Primary Signet-Ring-Cell Carcinoma in the Colorectum: A Case-Based Literature Review
Abstract
1. Introduction
2. Literature Review on Signet-Ring-Cell Carcinoma (SRCC) and Primary Colorectal Signet-Ring-Cell Carcinoma (PSRCCR)
2.1. Search Strategy
2.2. Epidemiology of PSRCCR
2.3. Histopathology
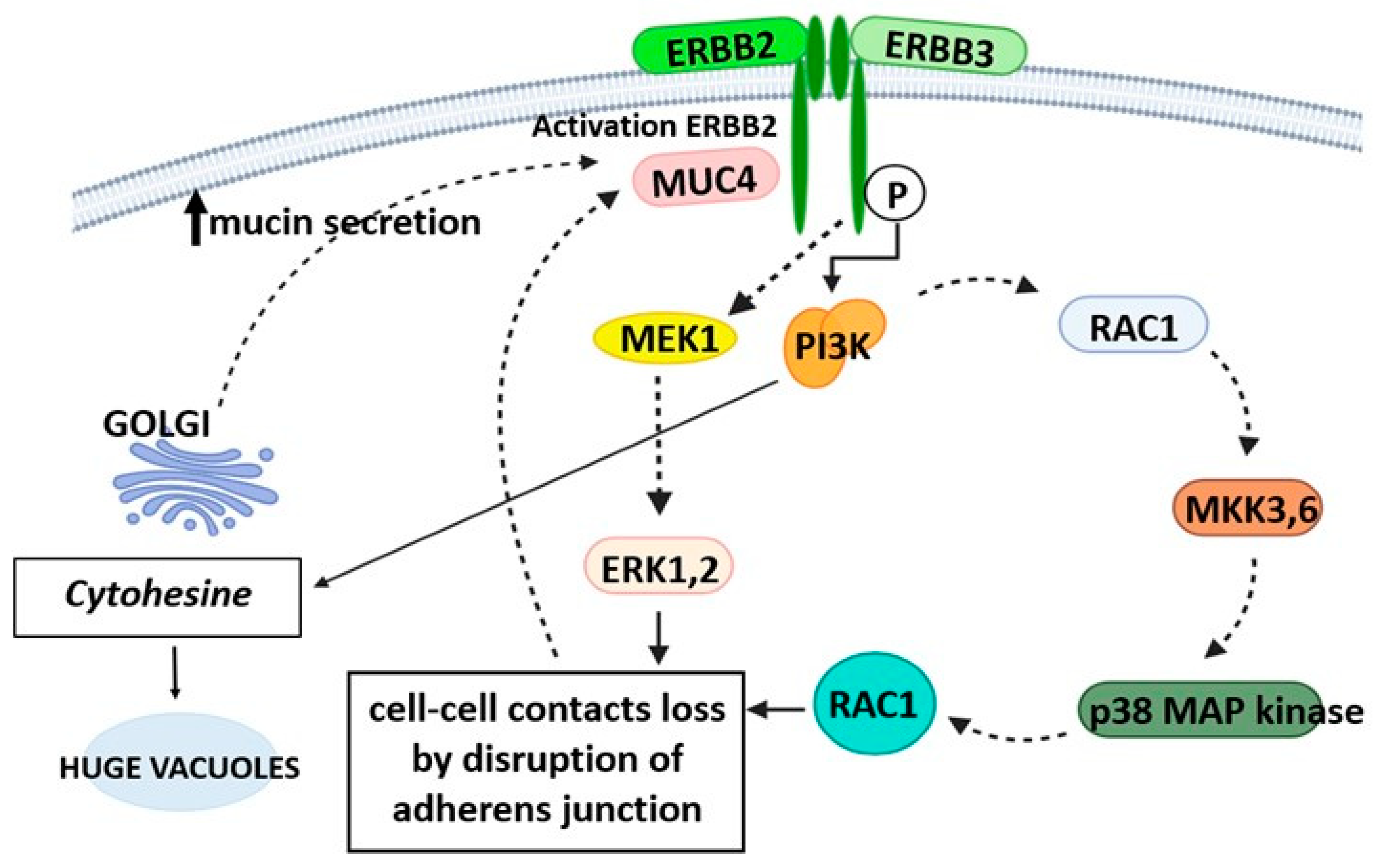
2.4. Genetics
2.5. Diagnosis
2.6. Clinical Course and Prognosis
2.7. Treatment Approaches
2.8. Pharmacological Treatments
3. Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laufman, H.; Saphir, O. Primary linitis plastica type of carcinoma of the colon. AMA Arch. Surg. 1951, 62, 79–91. [Google Scholar] [CrossRef] [PubMed]
- She, K.M.; Wang, H.M.; Chen, J.B.; Chou, D.S.; Mar, H.F.; Chen, C.C.; Chiang, F.F.; Chen, M.C.; Chen, Y.H. Colorectal cancer in younger than 30 years old group is not associated with poor prognosis. J. Soc. Colon Rectal Surg. 2011, 22, 93–98. [Google Scholar]
- Wang, R.; Wang, M.J.; Ping, J. Clinicopathological features and survival outcomes of colorectal cancer in young versus elderly: A population-based cohort study of SEER 9 registries data (1988–2011). Medicine 2015, 94, e1402. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.; Recio-Boiles, A.; Lotfollahzadeh, S.; Cagir, B. Colon Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470380 (accessed on 25 July 2024).
- Messerini, L.; Palomba, A.; Zampi, G. Primary signet-ring cell carcinoma of the colon and rectum. Dis. Colon Rectum 1995, 38, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Morson, B.C. International histological classification of tumours. In Histological Typing of Intestinal Tumours; World Health Organization: Geneva, Switzerland, 1976. [Google Scholar]
- Holtedahl, K.; Borgquist, L.; Donker, G.A.; Buntinx, F.; Weller, D.; Campbell, C.; Månsson, J.; Hammersley, V.; Braaten, T.; Parajuli, R. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: A prospective cohort study of diagnostic accuracy in primary care. BMC Fam. Pract. 2021, 22, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, B.; Zhao, Y.; Bai, J.; Wang, J.; Duan, X.; Luo, X.; Zhang, R.; Pu, Y.; Kou, M.; Lei, J.; et al. Colorectal Cancer: An Overview. In Gastrointestinal Cancers; Morgado-Diaz, J.A., Ed.; Exon Publications: Brisbane, Australia, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK586003/ (accessed on 25 July 2024).
- Richer, V.; Bouffard, D.; Provost, N. Signet-ring cell colon cancer in a 19-year-old patient with giant congenital cellular blue nevus of the scalp. Int. J. Dermatol. 2013, 52, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Kumar, N.; Krishna, S.; Shenoy, R. Primary colonic signet ring cell carcinoma in a young patient. Case Rep. 2014, 2014, bcr2013200587. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Korphaisarn, K.; Saif, A.; Foo, W.C.; Kopetz, S. Early-onset signet-ring cell adenocarcinoma of the colon: A case report and review of the literature. Case Rep. Oncol. Med. 2017, 2017, 2832180. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, J.; Ferdinande, L.; Demetter, P.; Ceelen, W. Mucinous subtype as prognostic factor in colorectal cancer: A systematic review and meta-analysis. J. Clin. Pathol. 2012, 65, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R.; Smith, M. Sialic acid and epithelial differentiation in colorectal polyps and cance—A morphological, mucin and lectin histochemical study. Pathology 1992, 24, 233–242. [Google Scholar] [CrossRef]
- Peruhova, M.; Peshevska-Sekulovska, M.; Krastev, B.; Panayotova, G.; Georgieva, V.; Konakchieva, R.; Nikolaev, G.; Velikova, T.V. What could microRNA expression tell us more about colorectal serrated pathway carcinogenesis? World J. Gastroenterol. 2020, 26, 6556–6571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuytens, F.; Drubay, V.; Eveno, C.; Renaud, F.; Piessen, G. Systematic review of risk factors, prognosis, and management of colorectal signet-ring cell carcinoma. World J. Gastrointest. Oncol. 2024, 16, 2141–2158. [Google Scholar] [CrossRef] [PubMed]
- Mora-Guzmán, I.; Di Martino, M.; Muñoz de Nova, J.L.; Viamontes Ugalde, F.E.; Rodríguez Sánchez, A. Primary signet ring cell carcinoma of the colon: A rare condition with a poor prognosis. A report on two cases. Rev. Gastroenterol. Mex. 2018, 83, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Benedix, F.; Kuester, D.; Meyer, F.; Lippert, H. Einfluss des muzinösen und siegelringzelligen Subtyps auf epidemiologische, histologische und molekularbiologische Eigenschaften sowie auf die Prognose des kolorektalen Karzinoms [Influence of mucinous and signet-ring cell differentiation on epidemiological, histological, molecular biological features, and outcome in patients with colorectal carcinoma]. Zentralbl Chir. 2013, 138, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Stanley, R.H. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System; IARC Press: Lyon, France, 2000. [Google Scholar]
- Pozos-Ochoa, L.I.; Lino-Silva, L.S.; León-Takahashi, A.M.; Salcedo-Hernández, R.A. Prognosis of Signet Ring Cell Carcinoma of the Colon and Rectum and their Distinction of Mucinous Adenocarcinoma with Signet Ring Cells. A Comparative Study. Pathol. Oncol. Res. 2018, 24, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Matkovčík, Z.; Hlad, J. Kolorektální karcinom z prstenčitých buněk—Kazuistika [Signet ring cell colorectalarcinomam—Case report]. Rozhl. Chir. 2015, 94, 333–336. [Google Scholar] [PubMed]
- Makino, T.; Tsujinaka, T.; Mishima, H.; Ikenaga, M.; Sawamura, T.; Nakamori, S.; Fujitani, K.; Hirao, M.; Kashiwazaki, M.; Masuda, N.; et al. Primary signet-ring cell carcinoma of the colon and rectum: Report of eight cases and review of 154 Japanese cases. Hepatogastroenterology 2006, 53, 845–849. [Google Scholar] [PubMed]
- Pande, R.; Sunga, A.; LeVea, C.; Wilding, G.E.; Bshara, W.; Reid, M.; Fakih, M.G. Significance of Signet-Ring Cells in Patients with Colorectal Cancer. Dis. Colon Rectum 2008, 51, 50–55. [Google Scholar] [CrossRef]
- Belli, S.; Aytac, H.O.; Karagulle, E.; Yabanoglu, H.; Kayaselcuk, F.; Yildirim, S. Outcomes of surgical treatment of primary signet ring cell carcinoma of the colon and rectum: 22 cases reviewed with literature. Int. Surg. 2014, 99, 691–698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thota, R.; Fang, X.; Subbiah, S. Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: Retrospective analysis of VACCR database. J. Gastrointest. Oncol. 2014, 5, 18–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, C.O.; Seo, J.W.; Kim, K.M.; Do, I.G.; Kim, S.W.; Park, C.K. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod. Pathol. 2008, 21, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.L.; Tan, K.Y.; Poon, P.L.; Cheng, A. Primary rectal signet ring cell carcinoma with peritoneal dissemination and gastric secondaries. World J. Gastroenterol. 2008, 14, 2118–2120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamilton, S.R.; Bosman, F.T.; Boffetta, P.; Ilyas, M.; Morreau; Nakamura, S.I. Carcinoma of the colon and rectum. In WHO Classification of Tumours of the Digestive System; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC Press: Lyon, France, 2010; pp. 134–146. [Google Scholar]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, C.Y.; Lee, W.H.; Wang, J.Y.; Chiang, H.; Chang, J.L.; Tsai, W.C.; Sheu, L.F.; Jin, J.S. Tissue microarray-determined expression profiles of cyclooxygenase-2 in colorectal adenocarcinoma: Association with clinicopathological parameters. Chin. J. Physiol. 2006, 49, 298–304. [Google Scholar] [PubMed]
- Vang, R.; Gown, A.M.; Barry, T.S.; Wheeler, D.T.; Yemelyanova, A.; Seidman, J.D.; Ronnett, B.M. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: Analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am. J. Surg. Pathol. 2006, 30, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.Y.; Frierson, H.F.; Moskaluk, C.A.; Li, Y.J.; Clegg, L.; Cote, T.R.; McCusker, M.E.; Hankey, B.F.; Edwards, B.K.; Goodman, M.T. CK20 and CK7 protein expression in colorectal cancer: Demonstration of the utility of a population-based tissue microarray. Hum. Pathol. 2005, 36, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Werling, R.W.; Yaziji, H.; Bacchi, C.E.; Gown, A.M. CDX2, a highly sensitive and specific marker of adenocarcinoma of intestinal origin, is an immunohistochemical survey of 476 primary and metastatic carcinomas. Am. J. Surg. Pathol. 2003, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yang, Y.; Shi, J.Y.; Li, Y.K.; Xu, J.X.; Cheng, Y.; Gu, J. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World J. Gastrointest. Surg. 2021, 13, 1567–1583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pernot, S.; Voron, T.; Perkins, G.; Lagorce-Pages, C.; Berger, A.; Taieb, J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 2015, 21, 11428–11438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, S.; Park, S.; Kim, H.; Kang, S.Y.; Ahn, S.; Kim, K.M. Gastric Cancer: Mechanisms, Biomarkers, and Therapeutic Approaches. Biomedicines 2022, 10, 543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopalan, V.; Smith, R.A.; Ho, Y.H.; Lam, A.K.Y. Signet-ring cell carcinoma of colorectum—Current perspectives and molecular biology. Int. J. Color. Dis. 2011, 26, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Kim, H.J.; Kim, J.C. Reduced E-cadherin expression as a cause of distinctive signet-ring cell variant in colorectal carcinoma. J. Korean Med. Sci. 2002, 17, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Oh, B.Y.; Hong, H.K.; Bae, J.S.; Kim, T.W.; Ha, S.Y.; Park, D.; Lee, W.Y.; Kim, H.C.; Yun, S.H.; et al. Molecular characterization of colorectal signet-ring cell carcinoma using whole-exome and RNA sequencing. Transl. Oncol. 2018, 11, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Korphaisarn, K.; Morris, V.; Davis, J.S.; Overman, M.J.; Fogelman, D.R.; Kee, B.K.; Dasari, A.; Raghav, K.P.; Shureiqi, I.; Trupti, M.; et al. Signet ring cell colorectal cancer: Genomic insights into a rare subpopulation of colorectal adenocarcinoma. Br. J. Cancer 2019, 121, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Graham, C.M.; Elci, O.; Griswold, M.E.; Zhang, X.; Khan, M.A.; Pitman, K.; Caudell, J.J.; Hamilton, R.D.; Ganeshan, B.; et al. Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 2013, 269, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Hu, F.; Hu, T.; Sun, Y.; Tong, T.; Gu, Y. Three-Dimensional CT Texture Analysis to Differentiate Colorectal Signet-Ring Cell Carcinoma and Adenocarcinoma. Cancer Manag. Res. 2019, 11, 10445–10453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siqueira, M.A.; Hayashi, T.; Yoshinaga, K.; Saisho, S.; Utsunomiya, J.; Sugihara, K. Colon cancer in a 14-year-old female with turner syndrome: Report of a case. Dis. Colon Rectum 2003, 46, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.T.; Chao, K.H.; Tung, C.C.; Chang, H.C.; Shih, I.L.; Lin, B.R.; Shieh, M.J.; Shun, C.T.; Wong, J.M.; Wei, S.C. Characteristics of primary signet ring cell carcinoma of colon and rectum: A case control study. BMC Gastroenterol. 2022, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Connell, J.B.; Maggard, M.A.; Sack, J.; Ko, C.Y. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis. Colon Rectum 2005, 48, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, U.; Zimmermann, A.; Späth, C.; Müller, T.; Maak, M.; Schuster, T.; Slotta-Huspenina, J.; Käser, S.A.; Michalski, C.W.; Janssen, K.P.; et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 2013, 258, 775–782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Taee, A.; Almukhtar, R.; Lai, J.; Jallad, B. Metastatic signet ring cell carcinoma of unknown primary origin: A case report and review of the literature. Ann. Transl. Med. 2016, 4, 283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tung, S.Y.; Wu, C.S.; Chen, P.C. Primary signet ring cell carcinoma of colorectum: An age- and sex-matched controlled study. Am. J. Gastroenterol. 1996, 91, 2195–2199. [Google Scholar] [PubMed]
- Huang, B.; Ni, M.; Chen, C.; Feng, Y.; Cai, S. Younger age is associated with poorer survival in patients with signet-ring cell carcinoma of the colon without distant metastasis. Gastroenterol. Res. Pract. 2016, 2016, 2913493. [Google Scholar] [CrossRef] [PubMed]
- Hyngstrom, J.R.; Hu, C.Y.; Xing, Y.; You, Y.N.; Feig, B.W.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Cormier, J.N.; Chang, G.J. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: Analysis from the National Cancer Data Base. Ann. Surg. Oncol. 2012, 19, 2814–2821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimaoka, S.; Niihara, T.; Tashiro, K.; Matsuda, A.; Nioh, T.; Ohi, H.; Nishimata, Y.; Nishimata, H.; Fujita, H.; Ohkura, Y.; et al. Signet-ring cell carcinoma of the colon 7mm in size with peritonitis carcinomatosa. J. Gastroenterol. 2002, 37, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Law, J.; Singh, S. Rings Round the Colon: An Early Diagnosis of Signet Ring Cell Carcinoma of the Colon. In Proceedings of UCLA Health, 2020; Volume 24. Available online: https://www.proceedings.med.ucla.edu/wp-content/uploads/2020/08/Singh-A200420SS-2-cc-rko-final-BLM-formatted.pdf (accessed on 25 July 2024).
- Hugen, N.; Verhoeven, R.H.; Lemmens, V.E.; van Aart, C.J.; Elferink, M.A.; Radema, S.A.; Nagtegaal, I.D.; de Wilt, J.H. Colorectal signet-ring cell carcinoma: Benefit from adjuvant chemotherapy but a poor prognostic factor. Int. J. Cancer 2015, 136, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Farraj, F.A.; Sabbagh, H.; Aridi, T.; Fakhruddin, N.; Farhat, F. Signet Ring Cell Carcinoma of the Colon in Young Adults: A Case Report and Literature Review. Case Rep. Oncol. Med. 2019, 2019, 3092674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, B.; Liu, B.; Yuan, Z.; Sun, K.; Chung, H.; Zheng, B.; Cordeiro, C.; Virmani, C.; Shapsis, A. Colorectal Signet Ring Cell Carcinoma Presenting as Ulcerating Rectosigmoid Stricture. ACG Case Rep. J. 2023, 10, e01130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaskin, D.A.; Reid, A.; Shea, M.; Gaskin, P.S. A Rare Case of Signet Ring Cell Colon Cancer Presenting as Adult Colorectal Intussusceptio. Case Rep. Pathol. 2022, 2022, 5271611. [Google Scholar] [CrossRef]
- Seog, W.J.; Dhruv, S.; Atodaria, K.P.; Polavarapu, A. An Extremely Rare Case of Rectal Signet Ring Cell Carcinoma. Gastroenterol. Res. 2022, 15, 106–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marone, J.; Patel, S.; Page, M.; Cheriyath, P. Signet cell carcinoma of the colon in a 17 year old child. J. Surg. Case Rep. 2012, 2012, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anas, D.; Tarik, D.; Yassine, M.M.; Haitam, S.; Ayoub, M.; Magouri, O.; Rachid, J.; Mohammed, B. Primary signet-ring cell carcinoma of the cecum: A rare case report. Int. J. Surg. Case Rep. 2022, 98, 107466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, P.Y.; Goldin, T.; Chang, J.; Markman, M.; Kundranda, M.N. Signet-Ring Cell Carcinoma of the Colon: A Case Report and Review of the Literature. Case Rep. Oncol. 2015, 8, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Pamukçu, O.; Selcukbiricik, F.; Bilici, A.; Sakız, D.; Ozdoğan, O.; Borlu, F. Signet cell carcinoma of colon in a nineteen-year-old patient: A case report. Case Rep. Oncol. Med. 2013, 2013, 695450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Simon, S. Signs and Symptoms of Colorectal Cancer. 2018. Available online: https://www.cancer.org/latest-news/signs-and-symptoms-of-colon-cancer.html (accessed on 25 July 2024).
- Sun, J.; Wang, X.; Gao, P.; Song, Y.; Chen, X.; Sun, Y.; Yu, D.; Lv, X.; Wang, Z. Prognosis and efficiency of adjuvant therapy in resected colon signet-ring cell carcinoma. Transl. Cancer Res. 2018, 7, 1006–1025. [Google Scholar] [CrossRef]



| Authors [Ref.] | Patient Description | Clinical Presentation | Histopathology | Treatment and Follow-Up |
|---|---|---|---|---|
| Farraj et al., 2019 [53] | 19-year-old male, Caucasian, non-smoker | Acute epigastric pain, radiating to the right upper quadrant, recurrent episodes of projectile and non-bloody vomiting, watery non-bloody diarrhea for 2 months. | Poorly differentiated signet-ring-cell adenocarcinoma of the ascending colon with metastasis to the omentum and involvement of the pericolonic fat; stage IV (T4a N0 M1, with peritoneal seeding). No mutations detected in KRAS, NRAS, and BRAF genes. | Right hemicolectomy followed by an ileotransverse anastomosis. 11 cycles bevacizumab and oxaliplatin with capecitabine. On one-year follow-up, a PET scan revealed complete remission of the tumor. |
| Mora-Guzman et al., 2018 [16] | 89-year-old woman with no family history of colon cancer | 3-month anorexia, weight loss, and abdominal pain. | Signet-ring-cell adenocarcinoma—poorly differentiated adenocarcinoma of the colon with >50% signet-ring-cell pattern with multiple tumor nodules and countless lymphatic embolisms, disperse tumor implants, and stage T4aN2bM1b (2010 ICC/AJCC TNM classification, 7th Edition). Immunohistochemistry: microsatellite instability in the PMS-2 and MLH-1 genes. | Right oncologic hemicolectomy. Adjuvant oncologic treatment rejected. |
| Mora-Guzman et al., 2018 [16] | 38-year-old man with no family history of colon cancer, HIV positive | 10-day abdominal pain, nausea, vomiting, absence of bowel transit, and great abdominal distension. | Poorly differentiated adenocarcinoma of the colon with >50% signet-ring cells, multiple metastatic peritoneal implants, lymph node conglomerates, and stage T4aN2bM1b. Immunohistochemistry showed microsatellite instability in the PMS-2 and MLH-1 genes and mutated KRAS gene. | Emergency right hemicolectomy. Palliative chemotherapy was decided upon. |
| Chen et al., 2023 [54] | 41-year-old man | Bright-red blood per rectum caused by ulcerating rectosigmoid stricture. | Metastatic poorly cohesive signet-ring cells. Immunostains revealed neoplastic cells strongly and diffusely positive for CDX2 and cytokeratin 20 while negative for cytokeratin 7, confirming the colorectal origin of the cancer. Immunostains for neuroendocrine markers (synaptophysin and chromogranin)—negative. | After the small bowel obstruction improved with conservative management, the patient was discharged with outpatient oncology follow-up for palliative chemotherapy. |
| Gaskin et al., 2022 [55] | 68-year-old male | Intermittent periumbilical pain for two days, no symptoms of abdominal obstruction. | Mucinous adenocarcinoma: pT3 N0 Mx signet-ring-cell carcinoma; no evidence of lymphovascular or perineural invasion. Immunohistochemistry: proficiency for all MMR proteins. Absence of KRAS mutation in codons 12, 13, 59, 61, 117, and 146. | Anterior resection with anastomosis; patient referred to oncology for continued care. |
| Seog et al., 2022 [56] | 57-year-old male with a past medical history of cerebral palsy, autism, mental disability, and a 25-year history of Crohn’s disease with perirectal abscesses | Chronic rectal pain secondary to numerous perirectal abscesses drained 3 months prior; episodes of hematochezia during the admission. | Cytokeratin (CK) 7 positive, CK20 positive, and SATB2 positive. The tumor was positive for hMLH1, hMSH2, hMSH6, and PMS2. T3N2 disease. | Chemotherapy with folinic acid (leucovorin)–fluorouracil–oxaliplatin (mFOLFOX6) and then considering surgical resection and hospice care. |
| Marone et al., 2012 [57] | 17-year-old male child | One month of progressive abdominal pain, right-sided, aggravated by eating and playing sports. | Signet-ring-cell carcinoma, T4N2M1. | Right colon resection with ileocolic anastomosis followed by 20 cycles of FOLFOX-6. One year later, presented with symptoms and signs of bowel obstruction, underwent diagnostic laparoscopy and debulking—passed away 1 week after the debulking procedure. |
| Anas et al., 2022 [58] | 37-year-old man | At the emergency department with bowel obstruction and altered general condition. | Primary signet-ring-cell carcinoma of the cecum. | Right ileo-colectomy and ileocolostomy. In good health after six months of follow-up. After completing the chemotherapy, the patient was scheduled for a colonoscopy and reversal of colostomy. |
| Park et al., 2015 [59] | 36-year-old female | New-onset progressive right-lower-quadrant pain without any significant past medical or family history. | pT3, pN0, pMx-stage IIA; microsatellite instability testing showed the preservation of MLH1, PMS2, MSH2, and MSH6 proteins by IHC and PCR. | Right hemicolectomy plus adjuvant fluropyrimidine (5-FU)-based therapy with the single agent capecitabine for 6 months, disease free for approximately 18 months. |
| Pamukçu et al., 2013 [60] | 21-year-old boy | Abdominal pain at the emergency center. | Signet-ring-cell carcinoma of the sigmoid colon. | The patient was accepted as inoperable with an advanced stage and underwent a palliative colostomy. The FOLFOX regimen included 5 fluorouracil, calcium folinate, oxaliplatin, and bevacizumab (FOLFOX + bevacizumab), which was started with the diagnosis of metastatic colon cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulinac, M.; Mileva, N.; Miteva, D.; Velikova, T.; Dikov, D. Primary Signet-Ring-Cell Carcinoma in the Colorectum: A Case-Based Literature Review. Gastroenterol. Insights 2024, 15, 632-646. https://doi.org/10.3390/gastroent15030046
Gulinac M, Mileva N, Miteva D, Velikova T, Dikov D. Primary Signet-Ring-Cell Carcinoma in the Colorectum: A Case-Based Literature Review. Gastroenterology Insights. 2024; 15(3):632-646. https://doi.org/10.3390/gastroent15030046
Chicago/Turabian StyleGulinac, Milena, Niya Mileva, Dimitrina Miteva, Tsvetelina Velikova, and Dorian Dikov. 2024. "Primary Signet-Ring-Cell Carcinoma in the Colorectum: A Case-Based Literature Review" Gastroenterology Insights 15, no. 3: 632-646. https://doi.org/10.3390/gastroent15030046
APA StyleGulinac, M., Mileva, N., Miteva, D., Velikova, T., & Dikov, D. (2024). Primary Signet-Ring-Cell Carcinoma in the Colorectum: A Case-Based Literature Review. Gastroenterology Insights, 15(3), 632-646. https://doi.org/10.3390/gastroent15030046








