Enhancing the Quality of Upper Gastrointestinal Endoscopy: Current Indicators and Future Trends
Abstract
1. Introduction
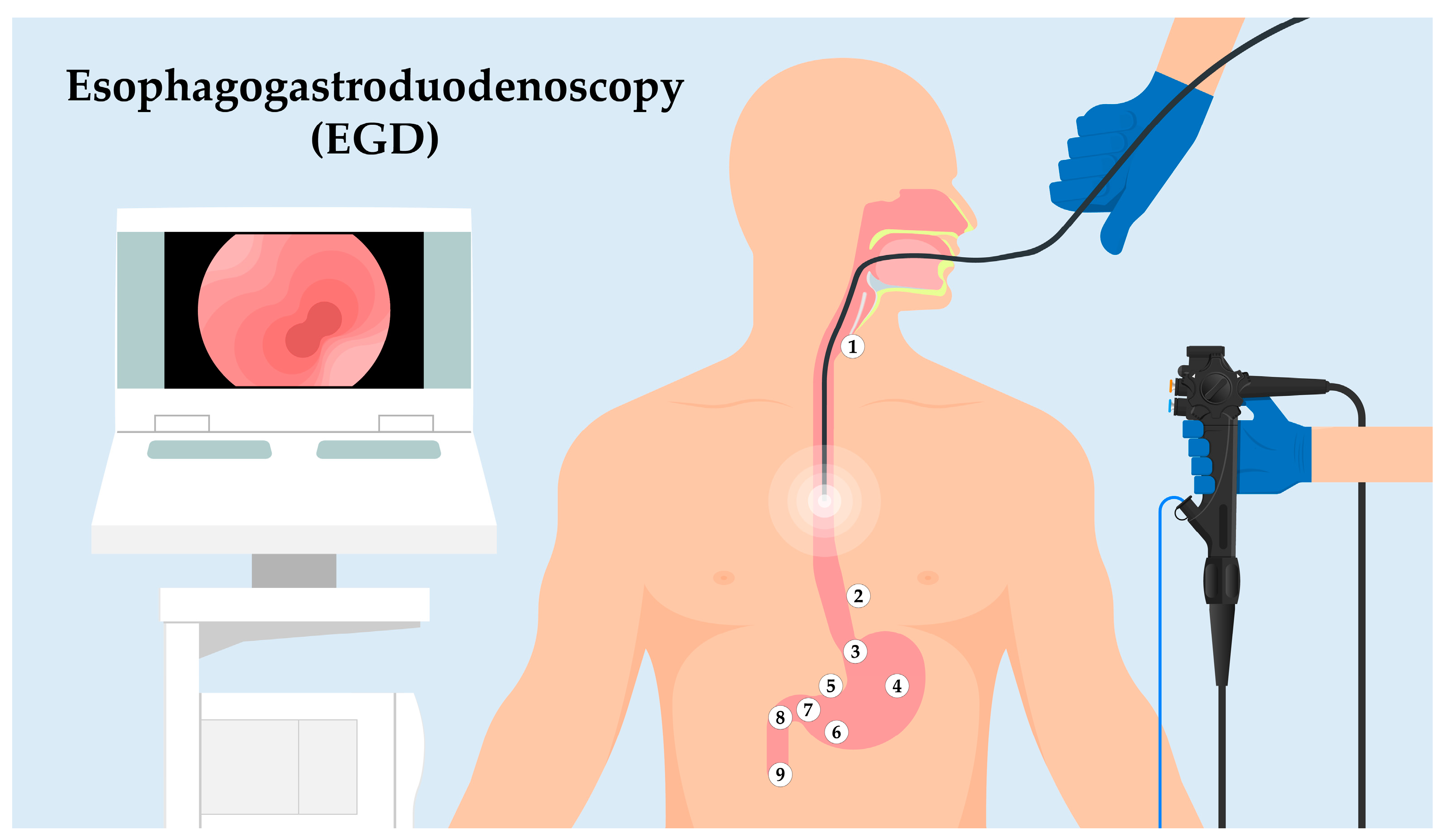
2. EGD Procedure: A Landmark-Driven Examination
- ⮚
- Upper Esophageal Sphincter (UES): As the endoscope enters the esophagus, the first landmark encountered is the UES. This muscular ring divides the pharynx from the esophagus and acts as a valve, ensuring a unidirectional flow of ingested contents.
- ⮚
- Z-line: Moving distally, the endoscope will visualize the “Z-line” or “squamocolumnar junction.” This zone demarcates the junction between the esophagus’s squamous epithelium and the stomach’s columnar epithelium. The appearance and location of the Z-line can offer insights into conditions like Barrett’s esophagus.
- ⮚
- Cardia: As the endoscope progresses into the stomach, the cardia is encountered, a small area surrounding the esophagogastric junction.
- ⮚
- Body of the Stomach: The main, central region of the stomach is examined next, noting the appearance of the gastric folds and assessing for abnormalities like ulcers or masses.
- ⮚
- Angularis Incisure: A notable bend in the stomach’s structure, this landmark can be a reference for the division between the body of the stomach and the antrum.
- ⮚
- Antrum: This portion of the stomach is closer to the pyloric canal and is essential to assess as it is a common site for peptic ulcers.
- ⮚
- Pyloric Canal and Pyloric Ring: The distal stomach section leading into the duodenum. It acts as a valve to regulate the release of gastric contents into the duodenum.
- ⮚
- Duodenal Bulb: The first part of the duodenum, immediately after the pylorus. It is a common site to inspect for ulcers, especially in patients with Helicobacter pylori infection.
- ⮚
- Descending (Second) Part of the Duodenum: The endoscope can typically be advanced a short distance beyond the bulb to visualize this segment. The presence of the ampulla of Vater, the joint opening for the bile and pancreatic ducts, can be identified in this region.
3. Best Practice Guidelines for High-Quality EGD: Guidelines from Leading Medical Societies
4. QIs for Upper Gastrointestinal Endoscopy
4.1. Fundamental Elements of High-Quality EGD: Patient Selection, Patient Preparation, Procedure, Follow-Up
- Patient Selection: A judicious evaluation of the patient’s symptoms, medical history, physical examination, and, where appropriate, non-invasive tests are critical in deciding when EGD is indicated.
- Patient Preparation: Effective communication with the patient about the purpose, process, and potential risks of the procedure, in addition to providing clear instructions for pre-procedure fasting and medication management, is essential to minimize the risk and maximize the diagnostic or therapeutic yield.
- Procedure: The endoscopist should adhere to established procedural guidelines, which include a systematic examination of the upper gastrointestinal tract, adequate documentation of findings, taking biopsies when indicated, and performing therapeutic interventions safely and effectively.
- Follow-Up: Post-procedure care includes monitoring for complications, communicating findings to the patient and their primary care provider, arranging for pathological evaluation of biopsies, and scheduling appropriate follow-up based on the results of the EGD.
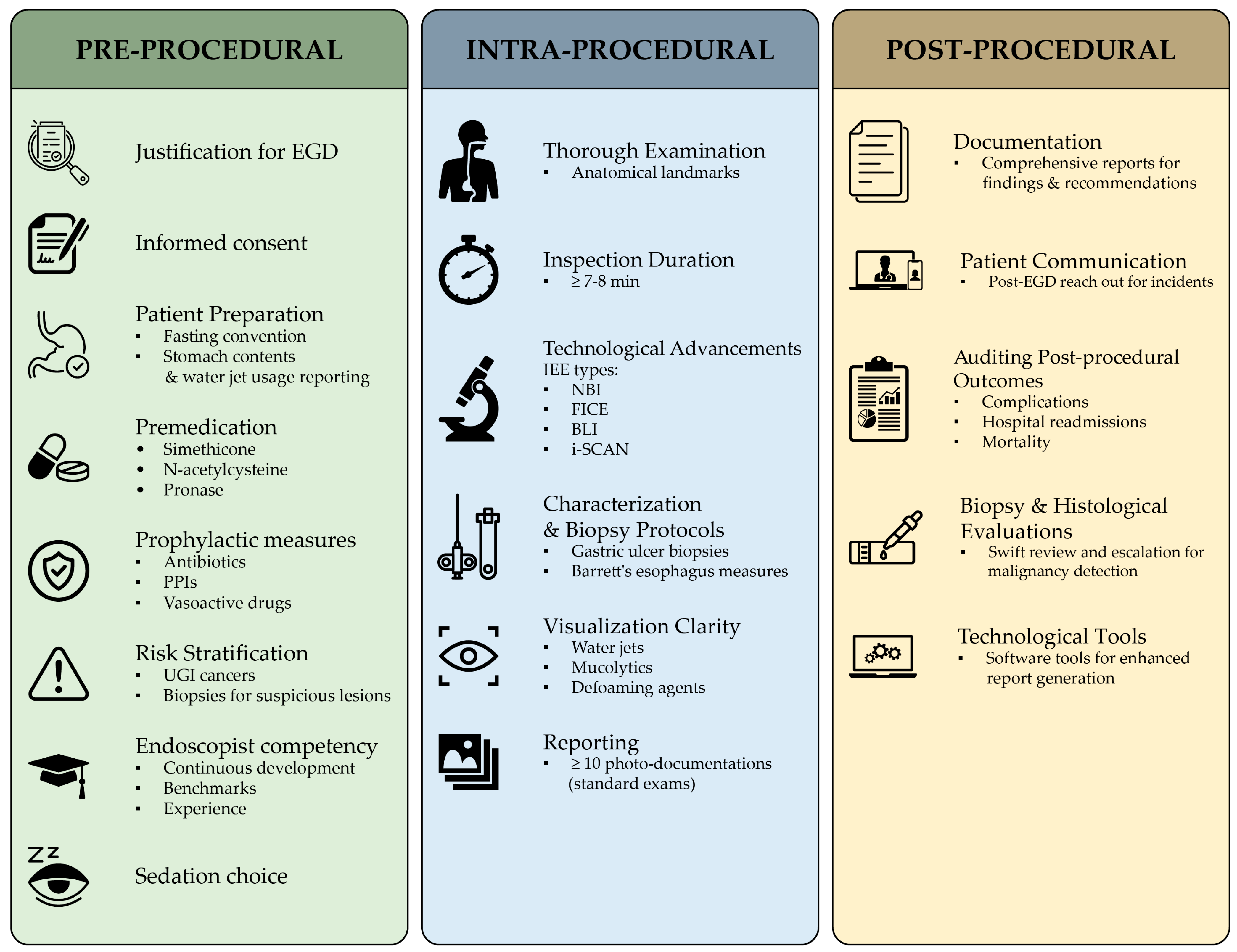
4.2. Pre-Procedural QIs
4.3. Intra-Procedural QIs
4.4. Post-Procedural QIs
4.5. Role of Advanced Endoscopic Techniques and Equipment
5. Challenges in Ensuring High-Quality EGD
5.1. Variability in Practitioner Training and Technique
5.2. Patient Compliance with Pre- and Post-Procedural Instructions
5.3. Limitations of Current QIs
5.4. Barriers to the Adoption of Advanced Techniques and Equipment
6. Future Directions for High-Quality EGD
6.1. Technological Innovations and Developments
6.2. Training and Credentialing of Endoscopists
6.3. Evolving QIs: From Procedure-Based to Patient-Centered Metrics
6.4. Role of AI in EGD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bisschops, R.; Areia, M.; Coron, E.; Dobru, D.; Kaskas, B.; Kuvaev, R.; Pech, O.; Ragunath, K.; Weusten, B.; Familiari, P.; et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2016, 48, 843–864. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Manabe, N.; Ayaki, M.; Nakamura, J.; Murao, T.; Fujita, M.; Kuinose, M.; Yamatsuji, T.; Naomoto, Y.; Haruma, K. Clinical significance of esophagogastroduodenoscopy in patients with esophageal motility disorders. Dig. Endosc. 2021, 33, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Uedo, N.; Gotoda, T.; Yoshinaga, S.; Tanuma, T.; Morita, Y.; Doyama, H.; Aso, A.; Hirasawa, T.; Yano, T.; Uchita, N.; et al. Differences in routine esophagogastroduodenoscopy between Japanese and international facilities: A questionnaire survey. Dig. Endosc. 2016, 28, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yalamarthi, S.; Witherspoon, P.; McCole, D.; Auld, C.D. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy 2004, 36, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Inoue, M.; Lin, J.-T.; Khor, C.; Yang, H.-K.; Ohtsu, A. Gastric Cancer Working Group Report. Ultrasound Med. Biol. 2010, 40 (Suppl. S1), i28–i37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.Y.; Park, J.M. Quality indicators in esophagogastroduodenoscopy. Clin. Endosc. 2022, 55, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C.; Ogoshi, K.; Narisawa, R.; Kishi, T.; Kato, T.; Fujita, K.; Sano, M.; Tsukioka, S. Impact of endoscopic screening on mortality reduction from gastric cancer Observational Study. World J. Gastroenterol. 2015, 21, 2460–2466. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.J.; Hyun, J.H.; Han, K.S.; Kim, B.C.; Hong, C.W.; Lee, S.-J.; Sohn, D.K. Correlation Between Bowel Preparation and the Adenoma Detection Rate in Screening Colonoscopy. Ann. Coloproctology 2017, 33, 93–98. [Google Scholar] [CrossRef]
- Brunner, K.T.; Calderwood, A.H. Quality in Colonoscopy. Curr. Gastroenterol. Rep. 2015, 17, 38. [Google Scholar] [CrossRef]
- Jain, R.; Chetty, R. Gastric Hyperplastic Polyps: A Review. Dig. Dis. Sci. 2009, 54, 1839–1846. [Google Scholar] [CrossRef]
- Kavic, S.M.; Basson, M.D. Complications of endoscopy. Am. J. Surg. 2001, 181, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K. Sedation in gastrointestinal endoscopy: Current issues. World J. Gastroenterol. 2013, 19, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Kelly, M.E.; Khan, A.; Irwin, R.; Khan, W.; Barry, K.; Waldron, R.; Khan, I.Z. Sedation for gastroscopy: Is it an adequately understood and informed choice? Ir. J. Med. Sci. 2016, 185, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Meining, A.; Semmler, V.; Kassem, A.; Sander, R.; Frankenberger, U.; Burzin, M.; Reichenberger, J.; Bajbouj, M.; Prinz, C.; Schmid, R. The effect of sedation on the quality of upper gastrointestinal endoscopy: An investigator-blinded, randomized study comparing propofol with midazolam. Endoscopy 2007, 39, 345–349. [Google Scholar] [CrossRef]
- Park, K.S. Introduction to Starting Upper Gastrointestinal Endoscopy: Proper Insertion, Complete Observation, and Appropriate Photographing. Clin. Endosc. 2015, 48, 279–284. [Google Scholar] [CrossRef]
- Waddingham, W.; Kamran, U.; Kumar, B.; Trudgill, N.J.; Tsiamoulos, Z.P.; Banks, M. Complications of diagnostic upper Gastrointestinal endoscopy: Common and rare—Recognition, assessment and management. BMJ Open Gastroenterol. 2022, 9, e000688. [Google Scholar] [CrossRef] [PubMed]
- Quality Indicators for Gastrointestinal Endoscopic Procedure…: Official Journal of the American College of Gastroenterology|ACG. Available online: https://journals.lww.com/ajg/Citation/2006/04000/Quality_Indicators_for_Gastrointestinal_Endoscopic.31.aspx (accessed on 20 July 2023).
- Cohen, J.; Safdi, M.A.; Deal, S.E.; Baron, T.H.; Chak, A.; Hoffman, B.; Jacobson, B.C.; Mergener, K.; Petersen, B.T.; Petrini, J.L.; et al. Quality indicators for esophagogastroduodenoscopy. Gastrointest. Endosc. 2006, 63, S10–S15. [Google Scholar] [CrossRef]
- Waschke, K.A.; Anderson, J.; Valori, R.M.; MacIntosh, D.G.; Kolars, J.C.; DiSario, J.A.; Faigel, D.O.; Petersen, B.T.; Cohen, J. ASGE principles of endoscopic training. Gastrointest. Endosc. 2019, 90, 27–34. [Google Scholar] [CrossRef]
- Januszewicz, W.; Kaminski, M.F. Quality indicators in diagnostic upper gastrointestinal endoscopy. Ther. Adv. Gastroenterol. 2020, 13, 1756284820916693. [Google Scholar] [CrossRef]
- Park, W.G.; Shaheen, N.J.; Cohen, J.; Pike, I.M.; Adler, D.G.; Inadomi, J.M.; Laine, L.A.; Lieb, J.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for EGD. Gastrointest. Endosc. 2015, 81, 17–30. [Google Scholar] [CrossRef]
- Chiu, P.W.Y.; Uedo, N.; Singh, R.; Gotoda, T.; Ng, E.K.W.; Yao, K.; Ang, T.L.; Ho, S.H.; Kikuchi, D.; Yao, F.; et al. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut 2019, 68, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Ragunath, K.; Wyman, A.; Banks, M.; Trudgill, N.; Pritchard, M.D.; Riley, S.; Anderson, J.; Griffiths, H.; Bhandari, P.; et al. Quality standards in upper gastrointestinal endoscopy: A position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017, 66, 1886–1899. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, Y.-K.; Cho, S.-M.; Kang, J.-K.; Lee, D.-J. Technical skills and training of upper gastrointestinal endoscopy for new beginners. World J. Gastroenterol. 2015, 21, 759–785. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Asplund, J.; Kauppila, J.H.; Mattsson, F.; Lagergren, J. Survival Trends in Gastric Adenocarcinoma: A Population-Based Study in Sweden. Ann. Surg. Oncol. 2018, 25, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Elvas, L.; Areia, M.; Brito, D.; Alves, S.; Saraiva, S.; Cadime, A.T. Premedication with simethicone and N-acetylcysteine in improving visibility during upper endoscopy: A double-blind randomized trial. Endoscopy 2017, 49, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Cho, Y.K.; Cha, J.M.; Lee, S.-Y.; Chung, I.-K. Effect of pronase as mucolytic agent on imaging quality of magnifying endoscopy. World J. Gastroenterol. 2015, 21, 2483–2489. [Google Scholar] [CrossRef]
- Lipp, A.; Lusardi, G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst. Rev. 2013, 11, CD005571.pub3. [Google Scholar] [CrossRef]
- Jain, N.K.; Larson, D.E.; Schroeder, K.W.; Burton, D.D.; Cannon, K.P.; Thompson, R.L.; DiMAGNO, E.P. Antibiotic Prophylaxis for Percutaneous Endoscopic Gastrostomy. Am. J. Gastroenterol. 1996, 91, 2301–2304. [Google Scholar] [CrossRef]
- Thomas, S.; Cantrill, S.; Waghorn, D.J.; Mcintyre, A. The role of screening and antibiotic prophylaxis in the prevention of percutaneous gastrostomy site infection caused by methicillin-resistant Staphylococcus aureus. Aliment. Pharmacol. Ther. 2007, 25, 593–597. [Google Scholar] [CrossRef]
- Leung, W.K.; Wu, M.-S.; Kakugawa, Y.; Kim, J.J.; Yeoh, K.-G.; Goh, K.L.; Wu, K.-C.; Wu, D.-C.; Sollano, J.; Kachintorn, U.; et al. Screening for gastric cancer in Asia: Current evidence and practice. Lancet Oncol. 2008, 9, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R.L.; Vicari, J.J.; Doughty, A.S.; Johanson, J.F.; Greenlaw, R.L. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. New Engl. J. Med. 2006, 355, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Teh, J.L.; Tan, J.R.; Lau, L.J.F.; Saxena, N.; Salim, A.; Tay, A.; Shabbir, A.; Chung, S.; Hartman, M.; So, J.B.-Y. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin. Gastroenterol. Hepatol. 2015, 13, 480–487.e2. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gaddam, S.; Wani, S.B.; Bansal, A.; Rastogi, A.; Sharma, P. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest. Endosc. 2012, 76, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, T.; Sano, Y.; Friedland, S.; Soetikno, R. American Gastroenterological Association (AGA) Institute Technology Assessment on Image-Enhanced Endoscopy. Gastroenterology 2008, 134, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, A.; Kaneko, K.; Konishi, K.; Ito, H.; Kushima, M.; Mitamura, K. Lugol staining pattern in background epithelium of patients with esophageal squamous cell carcinoma. Hepatogastroenterology 2004, 51, 713–717. [Google Scholar]
- Ishihara, R.; Yamada, T.; Iishi, H.; Kato, M.; Yamamoto, S.; Yamamoto, S.; Masuda, E.; Tatsumi, K.; Takeuchi, Y.; Higashino, K.; et al. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest. Endosc. 2009, 69, 213–218. [Google Scholar] [CrossRef]
- Shimizu, Y.; Omori, T.; Yokoyama, A.; Yoshida, T.; Hirota, J.; Ono, Y.; Yamamoto, J.; Kato, M.; Asaka, M. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: High-grade intra-epithelial neoplasia turns pink within a few minutes. J. Gastroenterol. Hepatol. 2008, 23, 546–550. [Google Scholar] [CrossRef]
- Peixoto, A.; Silva, M.; Pereira, P.; Macedo, G. Biopsies in Gastrointestinal Endoscopy: When and How. GE Port. J. Gastroenterol. 2016, 23, 19–27. [Google Scholar] [CrossRef]
- Sanghi, V.; Thota, P.N. Barrett’s esophagus: Novel strategies for screening and surveillance. Ther. Adv. Chronic Dis. 2019, 10, 204062231983785. [Google Scholar] [CrossRef]
- Husnoo, N.; Ahmed, W.; Shiwani, M.H. Duodenal biopsies for the diagnosis of coeliac disease: Are we adhering to current guidance? BMJ Open Gastroenterol. 2017, 4, e000140. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; Von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Dias, J.A.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Aruin, L.I. Importance of biopsy in stomach ulcer. Arkhiv Patol. 1989, 51, 70–76. [Google Scholar]
- Rugge, M.; Zaninotto, G.; Parente, P.; Zanatta, L.; Cavallin, F.; Germanà, B.; Macrì, E.; Galliani, E.; Iuzzolino, P.; Ferrara, F.; et al. Barrett’s Esophagus and Adenocarcinoma Risk: The experience of the North-Eastern Italian Registry (EBRA). Ann. Surg. 2012, 256, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, M.; Looman, C.W.N.; Steyerberg, E.W.; Kerkhof, M.; Kastelein, F.; van Dekken, H.; van Vuuren, A.J.; Bode, W.A.; van der Valk, H.; Ouwendijk, R.J.T.; et al. Predictors for Neoplastic Progression in patients with barrett’s esophagus: A prospective cohort study. Am. J. Gastroenterol. 2011, 106, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- García-Iglesias, P.; Villoria, A.; Suarez, D.; Brullet, E.; Gallach, M.; Feu, F.; Gisbert, J.P.; Barkun, A.; Calvet, X. Meta-analysis: Predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment. Pharmacol. Ther. 2011, 34, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Marmo, R.; Koch, M.; Cipolletta, L.; Capurso, L.; Grossi, E.; Cestari, R.; Bianco, M.A.; Pandolfo, N.; Dezi, A.; Casetti, T.; et al. Predicting mortality in non-variceal upper gastrointestinal bleeders: Validation of the italian pned score and prospective comparison with the rockall score. Am. J. Gastroenterol. 2010, 105, 1284–1291. [Google Scholar] [CrossRef]
- Chiu, P.W.; Ng, E.K.; Cheung, F.K.; Chan, F.K.; Leung, W.; Wu, J.C.; Wong, V.W.; Yung, M.; Tsoi, K.; Lau, J.Y.; et al. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin. Gastroenterol. Hepatol. 2009, 7, 311–316. [Google Scholar] [CrossRef]
- Kuo, C.H.; Sheu, B.S.; Kao, A.W.; Wu, C.H.; Chuang, C.H. A Defoaming agent should be used with pronase premedication to improve visibility in upper gastrointestinal endoscopy. Endoscopy 2002, 34, 531–534. [Google Scholar] [CrossRef]
- Neale, J.R.; James, S.; Callaghan, J.; Patel, P. Premedication with N-acetylcysteine and simethicone improves mucosal visualization during gastroscopy: A randomized, controlled, endoscopist-blinded study. Eur. J. Gastroenterol. Hepatol. 2013, 25, 778–783. [Google Scholar] [CrossRef]
- Chang, C.-C. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: An endoscopist-blinded, prospective, randomized study. World J. Gastroenterol. 2007, 13, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Barkun, A.N.; Saltzman, J.R.; Martel, M.; Leontiadis, G.I. ACG clinical guideline: Upper gastrointestinal and ulcer bleeding. Am. J. Gastroenterol. 2021, 116, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-H.; Wu, P.-Y.; Wu, T.-L.; Huang, S.-P.; Chen, Y.-Y.; Chen, M.-F.; Lin, W.-C.; Tsai, C.-L.; Lin, K.-P. Forrest classification for bleeding peptic ulcer: A new look at the old endoscopic classification. Diagnostics 2022, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Legros, R.; Chaussade, S.; Sautereau, D. Endoscopic haemostasis: An overview of procedures and clinical scenarios. Dig. Liver Dis. 2014, 46, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Management of Variceal Hemorrhage. Gastroenterol. Res. 2009. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, U.; Umapathy, C.; Halim, N.; Desai, M.; Nanjappa, A.; Arekapudi, S.; Theethira, T.; Wong, H.; Roytman, M.; Saligram, S. Update on the management of gastrointestinal varices. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Barkun, A.N.; Almadi, M.; Kuipers, E.J.; Laine, L.; Sung, J.; Tse, F.; Leontiadis, G.I.; Abraham, N.S.; Calvet, X.; Chan, F.K.; et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the international consensus group. Ann. Intern. Med. 2019, 171, 805. [Google Scholar] [CrossRef]
- Barkun, A.N.; Bardou, M.; Martel, M.; Gralnek, I.M.; Sung, J.J. Prokinetics in acute upper GI bleeding: A meta-analysis. Gastrointest. Endosc. 2010, 72, 1138–1145. [Google Scholar] [CrossRef]
- Dan, Y.Y.; So, J.; Yeoh, K.G. Endoscopic Screening for Gastric Cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 709–716. [Google Scholar] [CrossRef]
- Muto, M.; Horimatsu, T.; Ezoe, Y.; Morita, S.; Miyamoto, S. Improving visualization techniques by narrow band imaging and magnification endoscopy. J. Gastroenterol. Hepatol. 2009, 24, 1333–1346. [Google Scholar] [CrossRef]
- Brand, B.; Oesterhelweg, L.; Binmoeller, K.; Sriram, P.; Bohnacker, S.; Seewald, S.; De Weerth, A.; Soehendra, N. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig. Liver Dis. 2002, 34, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, A.; Gaiani, F.; Carra, M.C.; Brunetti, F.; Lévy, M.; Sobhani, I.; Azoulay, D.; Catena, F.; De’angelis, G.L.; De’angelis, N. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: A systematic review and meta-analysis. BioMed Res. Int. 2016, 2016, 4638683. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T.; Jung, H.-Y. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for early gastric cancer. Dig. Endosc. 2013, 25, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Parasher, G.; Wong, M.; Rawat, M. Evolving role of artificial intelligence in gastrointestinal endoscopy. World J. Gastroenterol. 2020, 26, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Gora, M.J.; Suter, M.J.; Tearney, G.J.; Li, X. Endoscopic optical coherence tomography: Technologies and clinical applications [Invited]. Biomed. Opt. Express 2017, 8, 2405–2444. [Google Scholar] [CrossRef] [PubMed]
- El Hajjar, A.; Rey, J.-F. Artificial intelligence in gastrointestinal endoscopy: General overview. Chin. Med. J. 2020, 133, 326–334. [Google Scholar] [CrossRef]
- Grover, S.C.; Walsh, C.M. Integrating artificial intelligence into endoscopy training: Opportunities, challenges, and strategies. Lancet Gastroenterol. Hepatol. 2023, 9, 11–13. [Google Scholar] [CrossRef]
- Mahmood, T.; Scaffidi, M.A.; Khan, R.; Grover, S.C. Virtual reality simulation in endoscopy training: Current evidence and future directions. World J. Gastroenterol. 2018, 24, 5439–5445. [Google Scholar] [CrossRef]
- Maulahela, H.; Annisa, N.G.; Konstantin, T.; Syam, A.F.; Soetikno, R. Simulation-based mastery learning in gastrointestinal endoscopy training. World J. Gastrointest. Endosc. 2022, 14, 512–523. [Google Scholar] [CrossRef]
- Khan, R.; Scaffidi, M.A.; Grover, S.C.; Gimpaya, N.; Walsh, C.M. Simulation in endoscopy: Practical educational strategies to improve learning. World J. Gastrointest. Endosc. 2019, 11, 209–218. [Google Scholar] [CrossRef]
- Gado, A.S.; Ebeid, B.A.; Axon, A.T. Quality assurance in gastrointestinal endoscopy: An Egyptian experience. Arab. J. Gastroenterol. 2016, 17, 153–158. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Alicante, S.; Amato, A.; Frazzoni, L.; Lombardi, G.; Manfredi, G.; Monica, F.; Sferrazza, S.; Vassallo, R.; Germanà, B.; et al. Quality performance measures in upper gastrointestinal endoscopy for lesion detection: Italian AIGO-SIED-SIGE joint position statement. Dig. Liver Dis. 2022, 54, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Kling, P.A.; Edin, K.; Domellouf, L. Observer Variability in Upper Gastrointestinal Fiber Endoscopy. Scand. J. Gastroenterol. 1985, 20, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, Z.; Castillo, G.; Jang, J.; Zaki, T.; Tzimas, D.; Guttentag, A.; Goodman, A.; Dikman, A.; Williams, R. Video Consent for Upper Endoscopy and Colonoscopy Improves Patient Comprehension in a Safety-net, Multi-lingual Population. J. Immigr. Minor. Health 2023, 25, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Teh, J.-L.; Shabbir, A.; Yuen, S.; So, J.B.-Y. Recent advances in diagnostic upper endoscopy. World J. Gastroenterol. 2020, 26, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.S.; Areia, M.; Ribeiro, M.D.; Rolanda, C. The impact of a structured virtual reality simulation training curriculum for novice endoscopists. GE Port. J. Gastroenterol. 2022, 29, 385–392. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.H.; Lee, G.H.; Kim, G.H.; Huh, G.; Hong, S.W.; Jung, H.-Y. Simulator-based training method in gastrointestinal endoscopy training and currently available simulators. Clin. Endosc. 2023, 56, 1–13. [Google Scholar] [CrossRef]
- Ekkelenkamp, V.E.; Koch, A.D.; De Man, R.A.; Kuipers, E.J. Training and competence assessment in GI endoscopy: A systematic review. Gut 2016, 65, 607–615. [Google Scholar] [CrossRef]
- Yoshimizu, S.; Hirasawa, T.; Horiuchi, Y.; Omae, M.; Ishiyama, A.; Yoshio, T.; Tsuchida, T.; Fujisaki, J. Differences in upper gastrointestinal neoplasm detection rates based on inspection time and esophagogastroduodenoscopy training. Endosc. Int. Open 2018, 06, E1190–E1197. [Google Scholar] [CrossRef]
- Nasiri, J.; Khatib, N.; Kheiri, S.; Najafi, M. The influence of escort during upper endoscopy and colonoscopy on patient satisfaction and anxiety. J. Fam. Med. Prim. Care 2016, 5, 134–138. [Google Scholar] [CrossRef]
- Okagawa, Y.; Abe, S.; Yamada, M.; Oda, I.; Saito, Y. Artificial intelligence in endoscopy. Dig. Dis. Sci. 2022, 67, 1553–1572. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; He, X.; Liu, M.; Xie, H.; An, P.; Zhang, J.; Zhang, H.; Ai, Y.; Tong, Q.; Guo, M.; et al. Evaluation of the effects of an artificial intelligence system on endoscopy quality and preliminary testing of its performance in detecting early gastric cancer: A randomized controlled trial. Endoscopy 2021, 53, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
| PRE-, INTRA-, AND POST- PROCEDURAL | CRITERIA/CATEGORY | ASGE/ACG [21] | ASIAN CONSENSUS [22] | BSG/AUGIS [23] | ESGE [1] |
|---|---|---|---|---|---|
| PRE-PROCEDURAL | EGD Indications | Accepted Indications | Risk stratification for UGI cancers; High-risk factors | Adequate preparation, indications, fitness assessment, and consent | - |
| Informed Consent | Risk discussion; proper documentation | Identification of high-risk patients for UGI cancers | Consent; endoscopist competency: JAG/JET accreditation with a minimum of 100 procedures/year | Proper instructions and informed consent | |
| Prophylactic Measures | Antibiotics for cirrhosis, PEG tube; PPI for suspected ulcer bleeding; vasoactive drugs for suspected variceal bleeding | - | Fasting protocol: 2 h for liquids, 6 h for solids; continuing professional development emphasizing lesion recognition | Water allowed until 2 h before procedure; safe fasting duration ≥6 h for solids | |
| INTRA-PROCEDURAL | Organ Examination | Complete organ examination, including stomach retroflexion | Sedation enhances detection of superficial neoplasms; systematic endoscopic mapping for detection of UGI superficial neoplasms; longer OGD times | Midazolam use; optimal procedure time: 7–8 min; high-definition systems for improved images and biopsies | Inclusion of esophagus, stomach, and duodenum in inspection; inspection duration should be ≥7 min |
| Biopsy Protocol | Gastric ulcer biopsy for malignancy; biopsy for suspected BE; adequate sample collection | Systematic photo-mapping; enhanced lesion recognition in high-risk and surveillance populations | Prague classification for Barrett’s lesions; Paris classification for lesion description | Minimum of 10 pictures for normal exam; use validated classifications for reporting | |
| Clinical Documentation and Visualization | Primary hemostasis; second treatment modality for bleeding ulcers | Iodine chromoendoscopy; NBI; indigo carmine chromoendoscopy | Photo mapping to enhance mucosal inspection; standardized terminology for reporting findings | Visualize major duodenal papilla; high-quality reporting with photo documentation | |
| POST-PROCEDURAL | Adverse Event Monitoring | Contact patients to document adverse events after EGD | Contact patients to document adverse events after EGD | Audit complications, readmissions, and mortality; review histology results from procedures | Implement software for reporting enhancement; monitor dysplasia incidence in Barrett’s surveillance |
| Patient Communication | - | - | Provide written and verbal post-EGD instructions; escalate malignant lesions promptly to multidisciplinary team meetings | Contact patients to document adverse events after EGD |
| Disease | Biopsy Site | Number of Biopsies | Method and Considerations | Reference |
|---|---|---|---|---|
| Barrett’s Esophagus | Esophagus above the GEJ | Every 1–2 cm in quadrants | Seattle protocol for quadrants; targeted biopsies of visible lesions; consider advanced imaging like NBI for identification of dysplastic areas; surveillance based on degree of dysplasia | [40,41] |
| Celiac Disease | Duodenal bulb and descending duodenum | 4–6 | Biopsies from the duodenal bulb and at least one other site in the duodenum; ensure adequate sampling of the intestinal mucosa for assessment of villous atrophy; orientation of biopsies for histological evaluation is important; four to six biopsies recommended, including one from the bulb | [40,42] |
| Eosinophilic Esophagitis | Esophagus | At least 6 | Biopsies from different locations focusing on areas with endoscopic mucosal abnormalities; eosinophil count ≥15 per high power field for diagnosis; Hematoxylin-eosin staining for assessment; two to four biopsies each from the proximal and distal esophagus recommended | [40,43] |
| Gastric Polyps | Polyp | Depends on polyp size | Small polyps (<5 mm): biopsy; larger polyps: removal and histological examination; multiple biopsies from large sessile polyps to rule out malignancy; polypectomy recommended for solitary polyps, with representative biopsies from smaller polyps in cases of multiple polyps | [40] |
| Helicobacter pylori | Antrum and corpus | Multiple from both sites | Sidney protocol: One biopsy each from lesser and greater curvatures of the antrum and body, and one from the incisura angularis; alternatively, three biopsies protocol: one from the incisura angularis, one from the greater curvature of the body, one from the greater curvature of the antrum | [40] |
| Infectious Esophagitis | Ulcers or lesions | As indicated | Biopsies from base of ulcers for CMV, edges for HSV; multiple biopsies may be needed for fungal esophagitis; consider PCR testing for definitive pathogen identification | [40] |
| Peptic Ulcer Disease | Ulcer and surrounding mucosa | ≥8 around the base | Biopsies of the ulcer margin and adjacent mucosa; consider testing for H. pylori; in cases of gastric ulcers, biopsy the ulcer base as well to rule out malignancy; recommended to perform multiple biopsies (≥8) in the base | [40] |
| Upper GI Neoplasia | Lesion site | 4–8 (optimal: 3–4) | Targeted biopsies of suspected malignant lesions; additional biopsies from the margins may be required for larger or irregular lesions; enhanced imaging techniques like chromoendoscopy may be used to identify subtle lesions; three or four biopsies considered optimal; exact targeting of appropriate site and viable tissue acquisition crucial for diagnosis; image enhanced endoscopy-assisted biopsy can aid in targeting and reduce the number of biopsies needed | [6] |
| Quality Indicator | Definition | Criteria Met? | |||
|---|---|---|---|---|---|
| PRE-PROCEDURAL | Justification for EGD | The EGD is performed for a valid indication. | Yes | No | N/A |
| Informed consent | The patient is informed of risks and benefits and provides consent. | Yes | No | N/A | |
| Patient preparation | The patient is properly prepared for the EGD, including fasting and taking prescribed medications. | Yes | No | N/A | |
| Premedication | The patient is given appropriate premedication to prevent complications. | Yes | No | N/A | |
| Prophylactic measures | Prophylactic antibiotics are administered to patients at risk for infection. | Yes | No | N/A | |
| Risk stratification | The patient’s risk of complications is assessed and mitigated. | Yes | No | N/A | |
| Endoscopist competency | The endoscopist is qualified and experienced to perform EGDs. | Yes | No | N/A | |
| Sedation | The patient is sedated safely and comfortably for the EGD procedure. | Yes | No | N/A | |
| INTRA-PROCEDURAL | Thoroughness of the examination | The entire UGI tract is examined thoroughly. | Yes | No | N/A |
| Duration of the examination | The examination is performed in a timely manner. | Yes | No | N/A | |
| Imaging enhancement equipment | Appropriate imaging enhancement is used to improve visualization. | Yes | No | N/A | |
| Adherence to biopsy protocols | Biopsies are taken from suspicious lesions and characterized appropriately. | Yes | No | N/A | |
| Clarity of visualization | The endoscopist can visualize the entire UGI tract clearly. | Yes | No | N/A | |
| Photo-documentation | The EGD is documented photographically. | Yes | No | N/A | |
| POST-PROCEDURAL | Reporting | The EGD findings are reported accurately and in a timely manner. | Yes | No | N/A |
| Documentation of findings | The EGD findings and recommendations are documented | Yes | No | N/A | |
| Patient communication | The patient is informed of the EGD findings and recommendations. | Yes | No | N/A | |
| Auditing post-procedural outcomes | The post-procedural outcomes are audited to ensure quality. | Yes | No | N/A | |
| Biopsy handling and review | Biopsies are handled and reviewed appropriately. | Yes | No | N/A | |
| Escalation of malignant lesions | Detected malignant lesions are escalated to the appropriate level of care. | Yes | No | N/A | |
| Report generation | The EGD report is generated accurately and in a timely manner. | Yes | No | N/A | |
| Surveillance for dysplasia | Patients with Barrett’s esophagus are monitored for dysplasia. | Yes | No | N/A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Tadros, M. Enhancing the Quality of Upper Gastrointestinal Endoscopy: Current Indicators and Future Trends. Gastroenterol. Insights 2024, 15, 1-18. https://doi.org/10.3390/gastroent15010001
Ferrari C, Tadros M. Enhancing the Quality of Upper Gastrointestinal Endoscopy: Current Indicators and Future Trends. Gastroenterology Insights. 2024; 15(1):1-18. https://doi.org/10.3390/gastroent15010001
Chicago/Turabian StyleFerrari, Caesar, and Micheal Tadros. 2024. "Enhancing the Quality of Upper Gastrointestinal Endoscopy: Current Indicators and Future Trends" Gastroenterology Insights 15, no. 1: 1-18. https://doi.org/10.3390/gastroent15010001
APA StyleFerrari, C., & Tadros, M. (2024). Enhancing the Quality of Upper Gastrointestinal Endoscopy: Current Indicators and Future Trends. Gastroenterology Insights, 15(1), 1-18. https://doi.org/10.3390/gastroent15010001






