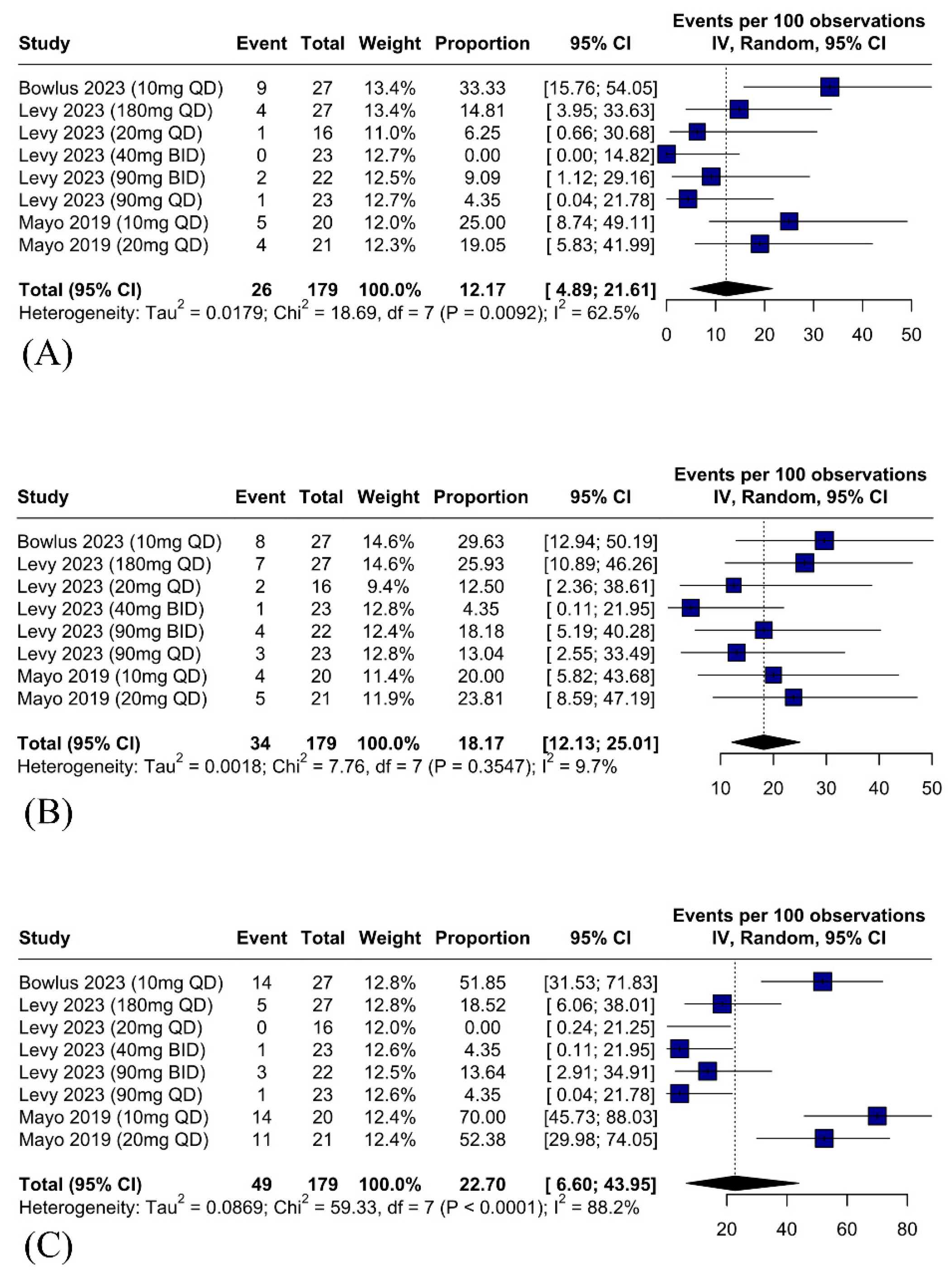
3.5.2. Safety Outcomes—Serious Adverse Events Related to IBAT Inhibitors Are Rare and Diarrhea Is the Most Common Adverse Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AICLD | autoimmune cholestatic liver disease |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ATX | autotaxin |
| BA | bile acid |
| BID | bis in die |
| C4 | 7-alpha-hydroxy-4-cholesten-3-one |
| CI | confidence interval |
| FGF19 | fibroblast growth factor 19 |
| GGT | gamma-glutamyl transferase |
| IBAT | ileal bile acid transporter inhibitors |
| IBD | inflammatory bowel disease |
| MD | mean difference |
| MOS-Sleep | medical outcomes study sleep scale |
| NRS | number rating scale |
| PBC | primary biliary cholangitis |
| PPAR | peroxisome proliferator-activated receptor |
| PSC | primary sclerosing cholangitis |
| QD | quaque die |
| RCT | randomized controlled trial |
| RoB2 | Cochrane risk of bias assessment tool |
| ROBINS-I | risk of bias in non-randomized studies of interventions |
| SD | standard deviation |
| SE | standard error |
| TB | total bilirubin |
| TEAEs | treatment-emergent adverse events |
| UDCA | ursodeoxycholic acid |
References
- Sarcognato, S.; Sacchi, D.; Grillo, F.; Cazzagon, N.; Fabris, L.; Cadamuro, M.; Cataldo, I.; Covelli, C.; Mangia, A.; Guido, M. Autoimmune biliary diseases: Primary biliary cholangitis and primary sclerosing cholangitis. Pathologica 2021, 113, 170–184. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; LaRusso, N.F. The cholangiopathies. Mayo Clin. Proc. 2015, 90, 791–800. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; Strazzabosco, M.; LaRusso, N.F. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology 2004, 127, 1565–1577. [Google Scholar] [CrossRef]
- Cançado, G.G.L.; Deeb, M.; Gulamhusein, A.F. Liver transplantation for cholestatic liver diseases: Timing & disease recurrence. Hepatology 2025. [Google Scholar] [CrossRef]
- Wunsch, E.; Krause, L.; Gevers, T.J.; Schramm, C.; Janik, M.K.; Krawczyk, M.; Willemse, J.; Uhlenbusch, N.; Löwe, B.; Lohse, A.W.; et al. Confidence in treatment is contributing to quality of life in autoimmune liver diseases: The results of ERN RARE-LIVER online survey. Liver Int. 2022, 43, 381–392. [Google Scholar] [CrossRef]
- Mells, G.F.; Pells, G.; Newton, J.L.; Bathgate, A.J.; Burroughs, A.K.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; Sandford, R.N.; et al. Impact of primary biliary cirrhosis on perceived quality of life: The UK-PBC national study. Hepatology 2013, 58, 273–283. [Google Scholar] [CrossRef]
- Schramm, C.; Wahl, I.; Weiler-Normann, C.; Voigt, K.; Wiegard, C.; Glaubke, C.; Brähler, E.; Löwe, B.; Lohse, A.W.; Rose, M. Health-related quality of life, depression, and anxiety in patients with autoimmune hepatitis. J. Hepatol. 2014, 60, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Haapamäki, J.; Tenca, A.; Sintonen, H.; Barner-Rasmussen, N.; Färkkilä, M.A. Health-related quality of life among patients with primary sclerosing cholangitis. Liver Int. 2014, 35, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y. Recent advances in the management of pruritus in chronic liver diseases. World J. Gastroenterol. 2017, 23, 3418. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Khan, T.M. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: A systematic review. J. Formos. Med. Assoc. 2016, 115, 689–702. [Google Scholar] [CrossRef]
- Beuers, U.; Wolters, F.; Oude Elferink, R.P.J. Mechanisms of pruritus in cholestasis: Understanding and treating the itch. Nat. Rev. Gastroenterol. Hepatol. 2022, 20, 26–36. [Google Scholar] [CrossRef]
- Verkade, H.J.; Felzen, A.; Keitel, V.; Thompson, R.; Gonzales, E.; Strnad, P.; Kamath, B.; van Mil, S. EASL Clinical Practice Guidelines on genetic cholestatic liver diseases. J. Hepatol. 2024, 81, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2022, 77, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology 2022, 75, 1012–1013. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Sohal, A.; Kowdley, K.V. Primary biliary cholangitis and primary sclerosing cholangitis therapy landscape. Am. J. Gastroenterol. 2024, 120, 151–158. [Google Scholar] [CrossRef]
- Corpechot, C.; Chazouillères, O.; Rousseau, A.; Le Gruyer, A.; Habersetzer, F.; Mathurin, P.; Goria, O.; Potier, P.; Minello, A.; Silvain, C.; et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N. Engl. J. Med. 2018, 378, 2171–2181. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Bowlus, C.L.; Mayo, M.J.; Kremer, A.E.; Vierling, J.M.; Kowdley, K.V.; Levy, C.; Villamil, A.; Ladrón de Guevara Cetina, A.L.; Janczewska, E.; et al. A phase 3 trial of seladelpar in primary biliary cholangitis. N. Engl. J. Med. 2024, 390, 849–861. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Bowlus, C.L.; Levy, C.; Akarca, U.S.; Alvares-Da-Silva, M.R.; Andreone, P.; Arrese, M.; Corpechot, C.; Francque, S.M.; Heneghan, M.A.; et al. Efficacy and safety of elafibranor in primary biliary cholangitis. N. Engl. J. Med. 2024, 390, 795–805. [Google Scholar] [CrossRef]
- de Vries, E.; Bolier, R.; Goet, J.; Parés, A.; Verbeek, J.; de Vree, M.; Drenth, J.; van Erpecum, K.; van Nieuwkerk, K.; van der Heide, F.; et al. Fibrates for itch (FITCH) in fibrosing cholangiopathies: A double-blind, randomized, placebo-controlled trial. Gastroenterology 2021, 160, 734–743.e6. [Google Scholar] [CrossRef]
- McNally, B.B.; Carey, E.J. Cholestatic liver diseases: Modern therapeutics. Expert Rev. Gastroenterol. Hepatol. 2025, 19, 365–370. [Google Scholar] [CrossRef]
- Al-Dury, S.; Marschall, H.U. Ileal bile acid transporter inhibition for the treatment of chronic constipation, cholestatic pruritus, and NASH. Front Pharmacol. 2018, 9, 931. [Google Scholar] [CrossRef]
- Muntaha, H.S.T.; Munir, M.; Sajid, S.H.; Sarfraz, Z.; Sarfraz, A.; Robles-Velasco, K.; Sarfraz, M.; Felix, M.; Cherrez-Ojeda, I. Ileal bile acid transporter blockers for cholestatic liver disease in pediatric patients with Alagille syndrome: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 7526. [Google Scholar] [CrossRef]
- Imran, M.; Elsnhory, A.B.; Ibrahim, A.A.; Elnaggar, M.; Tariq, M.S.; Mehmood, A.M.; Ali, S.; Khalil, S.; Khan, S.H.; Ali, M.; et al. Efficacy and safety of ileal bile acid transport inhibitors in inherited cholestatic liver disorders: A meta-analysis of randomized controlled trials. J. Clin. Exp. Hepatol. 2025, 15, 102462. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Kendrick, S.; Bowlus, C.L.; Tanaka, A.; Jones, D.; Kremer, A.E.; Mayo, M.J.; Haque, N.; von Maltzahn, R.; Allinder, M.; et al. GLIMMER: A randomized phase 2b dose-ranging trial of linerixibat in primary biliary cholangitis patients with pruritus. Clin. Gastroenterol. Hepatol. 2023, 21, 1902–1912.e13. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L.; Eksteen, B.; Cheung, A.C.; Thorburn, D.; Moylan, C.A.; Pockros, P.J.; Forman, L.M.; Dorenbaum, A.; Hirschfield, G.M.; Kennedy, C.; et al. Safety, tolerability, and efficacy of maralixibat in adults with primary sclerosing cholangitis: Open-label pilot study. Hepatol. Commun. 2023, 7, e0153. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.J.; Pockros, P.J.; Jones, D.; Bowlus, C.L.; Levy, C.; Patanwala, I.; Bacon, B.; Luketic, V.; Vuppalanchi, R.; Medendorp, S.; et al. A randomized, controlled, phase 2 study of maralixibat in the treatment of itching associated with primary biliary cholangitis. Hepatol. Commun. 2019, 3, 365–381. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.5; Cochrane: London, UK, 2024. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Lindor, K.D.; Gershwin, M.E.; Poupon, R.; Kaplan, M.; Bergasa, N.V.; Heathcote, E.J. American Association for the Study of Liver Diseases. Primary biliary cirrhosis. Hepatology 2009, 50, 291–308. [Google Scholar] [CrossRef]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef]
- Mayo, M.J.; Carey, E.; Smith, H.T.; Mospan, A.R.; McLaughlin, M.; Thompson, A.; Morris, H.L.; Sandefur, R.; Kim, W.R.; Bowlus, C.; et al. Impact of pruritus on quality of life and current treatment patterns in patients with primary biliary cholangitis. Dig. Dis. Sci. 2022, 68, 995–1005. [Google Scholar] [CrossRef]
- Smith, H.T.; de Souza, A.R.; Thompson, A.H.; McLaughlin, M.M.; Dever, J.J.; Myers, J.A.; Chen, J.V. Cholestatic pruritus treatments in primary biliary cholangitis and primary sclerosing cholangitis: A systematic literature review. Dig. Dis. Sci. 2023, 68, 2710–2730. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Sasaki-Tanaka, R.; Kimura, N.; Abe, H.; Yoshida, T.; Hayashi, K.; Sakamaki, A.; Yokoo, T.; Kamimura, H.; Tsuchiya, A.; et al. Pruritus in chronic cholestatic liver diseases, especially in primary biliary cholangitis: A narrative review. Int. J. Mol. Sci. 2025, 26, 1883. [Google Scholar] [CrossRef] [PubMed]
- Karatza, E.; Swift, B.; Carreño, F.; Mukherjee, S.; Casillas, L.; Lennie, J.; Fettiplace, J.; McLaughlin, M.M.; Kremer, A.E. Serum bile acid change correlates with improvement in pruritus in patients with primary biliary cholangitis receiving linerixibat. Liver Int. 2024, 44, 2293–2302. [Google Scholar] [CrossRef]
- Hegade, V.S.; Pechlivanis, A.; McDonald, J.A.K.; Rees, D.; Corrigan, M.; Hirschfield, G.M.; Taylor-Robinson, S.D.; Holmes, E.; Marchesi, J.R.; Kendrick, S.; et al. Autotaxin, bile acid profile and effect of ileal bile acid transporter inhibition in primary biliary cholangitis patients with pruritus. Liver Int. 2019, 39, 967–975. [Google Scholar] [CrossRef]
- Jacoby, A. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut 2005, 54, 1622–1629. [Google Scholar] [CrossRef]
- Al-Dury, S.; Wahlström, A.; Wahlin, S.; Langedijk, J.; Elferink, R.O.; Ståhlman, M.; Marschall, H.-U. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci. Rep. 2018, 8, 6658. [Google Scholar] [CrossRef]
- Tanaka, A.; Atsukawa, M.; Tsuji, K.; Notsumata, K.; Suyama, A.; Ito, H.; Das, S.; von Maltzahn, R.; McLaughlin, M.M. Japanese subgroup analysis of GLIMMER: A global Phase IIb study of linerixibat for the treatment of cholestatic pruritus in patients with primary biliary cholangitis. Hepatol. Res. 2023, 53, 629–640. [Google Scholar] [CrossRef]
- von Maltzahn, R.; Mayo, M.J.; Smith, H.T.; Thompson, A.; Das, S.; de Souza, A.R.; Lisi, E.; Levy, C.; McLaughlin, M.M.; Jones, D. Relationship between pruritus and sleep in participants with primary biliary cholangitis in the Phase 2b GLIMMER trial. J. Patient Rep. Outcomes 2024, 8, 60. [Google Scholar] [CrossRef]
- Carreño, F.; Karatza, E.; Mehta, R.; Collins, J.; Austin, D.; Swift, B. Population dose–response–time analysis of itch reduction and patient-reported tolerability supports Phase III dose selection for linerixibat. Clin. Pharmacol. Ther. 2023, 115, 288–298. [Google Scholar] [CrossRef]
- Carreño, F.; Mehta, R.; de Souza, A.R.; Collins, J.; Swift, B. Analysis of C4 concentrations to predict impact of patient-reported diarrhea associated with the ileal bile acid transporter inhibitor linerixibat. CPT Pharmacomet. Syst. Pharmacol. 2025, 14, 596–605. [Google Scholar] [CrossRef]
| Study | Design | AICLD | IBAT Inhibitor | Patient, n | Dose (Frequency) | Mean Age, Years (SD) | Female, n (%) | Receiving UDCA, n (%) | Receiving Lipid-Modifying Agents, n (%) | Receiving Antihistamine, n (%) | Mean Serum BA, µmol/L (SD) | Dose Escalation, Weeks | Follow-Up, Weeks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mayo 2019 [26] 1 | RCT | PBC | Maralixibat | 21 | 10 mg (QD) | 54.7 (12.7) | 20 (95.2) | 21 (100) | 4 (9.5) | 11 (26.2) | 33.1 (30.6) | 3 | 13 |
| Mayo 2019 [26] 2 | 21 | 5 or 20 mg (QD) | 53.5 (10.5) | 17 (81.0) | 19 (90.5) | 52.5 (94.4) | 2–4 | ||||||
| Bowlus 2023 [25] | Single-arm | PSC | Maralixibat | 27 | 10 mg (QD) | 43.7 (11.4) | 9 (33.3) | NA | 6 (22.2) | NA | 38.9 (NA) | 6 | 14 |
| Levy 2023 [24] (GLIMMER) 1 | RCT | PBC | Linerixibat | 16 | 20 mg (QD) | 58.5 (7.3) | 16 (100.0) | 14 (87.5) | NA | 2 (12.5) | 13.0 (13.8) | 0 | 12 |
| Levy 2023 [24] (GLIMMER) 2 | 23 | 90 mg (QD) | 52.5 (12.3) | 21 (91.1) | 22 (95.6) | NA | 3 (13.0) | 33.7 (55.8) | |||||
| Levy 2023 [24] (GLIMMER) 3 | 27 | 180 mg (QD) | 58.9 (11.1) | 25 (92.6) | 27 (100) | NA | 3 (11.1) | 26.6 (36.5) | |||||
| Levy 2023 [24] (GLIMMER) 4 | 23 | 40 mg (BID) | 55.6 (11.2) | 22 (95.6) | 21 (91.3) | NA | 4 (17.4) | 18.6 (21.8) | |||||
| Levy 2023 [24] (GLIMMER) 5 | 22 | 90 mg (BID) | 56.2 (11.3) | 20 (90.9) | 21 (95.4) | NA | 3 (13.6) | 19.3 (31.0) |
| IBAT Inhibitor | AICLD Studied | Mechanism of Action | Indication | Main Findings | Adverse Effects |
|---|---|---|---|---|---|
| Linerixibat | PBC | Interruption of intestinal bile acid reabsorption | Second-line therapy for pruritus in patients with AICLD | Significantly reduced pruritus severity (Levy 2023) [24], with improvements in itch intensity (MWDI score) and quality of life (PBC-40 itch, social, and emotional domains) compared to placebo; biomarker analysis also showed favorable changes. | Dose-related GI AEs, chiefly diarrhea (38–68% across doses) and abdominal pain (9–30%); nausea occurred less frequently. Some discontinuations due to diarrhea/abdominal pain; events resolved after stopping treatment. |
| Maralixibat | PBC and PSC | PBC (Mayo 2019) [26]: reduced pruritus severity with improvements in patient-reported itch scores (ItchRO, VAS) and quality of life. PSC (Bowlus 2023) [25]: reduced serum bile acids and modest pruritus improvements (ItchRO, 5-D itch), greater in those with more severe baseline itch. Both studies reported improvements in biomarkers analysis. | PBC (Mayo 2019) [26]: GI disorders in 78.6% vs. 50.0% with placebo; most frequent were diarrhea 61.9%, abdominal pain 23.8%, upper abdominal pain 23.8%, nausea 23.8%; fewer events during stable dosing and most resolved without discontinuation. PSC (Bowlus 2023) [25]: GI TEAEs in 81.5%; diarrhea 51.9%, nausea 33.3%, abdominal pain 29.6%; GI events were more common during dose escalation than stable dosing; one treatment-related serious AE (cholangitis). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, I.B.; Rezende, R.A.A.; Neto, C.A.M.L.; Rossi, Y.I.; Leite, M.d.A.B.C.; Cançado, G.G.L. Ileal Bile Acid Transporter Inhibitors for Adult Patients with Autoimmune Cholestatic Liver Diseases: A Systematic Review and Meta-Analysis. Gastroenterol. Insights 2025, 16, 30. https://doi.org/10.3390/gastroent16030030
Silveira IB, Rezende RAA, Neto CAML, Rossi YI, Leite MdABC, Cançado GGL. Ileal Bile Acid Transporter Inhibitors for Adult Patients with Autoimmune Cholestatic Liver Diseases: A Systematic Review and Meta-Analysis. Gastroenterology Insights. 2025; 16(3):30. https://doi.org/10.3390/gastroent16030030
Chicago/Turabian StyleSilveira, Igor Boechat, Rodolfo Augusto Assis Rezende, Carlos Alberto Monteiro Leitão Neto, Yohanna Idsabella Rossi, Marina de Assis Bezerra Cavalcanti Leite, and Guilherme Grossi Lopes Cançado. 2025. "Ileal Bile Acid Transporter Inhibitors for Adult Patients with Autoimmune Cholestatic Liver Diseases: A Systematic Review and Meta-Analysis" Gastroenterology Insights 16, no. 3: 30. https://doi.org/10.3390/gastroent16030030
APA StyleSilveira, I. B., Rezende, R. A. A., Neto, C. A. M. L., Rossi, Y. I., Leite, M. d. A. B. C., & Cançado, G. G. L. (2025). Ileal Bile Acid Transporter Inhibitors for Adult Patients with Autoimmune Cholestatic Liver Diseases: A Systematic Review and Meta-Analysis. Gastroenterology Insights, 16(3), 30. https://doi.org/10.3390/gastroent16030030