Abstract
The process of development, recurrence, and exacerbation of the inflammatory process depends on the cytokine levels in IBD. For that reason, many cytokine therapies have been developed for treating IBD patients. Researchers employ various techniques and methodologies for cytokine profiling to identify cytokine signatures in inflamed mucosa. These include enzyme-linked immunosorbent assays (ELISA), multiplex immunoassays, flow cytometry, and gene expression analysis techniques (i.e., microarray, RNA-seq, single-cell RNA-seq (scRNA-seq), mass cytometry (CyTOF), Luminex). Research knowledge so far can give us some insights into the cytokine milieu associated with mucosal inflammation by quantifying cytokine levels in mucosal tissues or biological fluids such as serum or stool. The review is aimed at presenting state-of-the-art techniques for cytokine profiling and the various biomarkers for follow-up and treatment.
1. Introduction
An increasing number of people are affected by inflammatory bowel disease (IBD) worldwide, including Crohn’s disease (CD), ulcerative colitis (UC), and unclassified IBD. Their incidence is increasing worldwide, significantly affecting young people and negatively impacting quality of life [1].
IBD has a diverse clinical presentation that includes episodes of abdominal pain, chronic diarrhea, rectal bleeding, and weight loss. The clinical manifestation of IBD is characterized by a relapsing–remitting course, which is often associated with systemic symptoms [2].
The chronic course of IBD sometimes leads to significant complications such as strictures, fistulas, infections, and cancer, which increase the morbidity and mortality of this entity. As a result, substantial healthcare costs are generated, leading to stress in the healthcare system [3].
The pathogenesis of IBD is associated with genetic susceptibility of the host, dysfunction of the intestinal immune system, disruption of intestinal epithelial barrier integrity, and some environmental factors [4]. In line with this, the intestinal epithelium is essential in maintaining the average balance of intestinal homeostasis. The dysregulation of microbial content and the impaired protein response are implicated in the impaired barrier function of IBD etiology [5].
On the other side, the innate and adaptive immune systems are the key factors that control intestinal inflammation in IBD patients. The process of development, recurrence, and exacerbation of the inflammatory process depends on the cytokine levels in IBD. For that reason, a plethora of cytokine therapies have been developed for the treatment of IBD patients [6].
Cytokines represent small proteins, which are synthesized mainly by immune cells. They implement the communication between cells by activating the antigen-specific effector cells, interfering with local and systemic inflammation. They realized this process by autocrine, paracrine, and endocrine pathways [7].
The innate immune system plays a central role in the pathogenesis of IBD. After being activated, macrophages and dendritic cells (DC) can potentially secrete several cytokines that regulate the inflammation process in IBD. The cytokine triggers many types of T-cells. Thus, the adaptive immune response is activated. It was established that in IBD patients, a dysregulation of T cells, particularly an imbalance of Treg/Th1, Th2, and Th17 cell populations, takes place in the development and exacerbation of the disease [8].
A complex network of different cytokines has been involved in IBD pathogenesis. These cellular interactions between the immune cells are modulated by inflammatory cytokines such as TNF-α, INF-γ, IL-1, IL-6, IL-4, IL-5, IL10, TGF-β. On the other hand, there are anti-proinflammatory cytokines like IL-13, IL-12, IL-18, and IL-23 [9]. The knowledge of the role that every different cytokine plays in inflammation in IBD is essential for establishing the correct treatment algorithm for each clinical setting. It must be underlined that cytokine interplay could be disease-specific and tissue-specific and may also change over time in the same patient. Thus, identifying the predictors of response to specific therapies should be the main point of future therapeutic interventions to determine the best personalized approach [10].
2. Search Strategy
We searched the relevant databases, including PubMed/MEDLINE, Embase, Scopus, and Web of Science, using both MeSH terms and free-text keywords as follows: “Inflammatory Bowel Diseases”, “Cytokines”, “Mucosa”, “Cytokine Signature”, “Biomarkers”. We combined search terms using Boolean operators and included the following: ((“Inflammatory Bowel Diseases” OR “IBD”) AND (“Cytokines” OR “Interleukins” OR “Tumor Necrosis Factor” OR “Interferons”) AND (“Mucosa” OR “Intestinal Mucosa” OR “Gastrointestinal Mucosa”) AND (“Biomarkers” OR “Diagnostic Markers” OR “Prognostic Markers”)). Additionally, some filters were applied as follows: Human studies, English language, and Publication date (e.g., last 10 years). Retrieved relevant articles were screened for titles, abstracts, and full texts for inclusion based on relevance to the topic and study objectives.
3. Role of Mucosal Immunology in IBD Pathogenesis
Cytokines are pivotal in orchestrating immune responses and driving inflammation, particularly in mucosal inflammation observed in conditions such as IBD. These small signaling proteins are secreted by various immune cells and regulate immune cell communication and activation. In inflamed mucosa, cytokines are key mediators that perpetuate inflammation and contribute to tissue damage [11].
Understanding the intricate role of cytokines in mucosal inflammation requires identifying cytokine signatures, which are unique patterns of cytokine expression associated with specific disease states or pathological conditions. Accurate identification of these signatures is crucial for elucidating disease mechanisms, predicting disease progression, and developing targeted therapeutic interventions [11].
Cytokines play a central role in immune responses and inflammation in inflamed mucosa, such as that which is observed in IBD. Identifying cytokine signatures is essential for elucidating disease mechanisms and guiding therapeutic interventions. Employing various techniques and methodologies for cytokine profiling, along with site-specific analysis, enables a comprehensive understanding of mucosal inflammation and holds promise for the development of personalized approaches to disease management.
The type of cytokines present in the intestinal mucosa significantly impacts the effector cell response. This article outlines the key cytokines involved in intestinal inflammation during IBD, including those related to innate immune responses (TNFα, TNF-like cytokine 1A, IL-8), adaptive immune responses (Th1 (IL-1β, IL-18, IFNγ, IL-12), Th2 (IL-4, IL-5, IL-13, IL-11, IL-33), Th17 (IL-17A, IL-17F, IL-21, IL-22, IL-25, IL-27)), and cytokines necessary for Th17 development (IL-6, TGFβ). Recently discovered innate lymphoid cells (ILCs) may produce IFN-γ, TNF, IL-5, IL-13, IL-17, and IL-22 [11].
The unique expression profile of IL-6, TGFβ1, IL-10, and FoxP3 may indicate IBD, as we have previously demonstrated [12]. Furthermore, cytokines such as IL-6, expressed in response to damaging factors (i.e., infections, etc.), can navigate the immunological processes in the mucosa to Th17 differentiation, along with chronic inflammation. Moreover, we demonstrated that IL-6 is the critical cytokine—common for chronic inflammation and carcinogenesis in colorectal cancer [13].
Researchers employ various techniques and methodologies for cytokine profiling to identify cytokine signatures in inflamed mucosa. These include enzyme-linked immunosorbent assays (ELISA), multiplex immunoassays, flow cytometry, and gene expression analysis techniques, such as quantitative real-time polymerase chain reaction (qPCR) and RNA sequencing. Researchers can gain insights into the cytokine milieu associated with mucosal inflammation by quantifying cytokine levels in mucosal tissues or biological fluids, such as serum or stool.
Importantly, site-specific analysis of cytokine expression in mucosal inflammation is paramount for an understanding of the heterogeneity of immune responses within different gastrointestinal tract regions. For instance, the cytokine profile observed in the inflamed mucosa of the colon may differ from that of the small intestine, reflecting distinct immunological microenvironments and disease phenotypes. Therefore, site-specific analysis allows for a more comprehensive characterization of cytokine signatures and facilitates the development of tailored therapeutic strategies for mucosal inflammatory disorders.
The cytokine network in IBD mucosa with the main groups of biomarkers is presented on Figure 1.
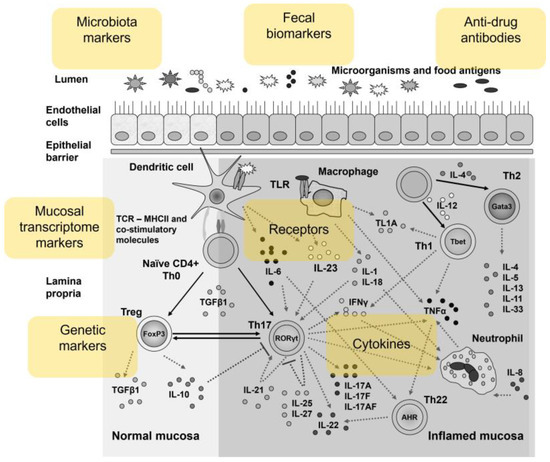
Figure 1.
Overview of the cytokine network in the inflamed mucosa in IBD patients as a source of biomarkers.
4. State-of-the-Art Techniques for Cytokine Profiling
The detection, study, and analysis of cytokines can be a challenge. The techniques used are different and specific, but also very sensitive [14]. The most commonly used samples for research are serum and plasma since they have the closest contact with all tissues and organs. In addition, synovial or cerebrospinal fluids are also used, as they may contain valuable biomarkers that can provide information about a specific, local area of the body. Cell and tissue culture supernatants are also often studied. However, they have quite a large amount of collagen, lipids, and other components that confuse the analysis [14]. Some cytokines are extremely sensitive in isolation and processing; others have multiple and different isoforms. This requires the use of highly specific antibodies that have been rigorously validated.
Profiling of cytokine levels of IBD patients can be quite challenging because a single cytokine can have many functions, and different cytokines can share the same function [15]. Although cytokines mediate many immunological responses in IBD, the specific pathogenic role of each can sometimes be ambiguous in different subtypes of the disease [16]. This requires precise, sensitive, and accurate detection and analysis methods, which will give a much deeper insight into a given disease.
4.1. Microarray Technology
The microarray technique makes it possible to quantify many cytokine profiles in serum or plasma. The aim is to investigate the expression profile of cytokines associated with a disease. The technique is accurate and validated and guarantees the reproducibility of results. Very often, serum is preferred as it gives a consistently lower background signal than plasma. Thus, with microarray assay, a large number of samples can be analyzed, after which some differentially expressed cytokines can be validated in plasma by ELISA, qRT-PCR, or with another technique [17,18]. The sensitivity limits of the analysis must be measured, as well as other indicators important for the accuracy of the results [19].
An earlier study used a high-throughput approach, microarray profiling in IBD patients, to identify predictive transcriptional signatures associated with intestinal inflammation, diagnosis, response to glucocorticoids, or disease prognosis [20]. The authors conclude that the discovery of gene expression profiles can greatly help in personalized therapy in patients with IBD.
A few studies based on microarray technology have also been published to identify effective biomarkers in ulcerative colitis (UC) [21,22,23,24]. In addition, the differences in platforms, protocols, sample sizes, and other factors make gene expression levels incomparable.
In summary, microarray techniques demonstrate accuracy and precision. They enable an extensive range of biomarker detection and simultaneous quantification using a smaller sample volume. The method is more sensitive than ELISA and has much lower running costs.
4.2. RNA Sequencing (RNA-Seq)
RNA sequencing (RNA-seq) precisely measures the qualitative and quantitative changes in expression levels of transcripts, transcriptional isoforms, gene fusions, single nucleotide variants, and other features. The method investigates transcriptomic differences and identifies significantly differentially expressed transcripts in patients with various diseases [25,26].
Numerous studies have been conducted to search for the molecular mechanism underlying the etiology and pathogenesis of IBD and for diagnosis and treatment. Over the years, research has been conducted to study transcriptomic signatures in IBD. Changes in transcribed RNAs in tissues or serum between the healthy and diseased or different IBD subtypes can enrich the available information and be used as diagnostic or prognostic biomarkers [27,28]. Transcriptomic studies are mainly used with sequencing technologies, where extensive information about the functions, expression levels, and biological pathways of different transcripts are analyzed in relation to IBD [29,30,31].
In a previous study, RNA-seq analysis was used to quantify the expression of 115 genes defined in the literature to be relevant to the pathogenesis of IBD in ulcerative colitis (UC) and Crohn’s disease (CD) patients [32]. Of these, 92 were significantly differentially expressed in inflamed mucosa of patients compared to healthy controls. The most upregulated genes shared by both diseases are REG1A, LCN2, NOS2, CXCL1, CXCL2 and S100A9.
A metagenomic study of the interaction between the microbiome and the transcriptome of IBD patients was performed to detect altered metabolic processes, disrupted alternative splicing patterns, or disrupted gene expression [33]. Alternative splicing patterns were increased in IBD patients, and genes for interleukin receptors, interleukins, and other inflammation regulators were dysregulated.
Impaired gene expression in the intestinal mucosa may contribute to the initiation and progression of CD [34]. In this study, genome-wide transcriptomic differences and significantly differentially expressed RNA-seq identified transcripts in normal, inflamed, and non-inflamed CD mucosa.
Both microarray and RNA-seq techniques are used to study the mechanism of molecular pathogenesis in human diseases. Initially, microarrays were predominant, but the use of the RNA-seq technique has gained popularity. Although both approaches are high-throughput and commonly used expression profiles in samples, each method has advantages and disadvantages [27,35,36].
4.3. Single-Cell RNA Sequencing (scRNA-Seq)
Single-cell RNA sequencing (scRNA-seq) is one of the most advanced techniques for detection and quantitative analysis of RNA transcripts in individual cells [37]. The technology is also applicable to reveal the complexity of messenger RNA molecules of different cell types, cellular subsets, and cell states in both healthy tissue and diseased states. scRNA-seq can provide substantial information to help us better understand diseases. Some challenges must be overcome to exploit its great potential in diagnosis and treatment [38,39]. The novelty of this technology compared to mass sequencing is the ability to detect rare subsets of cells that may be the causes of the disease pathogenesis [40].
Different studies have been conducted revealing the application of single-cell techniques in IBD. Kinchen et al. conducted a study using scRNA-seq and found SOX6, CD142, and WNT to be expressed by colonic mesenchymal cells consisting of fibroblast subsets [41]. If their regulation is disrupted, epithelial function is impaired, which leads to inflammation and UC. Similar studies of the epithelial layer of the colon were conducted [42,43,44]. They examined healthy tissue, inflamed UC tissue, and non-inflamed UC tissue and showed that scRNA-seq can be used to detect abnormalities at the tissue level before disease progression.
Studies have also been conducted on the application of scRNA-seq in CD [45,46,47]. They examine different cell subsets, some of which are studied; others have altered gene expression or dysregulation. Mitsialis et al. also used scRNA-seq technology to confirm their mass cytometry results in patients with two diseases (UC and CD) [48]. Within both the CD and UC groups, the authors found an expansion of several cell types that were characteristic of each disease.
All these data reveal the power of single-cell technologies that can help us understand the pathogenic signatures of IBD disease states.
4.4. Mass Cytometry (CyTOF)
Mass cytometry combines flow cytometry and mass spectrometry. It is also called CyTOF [49]. This technique allows for the quantitative analysis of a single cell in real-time and simultaneous measurement of up to 60 different biomarkers in a single cell. Cells are stained with specific antibodies that are conjugated to stable metal isotopes instead of fluorescent dyes. The technique is very sensitive and with high resolution, but it also has some disadvantages, which necessitates the use of other methods as well [50].
In a study by Rubin et al., mass cytometry revealed systemic and local immune signatures that could differentiate IBD [51]. They analyzed leukocyte subsets and examined gut-homing molecule expression in blood and intestinal tissue from IBD and healthy controls. The authors found greater cellular heterogeneity among CD compared with UC. Blood signatures could be used as non-invasive and safe biomarkers for disease diagnosis and monitoring [51].
A similar mass cytometry (CyTOF) study was conducted to compare immune cell populations in mucosa and blood from patients with IBD and healthy controls [48]. Mucosal and blood samples from IBD patients have an increased abundance of different types of immune cells compared to controls, which can be used to develop target therapies in patients with IBD.
4.5. Luminex Technology
The multiplex cytokine assay (Luminex) allows multiple biomarkers to be detected simultaneously. This technology is very useful because it can provide disease-specific biomarker models. A mixture of various antibodies is used, which must be highly specific and sensitive to limit cross-reactivity within the samples. The antibodies are attached to beads (microspheres) colored with red and infrared fluorophores of different intensity and can be mixed in the same assay [52]. Data from a meta-analysis study for IL-10 levels in patients with UC, Crohn’s disease and healthy controls showed that the relationship between the serum interleukin concentration in patients with UC does not differ when using the Luminex assay and ELISA [52]. There was no statistical difference in serum IL-10 levels between UC and CD patients.
Another study based on Luminex technology was conducted with prospectively collected samples from UC patients and control subjects [53]. Data showed that eotaxin-1 and G-CSF were elevated in the serum of UC patients compared to controls, 13 cytokines/chemokines were elevated in active UC compared to tissue controls, but only eotaxin-1 was elevated in both serum and tissue in all UC patients.
A very recent study evaluated the ability of Luminex multiplex technology to identify serum cytokine and/or chemokine markers of Crohn’s disease activity [54]. A total of 16 of 42 serum cytokines and chemokines were elevated compared to healthy controls. This indicates that CD is associated with changes in serum cytokines and chemokines levels, and their profiling may be a non-invasive strategy to assess disease activity. Although mass cytometry is a technique widely accepted by the scientific community, few studies have characterized cytokine production in immune cells using this method so far.
In Table 1, we present a summary of all methods and their advantages and limitations.

Table 1.
Techniques for Cytokine Profiling—benefits and limitations.
5. Clinical Implications and Therapeutic Opportunities
The treatment goal of IBD patients is to provide symptomatic relief, promote endoscopic remission, and avoid disease relapses. Thus, predicting the response to therapy is very important for the patient’s prognosis. Additionally, many IBD patients become intolerant or resistant to treatment over time. Therefore, the ability to predict treatment responses is of great importance for patients because it allows them to optimize future therapeutic options [55].
Furthermore, in past years, novel therapeutic approaches have been developed for IBD patients, including different new modulators of cytokine signaling events (such as JAK/TYK inhibitors), inhibitors of cytokines (IL-12/IL-23, IL-22, IL-36, and IL-6 inhibitors), and anti-adhesion and migration strategies (β7 integrin, sphingosine 1-phosphate receptors, and stem cells). Another interesting approach for IBD patients with promising results is related to microbial-based therapeutics, including fecal microbiota transplantation and bacterial inhibitors [56].
Some of the markers could be defined as predictive biomarkers for different IBD treatments.
5.1. Biomarkers for Response to Biological Treatments
Biologics has become a highly promising treatment option for patients with severe IBD over the past years. One of the disadvantages of this therapy is that around 13–46% of the patients become resistant or non-responders to this treatment [57].
For that reason, the early identification of patients who will lose clinical responses to biological therapies is crucial for their prognoses. Recently, in clinical practice, the implementation of different immune markers, microbiome, anti-drug antibodies, and genetics are used as a monitoring tool determining the outcome of biological therapy [58].
5.2. Immune Markers
Measurable compounds obtained from tissue or biofluid specimens are defined as biomarkers [59]. In patients with IBD, biomarkers are extremely useful as a diagnostic and prognostic tool in predicting disease relapse. They are less invasive and cost-effective than a colonoscopy [60]. The inflammation initiated by the disease agent stimulates the release of three essential cytokines (IL-6, TNF-α, and IL-1β), all of which induce the production of CRP from the hepatocytes. The synthesis of CRP is regulated by IL-6 cytokine, mainly produced by macrophages and T-cells [61]. However, serum CRP concentrations are non-specific in patients with IBD, and abnormal measurements can be detected in various immune-mediated diseases, obesity, trauma, cardiovascular events, infections, and neoplasia [62].
Fecal biomarkers are the proteins that are explicitly found in stool samples of patients with IBD. The fecal markers include fecal calprotectin (FC), calgranulin C, lactoferrin, and lipocalin-2 [63]. Unlike serum markers, fecal markers can be used as a non-invasive indicator strongly correlated with mucosal inflammation. The most widely used fecal biomarker in practice is FC. It represents a protein widely found in neutrophils, eosinophils, and macrophages. Changes in its concentration are observed in the body upon activation of granulocytes and mononuclear phagocytes [64]. Many studies have reported data related to the correlation between elevated levels of FC and the activity of IBD. In the active phase of the inflammation, a huge number of neutrophils are concentrated in the colonic mucosa; as a result, elevated levels of FC are detected [65]. It must be underlined that changes in fecal calprotectin levels are not exclusive to IBD. A lot of other pathological conditions, such as infectious colitis, colorectal cancer, diverticulitis, and those using non-steroidal anti-inflammatories or proton pump inhibitors, could lead to elevations of FC levels [66].
Recently, studies have shown a correlation between higher levels of FC and non-response to Infliximab in severe UC patients. Another critical point is that levels of FC could be a feasible predictor for histological and endoscopic response in patients on biological therapy [67,68].
5.3. Anti-Drug Antibody
An interesting fact is that some biological therapies can evoke an immune response with the production of anti-drug antibodies (ADA), which can lead to the loss of their responses in IBD patients. There is evidence that prolonged therapy with Infliximab could stimulate the synthesis of anti-Infliximab antibodies and cause an increased risk of treatment failure [69].
A study by Bartelds et al. showed that two-thirds of patients treated with adalimumab developed antibodies. Most often, the formation of ADA occurs within 28 weeks of treatment. Thus, an extended follow-up over 3 years established an increasing number of ADA. It has been proven that there is a correlation between high ADA levels and clinical non-response [70].
5.4. Genetic Markers
Some predictive genetic markers related to cytokines or their receptors could be feasibly used in predicting response to biological treatment. These markers include TNF/TNF-receptor genes, ATG16L1 gene, NOD2/CARD15 genes, IL23R, and IL12 genes [71,72].
A good example of the implementation of genetic markers as predictors for treatment response to biological therapy is when TNF-β and TNFRSF1B genes (rs1061624_A-rs3397_T) together with a minor allele (A) polymorphism of TNF gene (rs1800629) could predict a non-responsiveness to anti-TNF (Infliximab) therapy in CD patients [73,74].
5.5. Mucosal Transcriptomics Markers
Biologics therapy has the potential to inflect the expression level of mucosal cytokines and modulate inflammation. Thus, a change in cytokine transcript level can be used as a predictive therapeutic biomarker. There is a plethora of studies that have shown diminished mucosal TNF- α transcript levels in response to anti-TNF therapy, which have correlated positively with disease remission and mucosal healing in IBD patients [75,76].
Other transcriptomics markers such as IL-17A, IL-6, IL-7R, and interferon (IFN)-γ are as good as predictive therapeutic efficacy biomarkers of anti-TNF or anti-α4β7 therapies in IBD patients [77,78].
These markers are also presented in Figure 1.
6. Targeting Cytokines in IBD Therapy: Current Therapies and Their Impact on Cytokines
In the realm of IBD therapy, various treatments have been developed and utilized, each exerting its influence on cytokine activity within the body. These therapies aim to mitigate the inflammation characteristic of IBD by targeting critical cytokines involved in the disease process. Here, we explore the mainstay treatments for IBD and their impact on cytokine modulation.
Corticosteroids such as prednisone and budesonide are frequently used to induce remission in IBD due to their potent anti-inflammatory properties. They work by suppressing the activity of various cytokines, including interleukins (ILs) and tumor necrosis factor-alpha (TNF-α), thus dampening the inflammatory response [79].
Immunomodulatory drugs like azathioprine, mercaptopurine, and methotrexate are employed to maintain remission and reduce the need for corticosteroids in IBD. These agents act by modulating the activity of immune cells and cytokines, particularly TNF-α, IL-6, and interferon-gamma (IFN-γ), attenuating inflammation and preventing disease flares [80].
Aminosalicylates, including mesalamine and sulfasalazine, are commonly used for treating mild to moderate forms of IBD, primarily ulcerative colitis. While the exact mechanism of action remains unclear, these agents are thought to exert their anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, and promoting the release of anti-inflammatory mediators [81].
Biologic agents represent a significant advancement in IBD therapy, targeting specific cytokines involved in the pathogenesis of the disease. TNF-α inhibitors, such as Infliximab, adalimumab, and certolizumab pegol, neutralize TNF-α, a central cytokine in IBD inflammation, thereby reducing inflammation and promoting mucosal healing [82].
Moreover, agents targeting other cytokines, such as IL-12/23 inhibitors (ustekinumab), integrin antagonists (vedolizumab), and IL-23 inhibitors (risankizumab), have been developed to provide alternative treatment options for patients resistant to TNF-α inhibitors or those requiring a different mechanism of action [83].
Overall, current therapies in IBD therapy exert their effects by modulating cytokine activity, either directly or indirectly, to alleviate inflammation and achieve disease remission. Continued research into the intricate cytokine networks involved in IBD pathogenesis is essential for the development of novel therapeutic strategies aimed at improving treatment efficacy and patient outcomes.
Among the emerging cytokine-targeted therapies are the following observations. In recent years, the development of cytokine-targeted therapies has expanded rapidly, offering new avenues for the treatment of IBD. These emerging therapies aim to address the limitations of current treatments and provide alternative options for patients who do not respond adequately to existing regimens. Here, we explore some of the promising cytokine-targeted therapies that are under investigation for IBD:
Interleukin-23 (IL-23) inhibitor development is based on the critical role of IL-23 in the pathogenesis of IBD by promoting the differentiation and activation of Th17 cells, which are implicated in driving intestinal inflammation. Several IL-23 inhibitors, such as risankizumab and brazikumab, have shown promising results in clinical trials for both Crohn’s disease and ulcerative colitis. By specifically targeting IL-23, these biologic agents offer a novel approach to IBD therapy with the potential for improved efficacy and safety profiles [84].
Interleukin-17 (IL-17) inhibitors target IL-17, another critical cytokine involved in the inflammatory cascade in IBD, contributing to tissue damage and perpetuating chronic inflammation. Monoclonal antibodies targeting IL-17A, such as secukinumab and ixekizumab, have demonstrated efficacy in other autoimmune diseases and are currently being evaluated in clinical trials for IBD. These agents hold promise as potential therapeutics for patients with refractory disease or those who do not respond to conventional biologic therapies [85].
Janus Kinase (JAK) inhibitors represent a novel class of small-molecule drug that interferes with cytokine signaling pathways by targeting Janus kinases, enzymes involved in cytokine receptor signaling. Tofacitinib, a JAK inhibitor, has shown efficacy in treating moderate to severe ulcerative colitis and is currently approved for this indication. Other JAK inhibitors, such as upadacitinib and filgotinib, are also being investigated in clinical trials for Crohn’s disease and ulcerative colitis, offering potential oral alternatives to biologic therapies [86].
JAK inhibitors, including tofacitinib, interfere with cytokine-signaling pathways by targeting JAK enzymes which are critical for cytokine receptor activation. Tofacitinib inhibits the signaling of cytokines, such as IL-6, IL-12, IL-23, and IFN-γ, thereby reducing inflammation and improving clinical outcomes in patients with moderate to severe ulcerative colitis [86].
Tumor Necrosis Factor-alpha (TNF-α) receptor fusion proteins, in addition to traditional TNF-α inhibitors, are novel agents targeting the TNF-α pathway and are under development for treating IBD. TNF-α receptor fusion proteins, such as etanercept and certolizumab pegol, offer alternative mechanisms of inhibiting TNF-α activity and have shown promising results in clinical trials. These agents may provide additional options for patients who do not respond to conventional TNF-α inhibitors or experience treatment-related adverse effects [79].
Advances in cytokine biology have led to the identification of specific cytokine targets implicated in IBD pathogenesis. Selective cytokine modulators, such as anti-IL-13 antibodies and GM-CSF inhibitors, are currently being evaluated in preclinical and clinical studies for their potential efficacy in IBD. By targeting cytokines with more precise mechanisms of action, these agents aim to minimize off-target effects and improve treatment outcomes [82].
In conclusion, emerging cytokine-targeted therapies hold promise for the future of IBD treatment, offering novel approaches to modulating the inflammatory response and achieving disease remission. Continued research and clinical trials are needed to further evaluate the safety and efficacy of these therapies and to determine their optimal placement in the IBD treatment armamentarium.
7. Challenges and Future Directions
Current cytokine profiling techniques face several limitations that hinder their effectiveness in accurately characterizing cytokine signatures. Sensitivity and specificity issues are common, as some techniques may lack the sensitivity to detect low-abundance cytokines or the specificity to distinguish between closely related cytokine isoforms. Additionally, standardization challenges across different platforms and laboratories can lead to variability in results, making it difficult to compare findings between studies or establish robust diagnostic criteria [87].
Future research offers exciting opportunities to address these limitations and advance our understanding of cytokine biology in mucosal inflammation. One potential avenue is integrating multi-omics data, which would combine information from genomics, transcriptomics, proteomics, and metabolomics to provide a comprehensive view of cytokine regulation and function. This holistic approach could reveal novel cytokine networks and pathways involved in mucosal inflammation, offering new targets for therapeutic intervention [88].
Furthermore, personalized medicine approaches hold promise for tailoring cytokine-based therapies to individual patients based on their unique cytokine profiles and disease characteristics. Clinicians can optimize treatment strategies and improve outcomes for patients with mucosal inflammatory disorders by identifying specific cytokine signatures associated with different disease phenotypes or treatment responses. Overall, addressing the limitations of current cytokine profiling techniques and exploring innovative research directions offer exciting prospects for advancing our understanding and management of mucosal inflammation.
8. Conclusions
In conclusion, the limitations of current cytokine profiling techniques underscore the need for innovation in cytokine research for IBD. Addressing the challenges of sensitivity, specificity, and standardization is essential to enhance the accuracy and reliability of cytokine analysis. Advancements in cytokine research hold significant promise for improving the management and treatment of IBD by providing insights into disease mechanisms, guiding personalized therapeutic approaches, and ultimately optimizing patient outcomes.
Author Contributions
Conceptualization, M.P. and T.V.; methodology, D.M.; software, T.V.; validation, M.P., S.B. and M.K.; formal analysis, T.V.; investigation, S.B.; resources, D.M.; data curation, M.K.; writing—original draft preparation, M.P., S.B., M.K. and D.M.; writing—review and editing, T.V.; visualization, T.V.; supervision, T.V.; project administration, T.V.; funding acquisition, T.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union–NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study is financed by the European Union–NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0008.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, Regional and National Burden of Inflammatory Bowel Disease in 204 Countries and Territories from 1990 to 2019: A Systematic Analysis Based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Seyed Tabib, N.S.; Madgwick, M.; Sudhakar, P.; Verstockt, B.; Korcsmaros, T.; Vermeire, S. Big data in IBD: Big progress for clinical practice. Gut 2020, 69, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Kaistha, A.; Levine, J. Inflammatory bowel disease: The classic gastrointestinal autoimmune disease. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neuman, M.G. Immune dysfunction in inflammatory bowel disease. Transl. Res. 2007, 149, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Leon, F.; Smythies, L.E.; Smith, P.D.; Kelsall, B.L. Involvement of dendritic cells in the pathogenesis of inflammatory bowel disease. Adv. Exp. Med. Biol. 2006, 579, 117–132. [Google Scholar] [PubMed]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef]
- Felice, C.; Dal Buono, A.; Gabbiadini, R.; Rattazzi, M.; Armuzzi, A. Cytokines in Spondyloarthritis and Inflammatory Bowel Diseases: From Pathogenesis to Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 3957. [Google Scholar] [CrossRef]
- Velikova, T.; Kyurkchiev, D.; Ivanova-Todorova, E.; Spassova, Z.; Stanilova, S.; Altankova, I. Cytokines in Inflamed Mucosa of IBD Patients. In New Insights into Inflammatory Bowel Disease; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Velikova, T.; Karakolev, I.; Spassova, Z.; Kyurkchiev, D.; Altankova, I.; Stanilova, S. Upregulation of mRNA cytokine expression profile in inflamed colonic mucosa of patients with inflammatory bowel disease. Comptes Rendus L’académie Bulg. Sci. Sci. Mathématiques Nat. 2013, 66, 1769–1776. [Google Scholar]
- Velikova, T.V.; Miteva, L.; Stanilov, N.; Spassova, Z.; Stanilova, S.A. Interleukin-6 compared to the other Th17/Treg related cytokines in inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2020, 26, 1912–1925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selvarajah, S.; Negm, O.H.; Hamed, M.R.; Tubby, C.; Todd, I.; Tighe, P.J.; Harrison, T.; Fairclough, L.C. Development and validation of protein microarray technology for simultaneous inflammatory mediator detection in human sera. Mediat. Inflamm. 2014, 2014, 820304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bamias, G.; Nyce, M.R.; De La Rue, S.A.; Cominelli, F. New concepts in the pathophysiology of inflammatory bowel disease. Ann. Intern. Med. 2005, 143, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Műzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiswick, E.L.; Duffy, E.; Japp, B.; Remick, D. Detection and quantification of cytokines and other biomarkers. Methods Mol. Biol. 2012, 844, 15–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kupcova Skalnikova, H.; Cizkova, J.; Cervenka, J.; Vodicka, P. Advances in Proteomic Techniques for Cytokine Analysis: Focus on Melanoma Research. Int. J. Mol. Sci. 2017, 18, 2697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar] [PubMed]
- Montero-Meléndez, T.; Llor, X.; García-Planella, E.; Perretti, M.; Suárez, A. Identification of novel predictor classifiers for inflammatory bowel disease by gene expression profiling. PLoS ONE 2013, 8, e76235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, C.; Hua, J.; Tan, J.; Qian, W.; Zhang, L.; Hou, X. Identification of differentially expressed genes, associated functional terms pathways, and candidate diagnostic biomarkers in inflammatory bowel diseases by bioinformatics analysis. Exp. Ther. Med. 2019, 18, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Lai, L.; Feng, Q.; Shen, J. Identification of common differentially expressed genes and potential therapeutic targets in ulcerative colitis and rheumatoid arthritis. Front. Genet. 2020, 11, 572194. [Google Scholar] [CrossRef]
- Shi, L.; Han, X.; Li, J.X.; Liao, Y.T.; Kou, F.S.; Wang, Z.B.; Shi, R.; Zhao, X.J.; Sun, Z.M.; Hao, Y. Identification of differentially expressed genes in ulcerative colitis and verification in a colitis mouse model by bioinformatics analyses. World J. Gastroenterol. 2020, 26, 5983–5996. [Google Scholar] [CrossRef]
- Cao, F.; Cheng, Y.S.; Yu, L.; Xu, Y.Y.; Wang, Y. Bioinformatics analysis of differentially expressed genes and protein-protein interaction networks associated with functional pathways in ulcerative colitis. Med. Sci. Monit. 2021, 27, e927917. [Google Scholar] [CrossRef] [PubMed]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef]
- Ketkar, S.; Burrage, L.C.; Lee, B. RNA Sequencing as a Diagnostic Tool. JAMA 2023, 329, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.N.; Low, E.N.D.; Raja Ali, R.A.; Mokhtar, N.M. Delineating inflammatory bowel disease through transcriptomic studies: Current review of progress and evidence. Intest. Res. 2018, 16, 374–383. [Google Scholar] [CrossRef]
- Mirza, A.H.; Berthelsen, C.H.; Seemann, S.E.; Pan, X.; Frederiksen, K.S.; Vilien, M.; Gorodkin, J.; Pociot, F. Transcriptomic landscape of lncRNAs in inflammatory bowel disease. Genome Med. 2015, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Grisham, M.B.; Kevil, C.G. Application of comparative transcriptional genomics to identify molecular targets for pediatric IBD. Front. Immunol. 2015, 6, 165. [Google Scholar] [CrossRef]
- Planell, N.; Lozano, J.J.; Mora-Buch, R.; Masamunt, M.C.; Jimeno, M.; Ordás, I.; Esteller, M.; Ricart, E.; Piqué, J.M.; Panés, J.; et al. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut 2013, 62, 967–976. [Google Scholar] [CrossRef]
- Hong, S.N.; Joung, J.G.; Bae, J.S.; Lee, J.S.; Koo, J.S.; Park, S.J.; Im, J.P.; Kim, Y.S.; Kim, J.W.; Park, W.Y.; et al. RNA-seq reveals transcriptomic differences in Crohn’s disease patients’ inflamed and noninflamed intestinal mucosa compared with healthy controls’ normal mucosa. Inflamm. Bowel Dis. 2017, 23, 1098–1108. [Google Scholar] [CrossRef]
- Holgersen, K.; Kutlu, B.; Fox, B.; Serikawa, K.; Lord, J.; Hansen, A.K.; Holm, T.L. High-resolution gene expression profiling using RNA sequencing in patients with inflammatory bowel disease and in mouse models of colitis. J. Crohn’s Colitis 2015, 9, 492–506. [Google Scholar] [CrossRef]
- Häsler, R.; Sheibani-Tezerji, R.; Sinha, A.; Barann, M.; Rehman, A.; Esser, D.; Aden, K.; Knecht, C.; Brandt, B.; Nikolaus, S.; et al. Uncoupling of mucosal gene regulation, mRNA splicing and adherent microbiota signatures in inflammatory bowel disease. Gut 2017, 66, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, X.; Zhang, S.; Qi, C.; Zhang, Z.; Ma, R.; Xiang, L.; Chen, L.; Zhu, Y.; Tang, C.; et al. Oxidative stress gene expression, DNA methylation, and gut microbiota interaction trigger Crohn’s disease: A multi-omics Mendelian randomization study. BMC Med. 2023, 21, 179. [Google Scholar] [CrossRef]
- Shoemaker, D.D.; Schadt, E.E.; Armour, C.D.; He, Y.D.; Garrett-Engele, P.; McDonagh, P.D.; Loerch, P.M.; Leonardson, A.; Lum, P.Y.; Cavet, G.; et al. Experimental annotation of the human genome using microarray technology. Nature 2001, 409, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef]
- Haque, A.; Engel, J.; Teichmann, S.A.; Lönnberg, T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Yu, X.; Abbas-Aghababazadeh, F.; Chen, Y.A.; Fridley, B.L. Statistical and Bioinformatics Analysis of Data from Bulk and Single-Cell RNA Sequencing Experiments. Methods Mol. Biol. 2021, 2194, 143–175. [Google Scholar]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386.e17. [Google Scholar] [CrossRef]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell 2019, 178, 714–730.e22. [Google Scholar] [CrossRef]
- Serigado, J.M.; Foulke-Abel, J.; Hines, W.C.; Hanson, J.A.; In, J.; Kovbasnjuk, O. Ulcerative Colitis: Novel Epithelial Insights Provided by Single Cell RNA Sequencing. Front. Med. 2022, 9, 868508. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Chang, C.; Boschetti, G.; Ungaro, R.; Giri, M.; Grout, J.A.; Gettler, K.; Chuang, L.S.; Nayar, S.; Greenstein, A.J.; et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 2019, 178, 1493–1508.e20. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, N.; Gamini, R.; Cella, M.; Schettini, J.L.; Bugatti, M.; Zhao, S.; Rosadini, C.V.; Esaulova, E.; Di Luccia, B.; Kinnett, B.; et al. Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat. Commun. 2021, 12, 1921. [Google Scholar] [CrossRef] [PubMed]
- Rosati, E.; Rios Martini, G.; Pogorelyy, M.V.; Minervina, A.A.; Degenhardt, F.; Wendorff, M.; Sari, S.; Mayr, G.; Fazio, A.; Dowds, C.M.; et al. A novel unconventional T cell population enriched in Crohn’s disease. Gut 2022, 71, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Mitsialis, V.; Wall, S.; Liu, P.; Ordovas-Montanes, J.; Parmet, T.; Vukovic, M.; Spencer, D.; Field, M.; McCourt, C.; Toothaker, J.; et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology 2020, 159, 591–608.e10. [Google Scholar] [CrossRef]
- Bandura, D.R.; Baranov, V.I.; Ornatsky, O.I.; Antonov, A.; Kinach, R.; Lou, X.; Pavlov, S.; Vorobiev, S.; Dick, J.E.; Tanner, S.D. Mass cytometry: Technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 2009, 81, 6813–6822. [Google Scholar] [CrossRef]
- Cosma, A.; Nolan, G.; Gaudilliere, B. Mass cytometry: The time to settle down. Cytom. Part J. Int. Soc. Anal. Cytol. 2017, 91, 12–13. [Google Scholar] [CrossRef]
- Rubin, S.J.S.; Bai, L.; Haileselassie, Y.; Garay, G.; Yun, C.; Becker, L.; Streett, S.E.; Sinha, S.R.; Habtezion, A. Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nat. Commun. 2019, 10, 2686. [Google Scholar] [CrossRef]
- Meng, D.; Liang, L.; Guo, X. Serum interleukin-10 level in patients with inflammatory bowel disease: A meta-analysis. Eur. J. Inflamm. 2019, 17, 2058739219843405. [Google Scholar] [CrossRef]
- Coburn, L.A.; Horst, S.N.; Chaturvedi, R.; Brown, C.T.; Allaman, M.M.; Scull, B.P.; Singh, K.; Piazuelo, M.B.; Chitnavis, M.V.; Hodges, M.E.; et al. High-throughput multi-analyte Luminex profiling implicates eotaxin-1 in ulcerative colitis. PLoS ONE 2013, 8, e82300. [Google Scholar] [CrossRef]
- Raffa, G.; Tyree, R.; Carson, K.; Allaman, M.; Beaulieu, D.; Dalal, R.; Pabla, B.; Scoville, E.; Horst, S.; Schwartz, D.; et al. Non-invasive determination of disease activity in crohn’s disease by serum luminex profiling. Inflamm. Bowel Dis. 2024, 30, S12. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Elhag, D.A.; Kumar, M.; Saadaoui, M.; Akobeng, A.K.; Al-Mudahka, F.; Elawad, M.; Al Khodor, S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int. J. Mol. Sci. 2022, 23, 6966. [Google Scholar] [CrossRef]
- Kopylov, U.; Seidman, E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2016, 9, 513–526. [Google Scholar] [CrossRef]
- Billiet, T.; Cleynen, I.; Ballet, V.; Claes, K.; Princen, F.; Singh, S.; Ferrante, M.; Van Assche, G.; Gils, A.; Vermeire, S. Evolution of cytokines and inflammatory biomarkers during infliximab induction therapy and the impact of inflammatory burden on primary response in patients with Crohn’s disease. Scand. J. Gastroenterol. 2017, 52, 1086–1092. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Alghoul, Z.; Yang, C.; Merlin, D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines 2022, 10, 1492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. C-Reactive Protein as a Marker for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 661–665. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Lopez, R.N.; Leach, S.T.; Lemberg, D.A.; Duvoisin, G.; Gearry, R.B.; Day, A.S. Fecal biomarkers in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 577–582. [Google Scholar] [CrossRef]
- Johne, B.; Fagerhol, M.K.; Lyberg, T.; Prydz, H.; Brandtzaeg, P.; Naess-Andresen, C.F.; Dale, I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol. Pathol. 1997, 50, 113–123. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426. [Google Scholar] [CrossRef]
- Poullis, A.; Foster, R.; Mendall, M.A.; Fagerhol, M.K. Emerging role of calprotectin in gastroenterology. J. Gastroenterol. Hepatol. 2003, 18, 756–762. [Google Scholar] [CrossRef]
- Frin, A.C.; Filippi, J.; Boschetti, G.; Flourie, B.; Drai, J.; Ferrari, P.; Hebuterne, X.; Nancey, S. Accuracies of fecal calprotectin, lactoferrin, M2-pyruvate kinase, neopterin and zonulin to predict the response to Infliximab in ulcerative colitis. Dig. Liver Dis. 2017, 49, 11–16. [Google Scholar] [CrossRef]
- Ho, G.T.; Lee, H.M.; Brydon, G.; Ting, T.; Hare, N.; Drummond, H.; Shand, A.G.; Bartolo, D.C.; Wilson, R.G.; Dunlop, M.G.; et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 673–678. [Google Scholar]
- Bortlik, M.; Duricova, D.; Malickova, K.; Machkova, N.; Bouzkova, E.; Hrdlicka, L.; Komarek, A.; Lukas, M. Infliximab trough levels may predict sustained response to Infliximab in patients with Crohn’s disease. J. Crohn’s Colitis 2013, 7, 736–743. [Google Scholar] [CrossRef]
- Bartelds, G.M.; Krieckaert, C.L.; Nurmohamed, M.T. Development of anti-drug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011, 305, 1460–1468. [Google Scholar] [CrossRef]
- Koder, S.; Repnik, K.; Ferkolj, I.; Pernat, C.; Skok, P.; Weersma, R.K.; Potocnik, U. Genetic polymorphism in ATG16L1 gene influences the response to adalimumab in Crohn’s disease patients. Pharmacogenomics 2015, 16, 191–204. [Google Scholar] [CrossRef]
- Moroi, R.; Endo, K.; Kinouchi, Y.; Shiga, H.; Kakuta, Y.; Kuroha, M.; Kanazawa, Y.; Shimodaira, Y.; Horiuchi, T.; Takahashi, S.; et al. FCGR3A-158 polymorphism influences the biological response to Infliximab in Crohn’s disease through affecting the ADCC activity. Immunogenetics 2013, 65, 265–271. [Google Scholar] [CrossRef]
- Netz, U.; Carter, J.V.; Eichenberger, M.R.; Dryden, G.W.; Pan, J.; Rai, S.N.; Galandiuk, S. Genetic polymorphisms predict response to anti-tumor necrosis factor treatment in Crohn’s disease. World J. Gastroenterol. 2017, 23, 4958–4967. [Google Scholar] [CrossRef]
- Taylor, K.D.; Plevy, S.E.; Yang, H.; Landers, C.J.; Barry, M.J.; Rotter, J.I.; Targan, S.R. ANCA pattern and LTA haplotype relationship to clinical responses to anti-TNF antibody treatment in Crohn’s disease. Gastroenterology 2001, 120, 1347–1355. [Google Scholar] [CrossRef]
- Florholmen, J.R.; Johnsen, K.-M.; Meyer, R.; Olsen, T.; Moe, Ø.K.; Tandberg, P.; Gundersen, M.D.; Kvamme, J.-M.; Johnsen, K.; Løitegård, T.; et al. Discovery and validation of mucosal TNF expression combined with histological score—A biomarker for personalized treatment in ulcerative colitis. BMC Gastroenterol. 2020, 20, 321. [Google Scholar] [CrossRef]
- Cui, G.; Florholmen, J.; Goll, R. Could Mucosal TNF Transcript as a Biomarker Candidate Help Optimize Anti-TNF Biological Therapy in Patients with Ulcerative Colitis? Front. Immunol. 2022, 13, 881112. [Google Scholar] [CrossRef]
- Cui, G.; Fan, Q.; Li, Z.; Goll, R.; Florholmen, J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBioMedicine 2021, 66, 103329. [Google Scholar] [CrossRef]
- Perez-Sanchez, C.; Barbera Betancourt, A.; Lyons, P.A.; Zhang, Z.; Suo, C.; Lee, J.C.; McKinney, E.F.; Modis, L.K.; Ellson, C.; Smith, K.G.C. miR-374a-5p regulates inflammatory genes and monocyte function in patients with inflammatory bowel disease. J. Exp. Med. 2022, 219, e20211366. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Ford, A.C.; Achkar, J.P.; Khan, K.J.; Achkar, J.P.; Talley, N.J.; Marshall, J.K.; Moayyedi, P. Efficacy of 5-aminosalicylates in Crohn’s disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 617–629. [Google Scholar] [CrossRef]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic agents for IBD: Practical insights. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 537–545. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V.; Danese, S.; Colombel, J.F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sandborn, W.J.; D’Haens, G.; Panés, J.; Kaser, A.; Ferrante, M.; Louis, E.; Franchimont, D.; Dewit, O.; Seidler, U.; et al. Induction Therapy with the Selective Interleukin-23 Inhibitor Risankizumab in Patients with Moderate-to-Severe Crohn’s Disease: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study. Lancet 2017, 389, 1699–1709. [Google Scholar] [CrossRef]
- Hueber, W.; Sands, B.E.; Lewitzky, S.; Vandemeulebroecke, M.; Reinisch, W.; Higgins, P.D.; Wehkamp, J.; Feagan, B.G.; Yao, M.D.; Karczewski, M.; et al. Secukinumab, a Human Anti-IL-17A Monoclonal Antibody, for Moderate to Severe Crohn’s Disease: Unexpected Results of a Randomised, Double-Blind Placebo-Controlled Trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Vebr, M.; Pomahačová, R.; Sýkora, J.; Schwarz, J. A Narrative Review of Cytokine Networks: Pathophysiological and Therapeutic Implications for Inflammatory Bowel Disease Pathogenesis. Biomedicines 2023, 11, 3229. [Google Scholar] [CrossRef]
- Padoan, A.; Musso, G.; Contran, N.; Basso, D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 5534–5557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).