1. Introduction
Alcohol consumption causes progressive damage to various organs and tissues of the human body [
1,
2,
3,
4]. Worldwide there is excessive and varied consumption of alcohol, specifically in Mexico, where the main alcoholic beverages consumed are beer and spirits (brandy, tequila, rum, whiskey, cognac, vodka, etc.) [
3], which contain approximately 5% and 36–40% alcohol, respectively [
1]. Regarding alcohol consumption patterns, daily, excessive, and weekend consumption have been reported and have been associated with gender, age, socioeconomic level, consumption habits, and types of alcoholic beverages (wine and mixed drinks) [
5,
6]. According to the ENCODAT 2016 report, 2.9% of young Mexicans engage in daily alcohol consumption and 8.5% have habitual weekend consumption. It was also found that the main consumers of weekend alcohol are men; on the other hand, there are more and more women who consume weekend alcohol and are prone to liver damage due to alcohol [
3,
7].
There are few studies on ethanol consumption during the weekend. Morales-González et al. [
8] reported the harmful effects caused by alcohol consumption over the weekend, with a higher impact observed in the female group. They found that weekend alcohol consumption leads to liver damage, characterized by biochemical and histological alterations that initially present acutely. Prolonged consumption can result in more severe and irreversible damage [
8]. González-López et al. [
9] studied the effects of weekend alcohol consumption on the oral cavity, discovering that this pattern of alcohol intake leads to tissue alterations in the oral cavity, likely associated with an increase in oxidative stress (OS) [
9]. This is probably due to elevated oral peroxidase activity in individuals who regularly consume alcohol, producing oxidative stress and damage to the oral cavity [
10].
Liu et al. [
11] reported an increase in total cholesterol and HDL-C levels with daily versus weekend alcohol consumption and found a decrease in LDL-C levels with daily and moderate alcohol consumption, while LDL-C levels were significantly elevated with weekend alcohol consumption. Finally, the authors reported a greater increase in blood alcohol levels and weight gain with weekend alcohol consumption, concluding that weekend alcohol consumption had a greater impact on the body and favored the development of atherosclerotic plaque (increase plaque, decreased lumen diameter, etc.), while the opposite occurred with moderate daily alcohol consumption [
11]. Reports found in the literature demonstrate the damage to the liver caused by alcohol consumption [
12,
13,
14,
15], but the damage or alterations caused by weekend alcohol consumption to the liver have not been described to date; in particular, there are no reports on changes in the activity of antioxidant enzymes with this type of alcohol consumption in liver tissue.
The levels of antioxidant enzymes are influenced by the timing of ethanol administration [
16,
17]. Parra et al. [
18] administered a single ethanol dose of 1.5 g/kg bw and observed an increase in thiobarbituric acid reactive substances (TBARSs) content and a decrease in superoxide dismutase (SOD) enzyme activity. Morales-González et al. [
19] administered a daily ethanol dose of 1.5 g/Kg bw and found an increase in TBARSs, along with a surprising increase in antioxidant defenses [total antioxidant capacity, catalase, SOD, glutathione reductase (GR), and gluthathione peroxidase (GP)]. Ramirez et al. reported the increase in TBARS content in both serum and liver due to ethanol consumption [
20].
2. Materials and Methods
2.1. Experimental Design
Wistar rats (Rattus norvegicus) weighing 200 g were used. Rodents were housed at room temperature with access to water and food ad libitum (LabDiet Formulab diet A-8003-037). All procedures were approved by the research committees and by CICUAL, with registration numbers ESM.CI-01/13-06-2017 and ESM.CICUAL-12/23-06-2017, respectively. The procedures were carried out in accordance with the Mexican Official Standard for the Use and Care of Laboratory Animals (NOM-062-ZOO-1999).
The rats were divided into experimental groups based on alcohol consumption:
(a) control group: female or male rats that received only water and food ad libitum without ethanol.
(b) the 40% group: female or male rats that consumed 40% alcohol with ad libitum access in the drinking bowl, 2 days a week for 3 months.
(c) the 5% group: female or male rats that consumed 5% alcohol with ad libitum access in the drinking bowl, 2 days a week for 3 months.
After 3 months of treatment, the experimental rats were euthanized by decapitation after prior anesthesia with sodium pentobarbital (40 mg/kg of bw).
2.2. Liver Samples
The liver was briefly treated as usual: it was isolated, weighed, and placed in a PBS buffer, then homogenized using a sucrose buffer (0.25 M sucrose, 10 mM TRIS, and 0.3 mM EGTA at pH 7.4). The total protein concentration of the homogenate was determined using the Lowry method [
21], with a BSA solution as standard.
2.3. Determination of Antioxidant Enzymes
Catalase enzyme activity was measured using the Catalase Assay Kit (707002); superoxide dismutase enzyme activity was measured using the superoxide dismutase Assay Kit (706002); glutathione reductase enzyme activity was measured using the Glutathione Reductase Assay Kit (703202); and glutathione peroxidase enzyme activity was measured using the Glutathione Peroxidase Assay Kit (703102), following the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, USA). The results are expressed in nmol/mg (catalase and glutathione reductase) and as U/mg (superoxide dismutase and glutathione peroxidase).
2.4. Total Antioxidant Capacity in Liver and Determination of Thiobarbituric Acid Reactive Substances
Total antioxidant capacity (TAC) was determined using an Antioxidant Assay Kit (709001), and thiobarbituric acid reactive substances (TBARSs) were determined using the TBARS Assay Kit (10009055), following the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, USA) and reporting results in nmol/mg (Trolox) (TAC) and in nmol/mg of protein (TBARSs).
2.5. Statistical Analysis
The results were analyzed using the statistical program SigmaPlot ver. 12.3. Results are expressed as mean ± SEM, as appropriate. Statistical analysis was performed using Student’s t-test and/or analysis of variance (ANOVA). We considered differences between groups to be statistically significant when p < 0.05.
4. Discussion
Alcohol consumption is currently increasing dramatically among the young population, and the resulting damage and consequences significantly impact various aspects of an individual’s health and well-being. While there is vast evidence on the effects of alcohol consumption on different organs and tissues of the human body, many studies focus on models that consider chronic daily alcohol consumption, overlooking the more common weekend consumption pattern among the young population. In this study, we evaluated the effect of weekend alcohol consumption on the levels of antioxidant enzymes over a 12-week period using a female and male rodent model.
The profile of reactive oxygen species or the activity of antioxidant enzymes in the liver depends on the timing and dosage of ethanol administration. Morales-González et al. [
19] reported that after the administering ethanol intragastrically at a dose of 1.5 g/kg of body weight (using a 40% EtOH solution in isotonic saline solution) daily for 7 days, resulting in blood alcohol levels between 75 and 150 mg/dL, there was an increase in TBARSs as well as in endogenous antioxidant defenses (catalase, SOD, GPx, and GR). These findings suggest that the continuous ROS production induced by daily ethanol administration promotes the enhancement of antioxidant and detoxifying defenses within 7 days. Conversely, Namachivayam and Valsala Gopalakrishnan [
22] orally administered ethanol to rats for 56 days at a daily dose of 5 mL/kg b/w. They observed increased levels of reactive oxygen species and lipid peroxidation (MDA) in liver samples, along with decreased antioxidant activity (glutathione peroxidase, superoxide dismutase, and glutathione reductase). With prolonged ethanol administration, the antioxidant defenses do not recover, leading to cellular exhaustion.
Surprisingly, there is an elevation of all antioxidant enzymes by the consumption of weekend alcohol for 3 months, probably due to the fact that this consumption is two days a week, causing a certain recovery of the liver. But this elevation of the antioxidant systems is not enough to prevent the damage caused by weekend alcohol to various organs, such as the liver and the oral cavity, which has been previously reported by Morales-González [
8] and González-López [
9]. Excessive alcohol consumption causes liver damage (an elevation of transaminases), stimulates extensive damage to hepatocytes (morphological alterations), and mainly causes liver dysfunction (bilirubin and albumin) [
8,
12,
13,
19,
22].
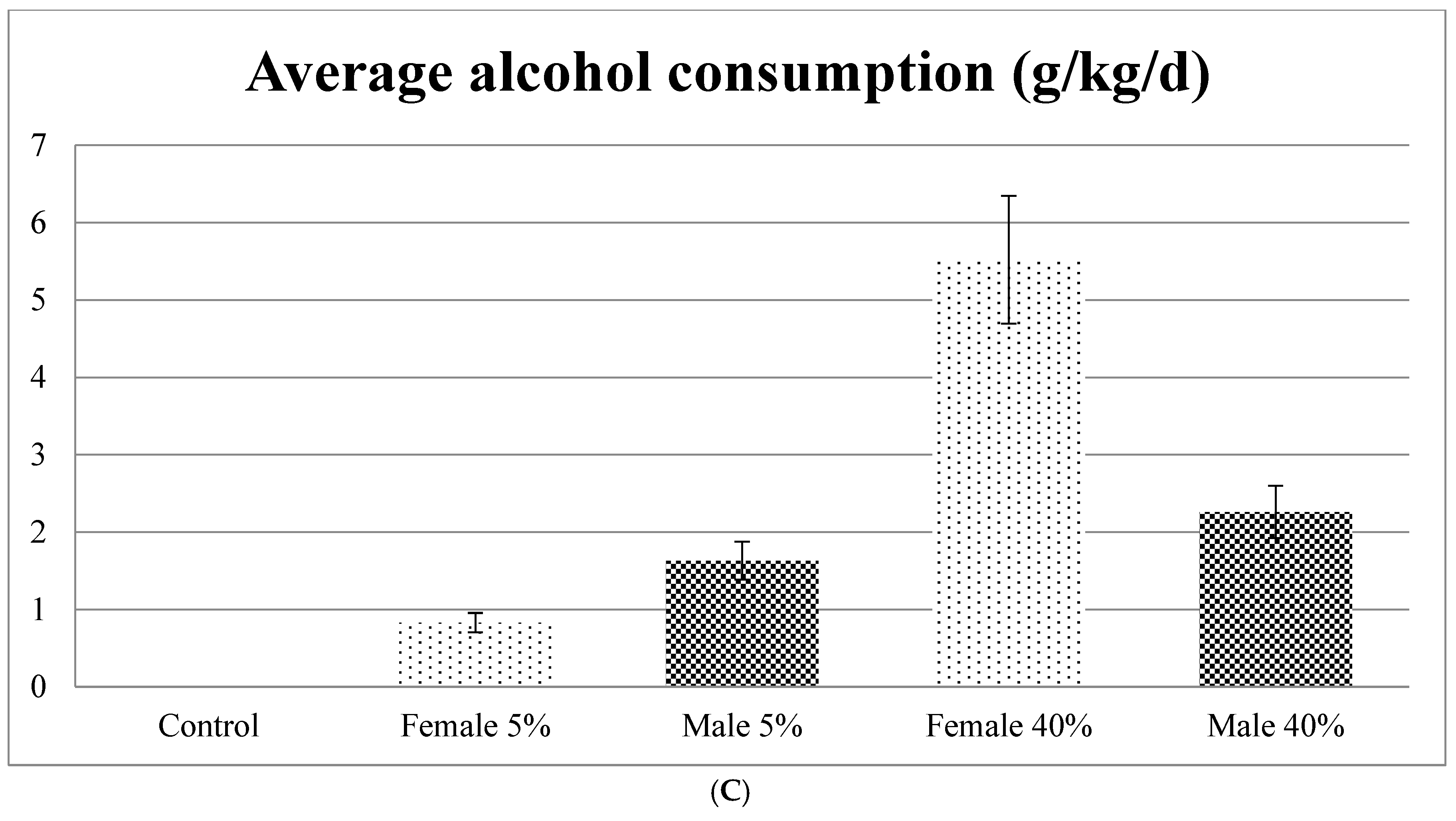
Importantly, it is observed that the group that consumed 40% ethanol showed the highest ethanol consumption (
Figure 2) and also showed the highest elevation of antioxidant defenses (
Table 1,
Table 2,
Table 3 and
Table 5). On the other hand, in the reports of Morales-González [
8] and González-López [
9], this group of females that consumed 40% alcohol is the group with the greatest damage to the liver and the oral cavity with weekend alcohol consumption. This is probably due to the fact that females have a lower metabolism of ethanol [
23,
24,
25], and therefore a delay in ethanol metabolism, a greater production of ROS (
Table 6), and greater liver damage, and therefore the cells try to defend themselves through the formation of antioxidant enzymes.
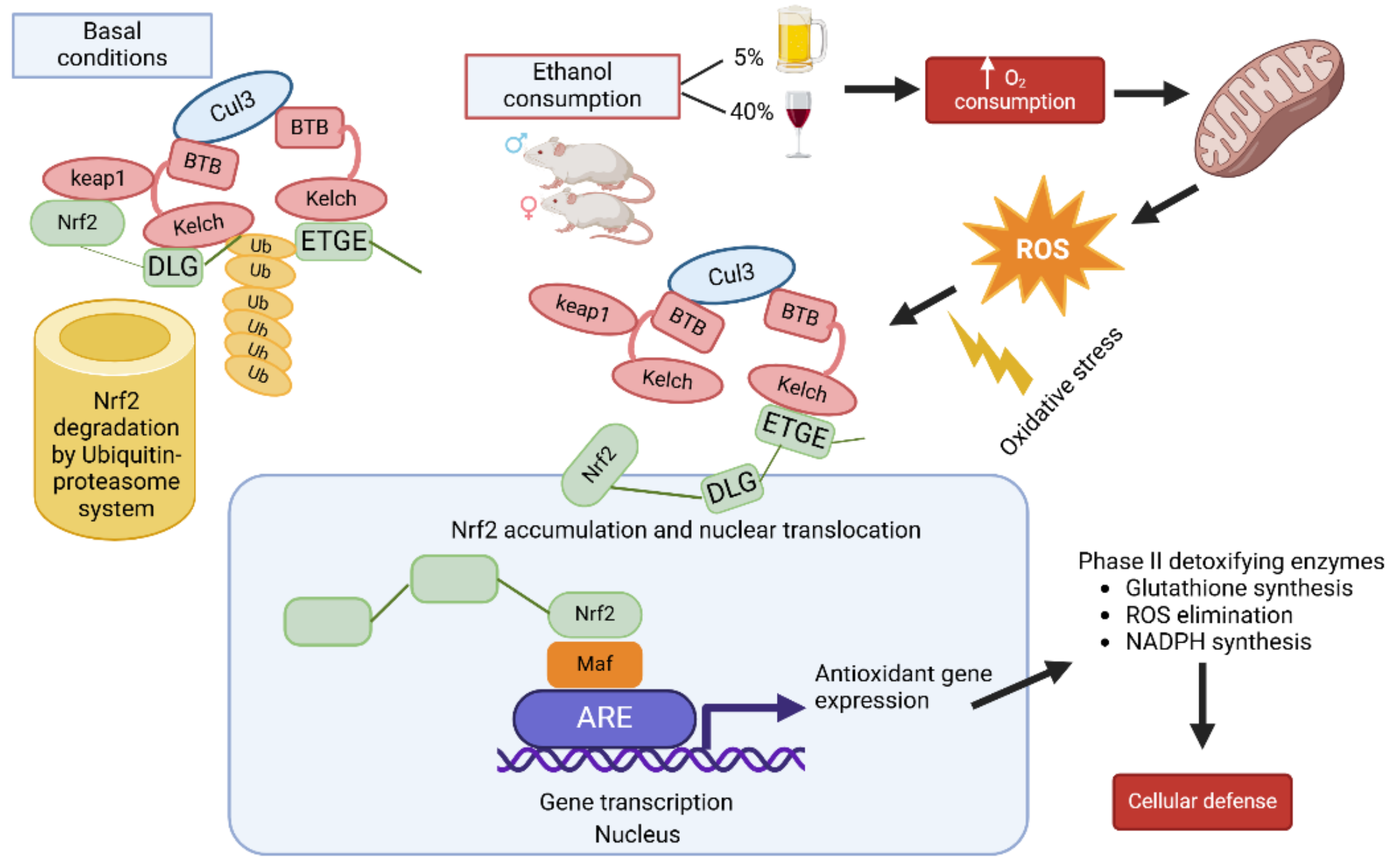
The product of alcohol metabolism in the liver is acetaldehyde, which promotes the formation of reactive oxygen species, which are highly toxic. The continuous consumption of alcohol and therefore its metabolism in the hepatocyte causes alterations in the redox state, as well as an imbalance between the formation of reactive oxygen species/endogenous antioxidant levels, which favors damage to this organ. [
26,
27]. On the other hand, it is known that the transcriptional factor Nrf2 has cytoprotective functions; for example, it controls the expression of numerous genes that encode antioxidant proteins (CAT, SOD, GR, and GPx) and detoxifying enzymes (Nqo1) [
28,
29]. And, it is likely that the elevation of reactive oxygen species causes the activation of Nrf2 caused by alcohol consumption [
19].
Finally, with the data reported in this research, we can hypothesize the participation of NRF2, which can be activated by the formation of free radicals from ethanol metabolism, and its NRF2 response will depend on the damage caused to the liver by the type of alcohol consumption (daily or weekend consumption), as proposed in
Figure 3.