From in Utero to Gut: The Unseen Impact of Early-Life Vitamin D Deficiency on the Gastrointestinal System—A Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Study Selection Criteria
- Inclusion Criteria:
- Study Design:
- ➢
- Randomized Controlled Trials (RCTs): Primary studies with high methodological rigor, providing evidence of causality between vitamin D deficiency and gastrointestinal health in neonates.
- ➢
- Cohort and Case–Control Studies: Well-designed observational studies assessing associations between maternal and/or neonatal vitamin D status and gastrointestinal outcomes in neonates.
- ➢
- Systematic Reviews and Meta-Analyses: Studies synthesizing evidence from multiple studies on the topic.
- Population:
- ➢
- Neonates (≤28 days of life): Including both preterm and term infants.
- ➢
- Mothers: With known vitamin D deficiency during pregnancy and their neonates.
- ➢
- Primary Outcome: Gastrointestinal disorders in neonates, including NEC, intestinal inflammation, and feeding intolerance.
- ➢
- Secondary Outcomes: Alterations in gut microbiome composition and immunological parameters (e.g., inflammatory cytokine levels).
- Language: Studies published in English.
- Exclusion Criteria:
- ➢
- Studies focusing on populations outside of neonates (e.g., adults).
- ➢
- Studies without clear diagnostic criteria for gastrointestinal disorders or without measurements of vitamin D status (e.g., studies without serum vitamin D levels).
- ➢
- Articles with a sample size too small to provide reliable conclusions.
- ➢
- Studies with low methodological quality, assessed by the Cochrane Risk of Bias Tool and Newcastle–Ottawa Scale (NOS).
2.2. Search Strategy
- ➢
- “maternal vitamin D deficiency”;
- ➢
- “neonatal vitamin D deficiency”;
- ➢
- “gastrointestinal disorders in neonates”;
- ➢
- “necrotizing enterocolitis”;
- ➢
- “gut microbiome”;
- ➢
- “intestinal inflammation”.
2.3. Study Selection and Data Extraction
- ➢
- Study characteristics (author, year of publication, sample size).
- ➢
- Methodological details (study design, intervention details, follow-up duration).
- ➢
- Outcome measures (vitamin D status, gastrointestinal disorders, microbiome composition).
- ➢
- Key findings (strength of association between vitamin D deficiency and gastrointestinal disorders).
Clarification on Review Types
2.4. Quality Assessment
- ➢
- Cochrane Risk of Bias Tool: Applied to RCTs, evaluating domains such as random sequence generation, allocation concealment, blinding, and incomplete outcome data.
- ➢
- Newcastle–Ottawa Scale (NOS): Applied to cohort and case–control studies, assessing the selection of participants, comparability of groups, and outcome assessment.
2.5. Data Analysis
- Statistical Considerations:
- ➢
- Heterogeneity: The I2 statistic will be used to assess statistical heterogeneity across studies. An I2 value greater than 50% will indicate significant heterogeneity.
- ➢
- Sensitivity Analysis: Sensitivity analysis will be conducted by excluding studies with a high risk of bias or small sample sizes.
- ➢
- Subgroup Analysis: Subgroup analyses will be conducted based on study design (RCTs vs. observational studies), population (preterm vs. term infants), and type of gastrointestinal disorder (e.g., NEC vs. feeding intolerance).
2.6. Final Study Selection Process
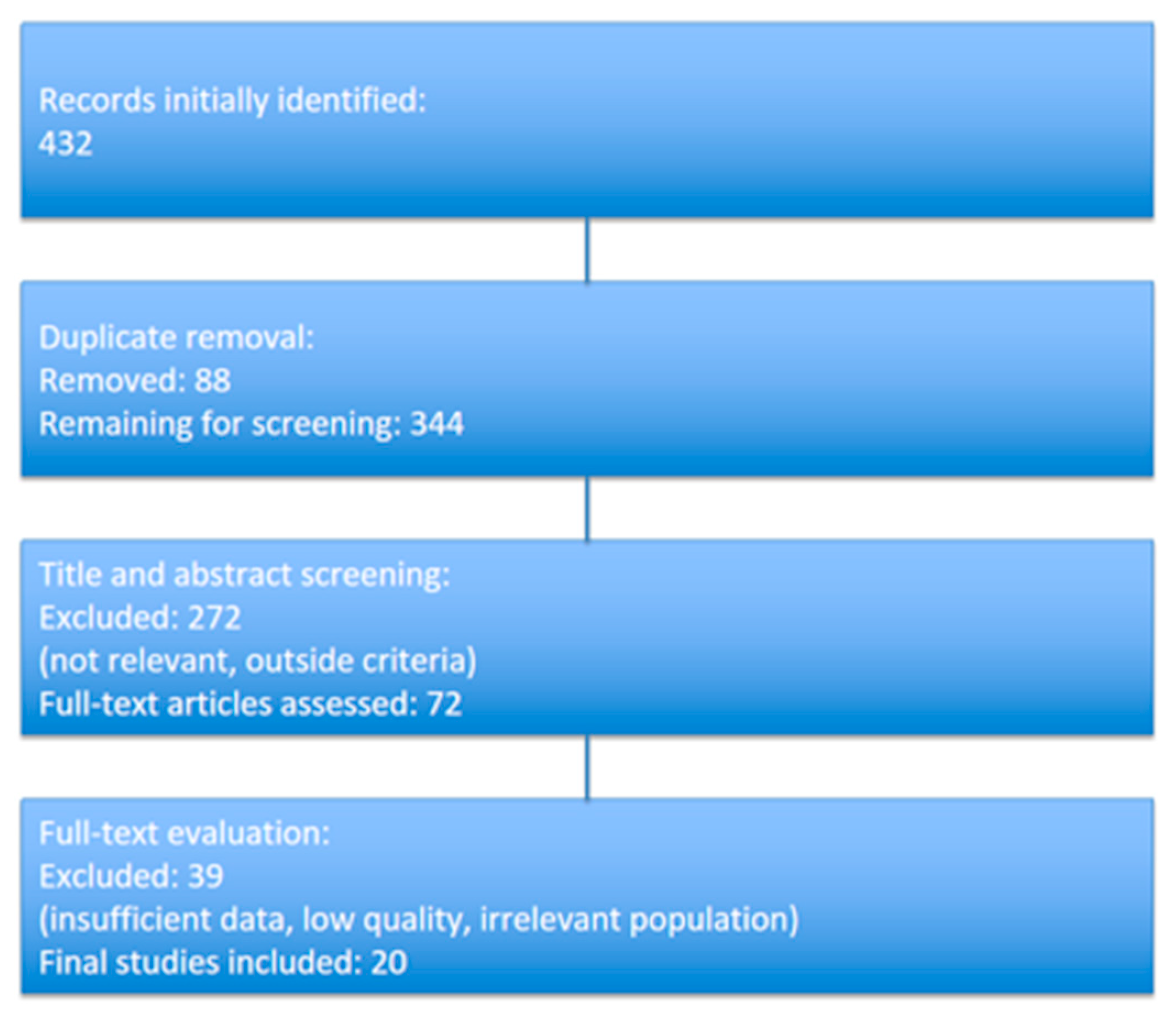
2.6.1. Study Selection and Screening Process
- Initial Search Results:
- Total articles identified: 432 studies (from PubMed, Scopus, Cochrane Library, and Google Scholar).
- 2.
- Duplicate Removal:
- After removing 88 duplicate records, 344 unique articles remained.
- 3.
- Title and Abstract Screening:
- Articles were screened based on their titles and abstracts. Studies not meeting the inclusion criteria or not addressing neonatal vitamin D deficiency or gastrointestinal disorders were excluded.
- A total of 272 articles were excluded at this stage, leaving 72 for full-text review.
- 4.
- Full-text Review:
- These 72 articles were further assessed for methodological quality and relevance.
- A total of 39 studies were excluded due to insufficient data, irrelevant outcomes, or low methodological quality.
- 5.
- Final Inclusion:
- Ultimately, 20 studies met all inclusion criteria and were included in the qualitative synthesis and, where possible, meta-analysis.
2.6.2. Reasons for Exclusion Included
- Lack of clear reporting on VDD or gastrointestinal outcomes.
- Small sample sizes or insufficient methodological rigor.
- Focus on non-neonatal populations or lack of specific gastrointestinal diagnostic criteria.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. Available online: https://pubmed.ncbi.nlm.nih.gov/21527855 (accessed on 1 August 2011). [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The impact of vitamin D levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. Available online: https://pubmed.ncbi.nlm.nih.gov/26528817 (accessed on 3 November 2015). [CrossRef]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018, 562, 589–594. Available online: https://pubmed.ncbi.nlm.nih.gov/30356183 (accessed on 1 October 2018). [CrossRef]
- Harvey, N.C.; Holroyd, C.; Ntani, G.; Javaid, K.; Cooper, P.; Moon, R.; Cole, Z.; Tinati, T.; Godfrey, K.; Dennison, E.; et al. Vitamin D supplementation in pregnancy: A systematic review. Health Technol. Assess. 2014, 18, 1–190. Available online: https://pubmed.ncbi.nlm.nih.gov/25025869 (accessed on 1 July 2014). [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. Available online: https://pubmed.ncbi.nlm.nih.gov/23533188 (accessed on 26 March 2013). [CrossRef]
- Sun, J.; Zhang, Y.G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. Available online: https://pubmed.ncbi.nlm.nih.gov/35406694 (accessed on 27 March 2022). [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. Available online: https://pubmed.ncbi.nlm.nih.gov/15985530 (accessed on 1 July 2005). [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. Available online: https://pubmed.ncbi.nlm.nih.gov/22503810 (accessed on 1 July 2012). [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. Available online: https://pubmed.ncbi.nlm.nih.gov/19172691 (accessed on 1 September 2008). [CrossRef]
- Talsness, C.E.; Penders, J.; Jansen, E.H.J.M.; Damoiseaux, J.; Thijs, C.; Mommers, M. Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA Birth Cohort Study. PLoS ONE 2017, 12, e0188011. Available online: https://pubmed.ncbi.nlm.nih.gov/29121673 (accessed on 9 November 2017). [CrossRef]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. Available online: https://pubmed.ncbi.nlm.nih.gov/39064795 (accessed on 20 July 2024). [CrossRef] [PubMed]
- Molani-Gol, R.; Rafraf, M. Maternal vitamin D in pregnancy and infant’s gut microbiota: A systematic review. Front. Pediatr. 2023, 11, 1248517. Available online: https://pubmed.ncbi.nlm.nih.gov/37915988 (accessed on 16 October 2023). [CrossRef]
- Song, Q.; Li, Y.; Zhou, T.; Xiao, M.; Xiao, B.; Wang, M.; Zhu, Y. Maternal vitamin D status during pregnancy and infant’s gut microbiota: A prospective cohort study. Front. Nutr. 2024, 11, 1428356. Available online: https://pubmed.ncbi.nlm.nih.gov/39135559 (accessed on 29 July 2024). [CrossRef] [PubMed]
- Fort, P.; Salas, A.A.; Nicola, T.; Craig, C.M.; Carlo, W.A.; Ambalavanan, N. A Comparison of 3 Vitamin D Dosing Regimens in Extremely Preterm Infants: A Randomized Controlled Trial. J. Pediatr. 2016, 174, 132–138. Available online: https://pubmed.ncbi.nlm.nih.gov/27079965 (accessed on 1 July 2016). [CrossRef] [PubMed]
- Shi, Y.; Liu, T.; Zhao, X.; Yao, L.; Hou, A.; Fu, J.; Xue, X. Vitamin D ameliorates neonatal necrotizing enterocolitis via suppressing TLR4 in a murine model. Pediatr. Res. 2018, 83, 1024–1030. Available online: https://pubmed.ncbi.nlm.nih.gov/29281615 (accessed on 1 May 2018). [CrossRef]
- Kamrani, K.; Mardani, M.; Zarkesh, M. The association between vitamin D levels and necrotizing enterocolitis in preterm neonates. Pediatr Dimens. 2020, 5, 1–4. [Google Scholar] [CrossRef]
- Yang, L.-R.; Li, H.; Zhang, T.; Zhao, R.-C. Relationship between vitamin D deficiency and necrotizing enterocolitis in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi 2018, 20, 178–183. Available online: https://pubmed.ncbi.nlm.nih.gov/29530115 (accessed on 1 March 2018).
- Cetinkaya, M.; Erener-Ercan, T.; Kalayci-Oral, T.; Babayigit, A.; Cebeci, B.; Semerci, S.Y. Maternal/neonatal vitamin D deficiency: A new risk factor for necrotizing enterocolitis in preterm infants? J. Perinatol. 2017, 37, 673–678. Available online: https://pubmed.ncbi.nlm.nih.gov/28333154 (accessed on 1 June 2017). [CrossRef]
- You, Z.; Mei, H.; Zhang, Y.; Song, D.; Zhang, Y.; Liu, C. The effect of vitamin D deficiency during pregnancy on adverse birth outcomes in neonates: A systematic review and meta-analysis. Front. Pediatr. 2024, 12, 1399615. Available online: https://pubmed.ncbi.nlm.nih.gov/38808102 (accessed on 14 May 2024). [CrossRef]
- Wang, Y.; Li, H.; Zheng, M.; Wu, Y.; Zeng, T.; Fu, J.; Zeng, D. Maternal vitamin D deficiency increases the risk of adverse neonatal outcomes in the Chinese population: A prospective cohort study. PLoS ONE 2018, 13, e0195700. [Google Scholar] [CrossRef]
- Sulistijono, E.; Vebrianti Corebima, B.I.R.; Pramitasari, Y.A. The correlation between levels of vitamin D (25(OH)D) and the occurrence of necrotizing enterocolitis (NEC)in preterm infants. Pediatr. Sci. J. 2023, 4, 6–15. [Google Scholar]
- Yangin Ergon, E.; Dorum, B.A.; Balki, H.G.; Bako, D.; Ozdemir, S.A. A Prospective Cross-Sectional Study on the Vitamin D Status of Neonates’ Standard Vitamin D Supplementation on Neonatal Morbidities. Children 2024, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.; Collins, C.E.; Holmes, A.; Lal, R.; Duncanson, K.; Taylor, R.; Gordon, A. Maternal exposures and the infant gut microbiome: A systematic review with meta-analysis. Gut Microbes 2021, 13, 1897210. [Google Scholar] [CrossRef] [PubMed]
- Drall, K.M.; Field, C.J.; Haqq, A.M.; de Souza, R.J.; Tun, H.M.; Morales-Lizcano, N.P.; Konya, T.B.; Guttman, D.S.; Azad, M.B.; Becker, A.B.; et al. Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: Implications for viral respiratory infections. Gut Microbes 2020, 12, 1799734. [Google Scholar] [CrossRef]
- Sordillo, J.E.; Zhou, Y.; McGeachie, M.J.; Ziniti, J.; Lange, N.; Laranjo, N.; Savage, J.R.; Carey, V.; O’Connor, G.; Sandel, M.; et al. Factors influencing the infant gut microbiome at age 3–6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J. Allergy. Clin. Immunol. 2017, 139, 482–491. Available online: https://pubmed.ncbi.nlm.nih.gov/27746239 (accessed on 1 February 2017). [CrossRef]
- Marsubrin, P.M.T.; Firmansyah, A.; Rohsiswatmo, R.; Purwosunu, Y.; Bardosono, S.; Malik, S.G.; Munasir, Z.; Timan, I.S.; Yuniati, T. Vitamin D and gut microbiome in preterm infants. BMC Pediatr. 2024, 24, 588. Available online: https://pubmed.ncbi.nlm.nih.gov/39285348 (accessed on 16 September 2024). [CrossRef]
- Tabassum, A.; Ali, A.; Zahedi, F.D.; Ismail, N.A.S. Immunomodulatory Role of Vitamin D on Gut Microbiome in Children. Biomedicines 2023, 11, 1441. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D and Pregnancy: Skeletal Effects, Nonskeletal Effects, and Birth Outcomes. Calcif. Tissue Int. 2013, 92, 128–139. Available online: https://pubmed.ncbi.nlm.nih.gov/22623177 (accessed on 1 February 2013). [CrossRef]
- Aparicio, A.; Gold, D.R.; Weiss, S.T.; Litonjua, A.A.; Lee-Sarwar, K.; Liu, Y.-Y. Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation. Nutrients 2023, 15, 2059. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10181263/ (accessed on 1 July 2025). [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. Available online: https://pubmed.ncbi.nlm.nih.gov/18676559 (accessed on 1 August 2008). [CrossRef]
- Motamed, S.; Anari, R.; Motamed, S.; Amani, R. Vitamin D and biomarkers of inflammation and oxidative stress among pregnant women: A systematic review of observational studies. BMC Immunol. 2023, 24, 41. Available online: https://bmcimmunol.biomedcentral.com/articles/10.1186/s12865-023-00577-w (accessed on 1 July 2025). [CrossRef] [PubMed]
- Mărginean, C.O.; Melit, L.E.; Balas, R.B.; Vasiesiu, A.M.; Flaseriu, T. The Crosstalk between Vitamin D and Pediatric Digestive Disorders. Diagnostics 2022, 12, 2328. Available online: https://www.mdpi.com/2075-4418/12/10/2328 (accessed on 1 July 2025). [CrossRef] [PubMed]
- Yang, K.; Zhu, J.; Wu, J.; Zhong, Y.; Shen, X.; Petrov, B. Maternal Vitamin D Deficiency Increases Intestinal Permeability and Programs Wnt/β-Catenin Pathway in BALB/C Mice. J. Parenter. Enteral. Nutr. 2021, 45, 102–114. Available online: https://pubmed.ncbi.nlm.nih.gov/32270535 (accessed on 1 January 2021). [CrossRef] [PubMed]
- Rosendahl, J.; Holmlund-Suila, E.; Helve, O.; Viljakainen, H.; Hauta-alus, H.; Valkama, S.; Enlund-Cerullo, M.; Hytinantti, T.; Tervahartiala, T.; Sorsa, T.; et al. 25-hydroxyvitamin D correlates with inflammatory markers in cord blood of healthy newborns. Pediatr. Res. 2017, 81, 731–735. Available online: https://pubmed.ncbi.nlm.nih.gov/28085793 (accessed on 1 May 2017). [CrossRef]
- Noakes, P.S.; Michaelis, L.J.; Williams, A.P.; Thornton, C.A.; Arshad, H.; Calder, P.C.; Harvey, N.; Cooper, C.; Roberts, G.C. Cord blood vitamin D status: Evidence for altered CD4+ naive and gut-homing T cell accumulation. In Proceedings of the 6th International Workshop on Immunonutrition, Palma de Mallorca, Spain, 15–17 October 2012; Cambridge University Press: Cambridge, UK, 2013; Volume 72. [Google Scholar] [CrossRef]
- Onwuneme, C.; Diya, B.; Uduma, O.; McCarthy, R.A.; Murphy, N.; Kilbane, M.T.; McKenna, M.J.; Molloy, E.J. Correction of vitamin D deficiency in a cohort of newborn infants using daily 200 IU vitamin D supplementation. Ir. J. Med. Sci. 2016, 185, 683–687. Available online: https://pubmed.ncbi.nlm.nih.gov/26210881 (accessed on 1 August 2016). [CrossRef]
| # | Author(s) [Ref] | Study Design | Population/Model | Participants (n) | Key Findings |
|---|---|---|---|---|---|
| 1 | Song J et al. [13] | Prospective cohort | Mother–infant dyads | 87 | Maternal vitamin D levels influence infant gut microbiota composition. |
| 2 | Kamrani B et al. [16] | Observational | Preterm infants | 64 (32 NEC, 32 control) | Lower vitamin D levels are significantly associated with increased risk of NEC. |
| 3 | Cetinkaya M et al. [18] | Observational | Preterm infants | 145 | Maternal/neonatal vitamin D deficiency is a potential risk factor for NEC. |
| 4 | Wang J et al. [20] | Prospective cohort | Mother–infant pairs | 1978 | Low maternal vitamin D levels increase the risk of adverse neonatal outcomes. |
| 5 | Sulistijono E et al. [21] | Observational | Preterm infants | 51 | Low vitamin D levels correlated with higher NEC incidence. |
| 6 | Yangin Ergon et al. [22] | Cross-sectional | Term infants | 86 | Standard vitamin D supplementation was associated with neonatal morbidity outcomes. |
| 7 | Grech A et al. [23] | Systematic review | 15 studies | — | Maternal factors, including vitamin D status, influence the infant gut microbiome. |
| 8 | Molani-Gol R et al. [24] | Systematic review | 18 studies | — | Maternal vitamin D affects infant gut microbiota composition. |
| 9 | Sordillo JE et al. [25] | Clinical trial | Infants | 333 | Vitamin D levels impact early infant gut microbiome development. |
| 10 | Marsubrin P et al. [26] | Observational | Preterm infants | 43 | Vitamin D modulates gut microbiome composition in preterm infants. |
| 11 | Tabassum A et al. [27] | Systematic review | Pediatric populations | — | Vitamin D has immunomodulatory effects on gut microbiota in children. |
| 12 | Hollis BW et al. [28] | Randomized controlled trial | Pregnant women | 494 randomized; ~350 completed | Vitamin D supplementation improves skeletal and nonskeletal pregnancy outcomes. |
| 13 | Aparicio A et al. [29] | Randomized controlled trial | Pregnant women | 120 | Antenatal vitamin D supplementation alters the maternal gut microbiome. |
| 14 | Misra M et al. [30] | Narrative review | Pediatric population | — | Comprehensive review of vitamin D deficiency and pediatric treatment guidelines. |
| 15 | Motamed S et al. [31] | Systematic review | Pregnant women | — | Vitamin D levels inversely correlate with inflammatory and oxidative markers. |
| 16 | Mărginean CO et al. [32] | Narrative review | Pediatric patients | — | Vitamin D plays a role in pediatric gastrointestinal disease pathophysiology. |
| 17 | Yang K et al. [33] | Animal cohort | BALB/C mice | 30 | Vitamin D deficiency increases intestinal permeability and alters Wnt/β-catenin signaling. |
| 18 | Rosendahl J et al. [34] | RCT | Healthy newborns | 81 | Cord blood vitamin D inversely correlates with inflammatory markers. |
| 19 | Noakes PS et al. [35] | Observational | Neonates | — | Cord vitamin D status affects immune cell populations in neonates. |
| 20 | Onwuneme C et al. [36] | Prospective study | Neonates | — | 200 IU/day of vitamin D effectively corrected neonatal deficiency. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinari, A.; Antoniou, E.; Gourounti, K.; Orovou, E.; Dagla, M.; Sarantaki, A.; Iatrakis, G. From in Utero to Gut: The Unseen Impact of Early-Life Vitamin D Deficiency on the Gastrointestinal System—A Systematic Review. Gastroenterol. Insights 2025, 16, 22. https://doi.org/10.3390/gastroent16030022
Kokkinari A, Antoniou E, Gourounti K, Orovou E, Dagla M, Sarantaki A, Iatrakis G. From in Utero to Gut: The Unseen Impact of Early-Life Vitamin D Deficiency on the Gastrointestinal System—A Systematic Review. Gastroenterology Insights. 2025; 16(3):22. https://doi.org/10.3390/gastroent16030022
Chicago/Turabian StyleKokkinari, Artemisia, Evangelia Antoniou, Kleanthi Gourounti, Eirini Orovou, Maria Dagla, Antigoni Sarantaki, and Georgios Iatrakis. 2025. "From in Utero to Gut: The Unseen Impact of Early-Life Vitamin D Deficiency on the Gastrointestinal System—A Systematic Review" Gastroenterology Insights 16, no. 3: 22. https://doi.org/10.3390/gastroent16030022
APA StyleKokkinari, A., Antoniou, E., Gourounti, K., Orovou, E., Dagla, M., Sarantaki, A., & Iatrakis, G. (2025). From in Utero to Gut: The Unseen Impact of Early-Life Vitamin D Deficiency on the Gastrointestinal System—A Systematic Review. Gastroenterology Insights, 16(3), 22. https://doi.org/10.3390/gastroent16030022











