Abstract
Left ventricular diastolic dysfunction (LVDD) is a hallmark of cirrhotic cardiomyopathy and has been linked to a poorer quality of life and worse outcomes in patients with end-stage liver disease. Its impact on survival after a liver transplant (LT) is not known, especially when using current diagnostic criteria to define LVDD. We conducted a systematic review and meta-analysis of the current published literature on mortality after a LT in patients with LVDD. We searched for articles in PubMed, Scopus, EMBASE, Web of Science, and the COCHRANE Central database. We included cohort studies that compared post-transplant outcomes between cirrhotic patients with and without LVDD. Our primary outcome of interest was all-cause mortality after a LT in relation to the presence of LVDD per the 2016 American Society of Echocardiography criteria. A total of 1029 articles were screened during the selection process. Two studies included in the meta-analysis showed no significant difference in mortality, but there was high heterogeneity. A narrative review of other studies that classified diastolic function (DD) using different criteria was also performed, revealing an association with worse outcomes in these patients. High-quality prospective studies using current criteria are needed to confirm these findings.
1. Introduction
Cardiovascular dysfunction is a hallmark of end-stage liver disease (ESLD). Multiple studies have detailed distinct structural and functional abnormalities in the hearts of these patients, including impaired systolic response to stress, resting left ventricular diastolic dysfunction (LVDD), and electrophysiologic alterations [1,2]. Clinical criteria for this condition, termed cirrhotic cardiomyopathy (CCM), were first proposed at the 2005 Montreal World Congress of Gastroenterology (WCG) [3]. The current recommendations to define LVDD, as proposed by the American Society of Echocardiography (ASE) in 2009 [4] and updated in 2016 [5], utilize tissue Doppler imaging (TDI) techniques to describe diastolic function more accurately. These same recommendations have been incorporated into new criteria for CCM proposed by an expert panel group [6].
LVDD has received special attention as an early marker of CCM [1], with its prevalence in patients with cirrhosis reported as high as 51.2% in one systematic review [7]. Evidence from multiple studies has linked LVDD to a poorer quality of life, a more advanced disease stage, decompensation, and reduced survival [7,8,9,10,11,12].
Liver transplantation (LT) remains the only cure for ESLD. Transplantation surgery is associated with acute hemodynamic changes, and LVDD is considered a risk factor for worse outcomes in patients undergoing LT [13]. Observational studies have indicated that cardiovascular complications are a leading cause of morbidity and mortality after LT [14]. Retrospective studies have been conducted to analyze the impact of LVDD during LT, but a consensus is still lacking regarding the importance of this clinical entity [15,16,17].
The aim of this study was to conduct a systematic review and meta-analysis of the published literature regarding the impact of LVDD, as defined using the latest ASE 2016 criteria, on survival and other outcomes such as graft failure (GF) and heart failure (HF) following LT.
2. Materials and Methods
2.1. Protocol Registration and Guidelines
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P) statement [18] and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under ID CRD42021277455.
2.2. Eligibility Criteria
We included cohort studies that compared post-transplant outcomes between cirrhotic patients with (w/) and without (w/o) LVDD. The inclusion criteria for study selection encompassed retrospective or prospective cohort studies involving adult patients (≥18 years) with ESLD who underwent LT, as well as a pretransplant echocardiogram to classify patients’ diastolic function according to the ASE 2016 criteria and a post-LT follow-up assessing survival and other outcomes.
2.3. Information Sources and Search Strategy
The following scientific databases were accessed: PubMed, Scopus, EMBASE, Web of Science, and the COCHRANE Central database. The search strategy was developed by an experienced librarian in collaboration with the study authors. Database searches were conducted from inception to June 2022 with no language restriction. The search strategy comprised a series of Medical Subject Heading terms and keywords related to the population (liver cirrhosis), intervention (liver transplant), and comparison (diastolic dysfunction) of interest. The complete search strategy is available in Appendix A.
2.4. Data Management and Selection Process
References obtained from the search were stored in Distiller Systematic Review software for the screening process, which comprised a title/abstract and full-text screening phase. Two independent reviewers collaborated in screening each reference. Any conflicts during the initial phase were resolved in the full-text phase, with remaining conflicts addressed through consensus or the intervention of a third reviewer. A pilot test was conducted before each phase to ensure inter-rater agreement, defined as a kappa index equal to or higher than 0.7.
2.5. Data Collection Process
Two independent members of the research team collected information from each included study. Any conflicts in data collection were resolved through consensus or, if necessary, via the intervention of a third reviewer. The following information was gathered from each study: study characteristics (first author, year, country where study was conducted, follow-up time, sample size, setting, and study design), population characteristics (age, gender, body mass index (BMI), comorbidities, and cirrhosis status as well as etiology), laboratory parameters (AST and ALT), and outcomes of interest, which are mentioned in the next section.
2.6. Outcomes
Our primary outcome was survival after LT. Additional outcomes included the overall length of hospital stay, length of ICU stay, days on mechanical ventilation, the incidence of acute kidney injury (AKI), days on vasopressor, cardiovascular outcomes, and 30-day mortality.
2.7. Risk of Bias in Individual Studies
The risk of bias assessment was conducted by two independent reviewers working in duplicate, utilizing the Newcastle–Ottawa scale. Any conflicts were resolved through consensus or the intervention of a third reviewer.
2.8. Data Synthesis
After data extraction, survival analysis, presented as Kaplan–Meier curves, had to be processed for the meta-analysis. Since none of the included studies provided survival data with relative risk, odds ratio, or hazard ratio (HR), we employed previously described methods [19] to derive the HR, log-rank observed–expected events (O-E), and log-rank variance (V) from the selected studies. These data were utilized for a random effects meta-analysis of the pooled HRs using RevMan software version 5.3.
For our primary outcome analysis of post-LT survival, only two references provided adequate data. Therefore, we conducted a narrative synthesis that included all other studies found in our search that analyzed post-LT mortality and other outcomes related to LVDD, irrespective of the criteria used to define it.
3. Results
3.1. Study Characteristics
A total of 1029 references were screened during the selection process. Sixteen studies, involving 4793 participants, met the inclusion criteria. The study selection process is depicted in Figure 1. Additional relevant information regarding the clinical characteristics of the study population and outcomes across studies is presented in Table 1 and Table 2. The assessment of study quality using the Newcastle–Ottawa scale is summarized in Table 3.
Figure 1.
Overview of the study selection.

Table 1.
Summary characteristics of included studies.

Table 2.
Summary outcomes of included studies.

Table 3.
Newcastle–Ottawa quality assessment scale.
3.2. Post-Transplant Mortality
Only two studies were suitable for our primary outcome analysis of post-LT mortality [23,32]. We did not find a statistically significant difference between patients with and without pre-transplant LVDD (OR 0.13, 95% IC −0.20–0.47). The heterogeneity between the studies was high (i2 = 80%) (Figure 2). References that used criteria other than the ASE 2016 definition to define LVDD and analyze post-LT mortality are presented in the following narrative synthesis.
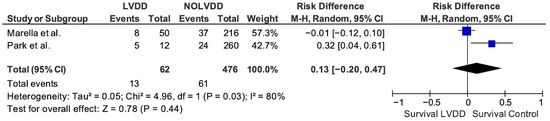
Figure 2.
Forest plot chart of random-effects meta-analysis of mortality after LT in patients with and without LVDD as per ASE 2016 criteria [23,32].
Park et al. [32] used both the 2016 and 2009 ASE criteria to classify patients undergoing LT. Post-LT mortality was increased for patients with LVDD using both criteria, but the 2016 criteria were superior, as they predicted higher mortality in the LVDD group (46% vs. 38%) and better survival in the normal diastolic function group (91% vs. 83%).
In a retrospective cohort of cirrhotic patients undergoing LT [15], a left atrial volume index (LAVI) > 40 mL/m2 was associated with worse survival (50% vs. 71% 5-year survival) compared to LAVI < 40 mL/m2 (p = 0.01). The same study also found that increased LAVI and mitral annular velocity were predictors of post-LT HF.
Another study [31] involving patients who underwent LT discovered a relationship between higher LAVI (>27 mL/m2) and post-transplant mortality (HR = 2.3; 95% CI, 1.04–5.20; and p = 0.04). However, this association was observed only among patients with MELD scores higher than 33, suggesting that LVDD, as measured via LAVI, has a greater impact on patients with more advanced liver disease.
A study of echocardiographic predictors of post-LT survival in patients with ESLD [20] found increased post-LT mortality in patients with tricuspid regurgitation (TR) higher than mild (HR, 1.68; 95% CI, 1.03–2.75; and p 0.04). Similar findings were reported by Kia et al. [34].
In a separate investigation [16], no difference in survival after LT was found between patients with and without LVDD, as defined using the 2005 WCG criteria. However, they observed a higher proportion of moderate to severe TR in patients who died during the follow-up compared to survivors (26% vs. 9%, p = 0.02).
Mittal et al. [25] reported a higher incidence of acute cellular rejection (ACR), GF, and mortality (p = 0.0001) in patients with LVDD, as defined by them using the E/A ratio and E/e’ ratio.
3.3. Prevalence of LVDD
We found a global prevalence of LVDD of 12.2% in studies using the 2016 ASE criteria (n = 677) [23,24,32]. The global prevalence of LVDD using the 2009 criteria was 32.4% (n = 555) [28,32]. Prevalence varied among other studies using different criteria (Table 1).
3.4. Immediate Postoperative Outcomes
The included studies showed conflicting results on the impact of LVDD on postoperative outcomes.
Two studies [23,24] that used the 2016 ASE criteria did not report differences between patients with and without LVDD concerning days on vasopressors, days on mechanical ventilation, and 30-day all-cause mortality. However, another study using the same criteria to define LVDD [32] found higher use of mechanical ventilation (40.8% vs. 100%, p ≤ 0.001) and higher continuous renal replacement therapy (CRRT) (6.2% vs. 25%, p = 0.006) between patients with and without LVDD. Nevertheless, no differences were found between early allograft dysfunction (EAD) and AKI during the first week. No differences were found using the 2009 ASE criteria. A different study [29] did find a statistically significant association between grade II LVDD and EAD, as well as cardiovascular artery disease (CAD). However, the study is notable for its incomplete echocardiographic data.
Mittal et al. [25] found that patients with grade 2 or 3 LVDD were more likely to develop ACR (HR 3.38; 95% CI 2.64–4.33; and p ≤ 0.0001) and GF (HR 2.26; 95% CI 1.46–3.51; and p ≤ 0.0001) on post-LT follow-up.
Other studies [21,30] that used different criteria to define LVDD (2005 WCG criteria, European Study Group on Diastolic Heart Failure criteria) in patients with ESLD and/or hepatocellular carcinoma (HCC) who underwent LT found no differences in post-LT ventilation time, renal failure, or serious adverse events in patients with and without LVDD. Additionally, cardiac arrest, intraoperative blood loss, and transfusion requirements were also similar between groups.
3.5. LVDD and Postoperative Cardiovascular Complications
One study [26] evaluated pre-LT systolic and diastolic HF predictors in patients with ESLD. LVDD was defined by the authors using the E/A and E/e’ ratios. They found that LVDD was an independent predictor of post-LT HF, with grade 3 LVDD being the strongest predictor (HR = 1.89, 95% CI = 1.11–3.13; and p = 0.02).
Dowsley et al. [15] found that patients who developed post-LT HF had higher E/E’ (9.1 [3.3] vs. 7.1 [2.3], p = 0.02) and increased LAVI (41.0 [12.9] mL/m2 vs. 31.4 [8.0] mL/m2, p = 0.008) compared with controls on the pre-LT echocardiogram. They also had a higher prevalence of E/E’ < 10 and LAVI > 40 mL/m2. In a multivariate analysis, elevated E/E’, increased LAVI, and low mean arterial pressure before LT remained predictors of HF after LT.
Sonny et al. [28] utilized the 2009 ASE criteria and found that patients with LVDD did not exhibit a higher incidence of the primary composite outcome of mortality, GF, and/or major cardiovascular events than those without LVDD (HR, 1.47; 95% CI: 0.85–2.56; and p = 0.17). However, most of the patients were classified as having grade 1 LVDD. LAVI was also analyzed but did not show any association with these outcomes.
Studies that used the 2016 ASE criteria to define LVDD found conflicting results regarding post-LT HF and cardiovascular outcomes. Some studies [23,24] reported no difference in the incidence of cardiac arrhythmias, cardiac adverse events, and HF, while another study [32] reported a higher incidence of HF between patients with and without LVDD.
In a study of patients with ESLD who underwent LT [22], the Cirrhotic Cardiomyopathy Consortium (CCMC) criteria were used to classify patients. They found that patients with CCM were at increased risk for the primary composite outcome of new-onset cardiovascular disease after transplant (HR, 2.57; 95% CI 1.19–5.54; and p = 0.016). Five-year cardiovascular disease-free survival was notably higher in patients without CCM compared to patients with CCM, but there was no impact of CCM on all-cause mortality. However, a subgroup analysis showed no statistically significant difference in individual post-transplant CV disease(s) (i.e., coronary artery disease, heart failure, arrhythmia, or stroke) between groups.
Another study [33] compared the WCG criteria to the CCMC criteria to determine which were better at predicting post-LT major adverse cardiac events (MACE). Multivariable analysis showed that patients fulfilling CCMC criteria were at increased risk of developing MACE (HR, 1.93; 95% CI, 1.05–3.56; and p = 0.04) after controlling for covariates. No association was found using the WCG criteria. The same study also analyzed individual diastolic function parameters in search of an association with MACE. Only a reduced median septal e’ was significantly associated with post-LT MACE (p = 0.002), with a cut-off value of < 7 predicting increased risk, even after adjusting for diabetes (HR, 3.16; 95% CI, 1.78–5.61; and p < 0.001).
3.6. LVDD and Length of Hospital Stay
Among the included studies, only one [19] found a statistically significant difference in the length of ICU stay between patients with and without LVDD when employing the 2016 ASE criteria. No significant difference was reported in the overall length of hospital stay, and there was no difference when using the 2009 criteria as well. Other studies [23,24,29] that utilized the 2016 ASE criteria did not find significant differences in this outcome. Similarly, another study [21] that used the European Study Group on Diastolic Heart Failure guidelines to define LVDD found no significant difference in the length of hospital and ICU stays between patients with and without LVDD.
3.7. LVDD and MELD Score
One study [16] that assessed the prevalence of LVDD using the 2005 WCG criteria and its association with post-LT outcomes in patients with ESLD found no significant difference in the MELD score between patients with and without LVDD. Similar results were observed in studies utilizing the 2016 ASE criteria [23,29]. However, in another study [32], a higher MELD score was correlated with the severity of LVDD. The correlation was stronger in the 2016 criteria than in the 2009 criteria (r = 0.219 vs. 0.180, respectively) and stronger in patients with a MELD score higher than 16 points (r = 0.165 and 0.223, respectively). These results were statistically significant for both the 2009 and 2016 criteria (p = 0.01 and 0.001, respectively).
3.8. Echocardiographic Changes after LT
Regarding echocardiographic changes after LT, several studies describe alterations in patients with ESLD following LT [15,28], including worsening LVDD grade, increased left ventricular mass index, decreased LVEF and stroke volume, and no changes in LAVI. In one study [22], only 6 out of 22 patients with post-LT echocardiograms exhibited normalization of DD post-LT after a median of 1.0 ± 0.9 years (4/11 with NASH, 2/8 with ALD, and p = 0.81).
4. Discussion
Our study did not find significant differences in long-term mortality after LT between patients with preoperative LVDD, as defined using the 2016 ASE criteria, and those without it. Nevertheless, it is crucial to note that only two studies met the inclusion criteria for analysis, and they presented conflicting results. However, we found significant differences in the length of hospital and ICU stays. Well-designed prospective studies are needed to draw stronger conclusions on the impact of LVDD on outcomes after LT and its role in better selecting the right candidate for transplant.
Initially, patients with CCM typically exhibited normal systolic function. This phenomenon was attributed to distinct cardiovascular changes seen in patients with ESLD, including hyperdynamic circulation and systemic vasodilation, which preserved the left ventricular ejection fraction [1]. As a result, early research in this field predominantly focused on diastolic function, with LVDD becoming a key factor in diagnosing CCM.
The original 2005 WGO criteria used to define LVDD often overestimated its prevalence because the parameters relied on measurements of blood flow across the cardiac valves, which are volume-dependent and frequently altered in patients with advanced liver disease [4]. Today, LVDD is recognized as the hallmark of CCM [6].
Most recently, researchers have adopted the 2016 ASE criteria for defining LVDD. These criteria are favored because they utilize TDI technology, which is less dependent on volume status than previous criteria, offering a more accurate reflection of impaired cardiac relaxation [35]. The 2016 ASE criteria employ the following measurements to define LVDD: septal e’ velocity < 7 cm/s, E/e’ ratio ≥ 15, LAVI ≥ 34 Ml/m2, and TR velocity ≥ 2.8 m/s. A patient meeting at least three of these criteria is classified as having LVDD. If only two criteria are present, the diastolic function is considered indeterminate. If only one or none of these criteria is found, the patient is not considered to have DD [4].
New criteria for CCM were introduced in 2019 by the Cirrhotic Cardiomyopathy Consortium, utilizing a modified version of the 2016 ASE criteria to define LVDD [6]. Subsequent retrospective studies have validated these new criteria, demonstrating their superiority in predicting new cardiovascular disease and MACE following LT. However, similar to our study, these studies did not observe differences in all-cause mortality [22,27,33].
Many studies identified in our systematic review reported conflicting results concerning the relationship between LVDD and adverse outcomes. Notably, there was significant heterogeneity in the criteria used to define LVDD across these studies, including the WGC criteria [15,30], the 2009 ASE criteria [28,32], and isolated parameters of diastolic function such as TR, LAVI, and E/e’ [20,25,26,31,34]. Due to the substantial variability in the criteria employed, most of these studies could not be included in our meta-analysis. Given the limited number of studies using these criteria, we chose to incorporate those using the previous 2009 ASE criteria; however, a mortality analysis was not possible due to insufficient information, despite our attempts to gather additional data from the authors.
As mentioned earlier, our analysis did not reveal a difference in post-LT mortality between patients with preoperative LVDD defined using the ASE 2016 criteria. Nonetheless, other studies have indicated an impact when using isolated diastolic function parameters. These studies suggest that patients with markers of LVDD experience worse postoperative outcomes, a higher incidence of acute heart failure, and increased mortality [20,25,26,31,34]. This observation may be attributed to the specific parameters used, which select patients with more severe LVDD (i.e., dysfunction severe enough to increase LA volume or induce significant TR) compared to those patients classified as having dysfunction under the ASE 2016 criteria. It has been theorized that LVDD in these patients could lead to the heart’s inability to accommodate preload following transplantation, resulting in concurrent graft congestion and dysfunction. This explanation offers insights into the increased post-LT mortality.
The variations in mortality observed between the two studies [23,32] included in our analysis could be explained by differences in patient etiologies for ESLD as well as comorbidities between the two cohorts. The study by Marella et al. [23] had a higher proportion of patients with NASH, diabetes, and hypertension, conditions also associated with LVDD in patients without liver disease. These additional comorbidities may introduce a distinct pathophysiological pathway, explaining the disparate outcomes. Notably, Park et al. [32] did not categorize LVDD according to its severity and might have included more patients with advanced disease in their cohort. Marella et al. [23] reported that most patients in their cohort had grade I and II LVDD, with only 4% of patients classified as grade III.
The variations in hospital and ICU length of stay may be attributed to patients with LVDD requiring extended periods of mechanical ventilation, heightened vasopressor usage, or experiencing more complications during their hospitalization.
The limitations of our study encompass several factors. Firstly, the retrospective nature of the selected studies introduces inherent limitations and potential bias in reporting and publication. Secondly, the studies included in our meta-analysis exhibited high heterogeneity, posing a challenge to the interpretation of the results. Moreover, we encountered difficulties in obtaining complete datasets for certain studies that could have been included in the final analysis. Lastly, the limited number of studies in our analysis and the varying outcomes underscore the historical lack of consensus regarding the criteria employed to define LVDD.
5. Conclusions
Our systematic review and meta-analysis did not find a significant difference in long-term mortality following LT between patients with preoperative LVDD and those without it, as defined using the 2016 ASE criteria. However, these findings warrant validation through well-designed prospective studies. Our literature search revealed variations in the criteria used to define LVDD in CCM. Given the emergence of new criteria showing significance in retrospective studies, we advocate for future research to adhere to these updated guidelines for defining LVDD and, more broadly, CCM.
Author Contributions
C.E.G.-M. made the initial manuscript and subsequent corrections as well as made substantial contributions to the data analysis and interpretation; D.R.-C. made the initial manuscript and subsequent corrections as well as made substantial contributions to the data analysis; S.M.-J. made the initial manuscript and subsequent corrections as well as made substantial contributions to the data analysis; A.R.-C. made the initial manuscript and subsequent corrections as well as made substantial contributions to the data interpretation; I.E.H.-P. made the initial manuscript and subsequent corrections as well as made substantial contributions to the data interpretation; J.R.A.-L. helped better define the initial idea, helped revise the initial manuscript, offered suggestions for the manuscript, provided his expertise as a cardiologist to select the best articles for the meta-analysis when in doubt, and approved the final version of this manuscript to be published; H.N.-T. revised the initial manuscript and made substantial contributions to the manuscript; and L.E.M.-E. conceptualized the initial idea, made substantial contributions to the interpretation of the data, offered suggestions for the manuscript, supervised the project, provided her expertise as a hepatologist to select the best articles for the meta-analysis, and approved the final version of this manuscript to be published. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The supporting data from these findings can be made available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Complete Search Strategy Employed in the Medical Databases
(((“Liver Cirrhosis” OR “Cirrhosis, Hepatic” OR “Cirrhosis, Liver” OR “Fibrosis, Liver” OR “Hepatic Cirrhosis” OR “Liver Fibrosis”) AND (“LTx” OR “Liver Transplantation” OR “Grafting, Liver” OR “Hepatic Transplantation” OR “Hepatic Transplantations” OR “Liver Grafting” OR “Liver Transplant” OR “Liver Transplantations” OR “Liver Transplants” OR “Transplant, Liver” OR “Transplantation, Hepatic” OR “Transplantation, Liver” OR “orthotopic liver transplantation” OR “liver tissue transplantation” OR “liver orthotopic transplantation” OR “liver heterotopic transplantation” OR “auxiliary liver transplantation”))) AND ((“diastolic dysfunction” OR “heart failure” OR “left ventricular diastolic dysfunction” OR “Ventricular Dysfunction, Left” OR “heart left ventricle failure”)) AND ((“Echocardiography” OR “2 D Echocardiography” OR “2-D Echocardiography” OR “2D Echocardiography” OR “Contrast Echocardiography” OR “Cross Sectional Echocardiography” OR “Cross-Sectional Echocardiography” OR “Echocardiography, 2 D” OR “Echocardiography, 2-D” OR “Echocardiography, 2D” OR “Echocardiography, Contrast” OR “Echocardiography, Cross Sectional” OR “Echocardiography, Cross-Sectional” OR “Echocardiography, M Mode” OR “Echocardiography, M-Mode” OR “Echocardiography, Transthoracic” OR “Echocardiography, Two Dimensional” OR “Echocardiography, Two- Dimensional” OR “M Mode Echocardiography” OR “M-Mode Echocardiography” OR “Transthoracic Echocardiography” OR “Two Dimensional Echocardiography” OR “Two-Dimensional Echocardiography” OR “2-dimensional echocardiography”))
References
- Wiese, S.; Hove, J.D.; Bendtsen, F.; Møller, S. Cirrhotic Cardiomyopathy: Pathogenesis and Clinical Relevance. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.; Danielsen, K.V.; Wiese, S.; Hove, J.D.; Bendtsen, F. An Update on Cirrhotic Cardiomyopathy. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.; Henriksen, J.H. Cardiovascular Complications of Cirrhosis. Gut 2008, 57, 268–278. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Izzy, M.; VanWagner, L.B.; Lin, G.; Altieri, M.; Findlay, J.Y.; Oh, J.K.; Watt, K.D.; Lee, S.S.; on behalf of The Cirrhotic Cardiomyopathy Consortium. Redefining Cirrhotic Cardiomyopathy for the Modern Era. Hepatology 2020, 71, 334–345. [Google Scholar] [CrossRef]
- Stundiene, I.; Sarnelyte, J.; Norkute, A.; Aidietiene, S.; Liakina, V.; Masalaite, L.; Valantinas, J. Liver Cirrhosis and Left Ventricle Diastolic Dysfunction: Systematic Review. World J. Gastroenterol. 2019, 25, 4779–4795. [Google Scholar] [CrossRef]
- Karagiannakis, D.S.; Vlachogiannakos, J.; Anastasiadis, G.; Vafiadis-Zouboulis, I.; Ladas, S.D. Diastolic Cardiac Dysfunction Is a Predictor of Dismal Prognosis in Patients with Liver Cirrhosis. Hepatol. Int. 2014, 8, 588–594. [Google Scholar] [CrossRef]
- Lee, S.K.; Song, M.J.; Kim, S.H.; Ahn, H.J. Cardiac Diastolic Dysfunction Predicts Poor Prognosis in Patients with Decompensated Liver Cirrhosis. Clin. Mol. Hepatol. 2018, 24, 409–416. [Google Scholar] [CrossRef]
- Premkumar, M.; Devurgowda, D.; Vyas, T.; Shasthry, S.M.; Khumuckham, J.S.; Goyal, R.; Thomas, S.S.; Kumar, G. Left Ventricular Diastolic Dysfunction Is Associated with Renal Dysfunction, Poor Survival and Low Health Related Quality of Life in Cirrhosis. J. Clin. Exp. Hepatol. 2019, 9, 324–333. [Google Scholar] [CrossRef]
- Ruíz-del-Árbol, L.; Achécar, L.; Serradilla, R.; Rodríguez-Gandía, M.Á.; Rivero, M.; Garrido, E.; Natcher, J.J. Diastolic Dysfunction Is a Predictor of Poor Outcomes in Patients with Cirrhosis, Portal Hypertension, and a Normal Creatinine. Hepatology 2013, 58, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Frigo, A.C.; Tonon, M.; Angeli, P. Cardiovascular Predictors of Death in Patients with Cirrhosis. Hepatology 2018, 68, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jayakumar, S.; Traboulsi, M.; Lee, S.S. Cirrhotic Cardiomyopathy: Implications for Liver Transplantation: Liu et Al. Liver Transpl. 2017, 23, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lee, S.S. Predicting Cardiovascular Complications after Liver Transplantation: 007 to the Rescue? Liver Transpl. 2011, 17, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Dowsley, T.F.; Bayne, D.B.; Langnas, A.N.; Dumitru, I.; Windle, J.R.; Porter, T.R.; Raichlin, E. Diastolic Dysfunction in Patients with End-Stage Liver Disease Is Associated with Development of Heart Failure Early after Liver Transplantation. Transplantation 2012, 94, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Raevens, S.; De Pauw, M.; Geerts, A.; Berrevoet, F.; Rogiers, X.; Troisi, R.I.; Van Vlierberghe, H.; Colle, I. Prevalence and Outcome of Diastolic Dysfunction in Liver Transplantation Recipients. Acta Cardiol. 2014, 69, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, F.; Rodrigues, C.; Adrego, T.; Viana, J.; Vieira, H.; Seco, C.; Pereira, L.; Pinto, F.; Eufrásio, A.; Bento, C.; et al. Diastolic Dysfunction in Liver Cirrhosis: Prognostic Predictor in Liver Transplantation? Transplant. Proc. 2016, 48, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Reprint—Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical Methods for Incorporating Summary Time-to-Event Data into Meta-Analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Bushyhead, D.; Kirkpatrick, J.N.; Goldberg, D. Pretransplant Echocardiographic Parameters as Markers of Posttransplant Outcomes in Liver Transplant Recipients: Echocardiographic Parameters and Outcomes. Liver Transpl. 2016, 22, 316–323. [Google Scholar] [CrossRef]
- Xu, Z.-D.; Xu, H.-T.; Li, W.-W.; Zou, Z.; Shi, X.-Y. Influence of Preoperative Diastolic Dysfunction on Hemodynamics and Outcomes of Patients Undergoing Orthotopic Liver Transplantation. Int. J. Clin. Exp. Med. 2013, 6, 351–357. [Google Scholar] [PubMed]
- Izzy, M.; Soldatova, A.; Sun, X.; Angirekula, M.; Mara, K.; Lin, G.; Watt, K.D. Cirrhotic Cardiomyopathy Predicts Posttransplant Cardiovascular Disease: Revelations of the New Diagnostic Criteria. Liver Transpl. 2021, 27, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Marella, H.; Yedlapati, N.; Kothadia, J.P.; Mupparaju, V.K.; Marella, S.; Nair, S. Impact of Left Ventricular Diastolic Dysfunction on Liver Transplantation Outcomes Based on the Latest American Society of Echocardiography/European Association of Cardiovascular Imaging Recommendations. Clin. Exp. Hepatol. 2021, 7, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Marella, H.K.; Kamal, F.; Peravali, R.; Jacob, J.; Nair, S.P. Left Ventricular Diastolic Dysfunction in Liver Transplantation: A Stronger Association with Non-Alcoholic Steatohepatitis. Clin. Exp. Hepatol. 2020, 6, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Mittal, C.; Qureshi, W.; Singla, S.; Ahmad, U.; Huang, M.A. Pre-Transplant Left Ventricular Diastolic Dysfunction Is Associated with Post Transplant Acute Graft Rejection and Graft Failure. Dig. Dis. Sci. 2014, 59, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.; Mittal, C.; Ahmad, U.; Alirhayim, Z.; Hassan, S.; Qureshi, S.; Khalid, F. Clinical Predictors of Post-Liver Transplant New-Onset Heart Failure: New-Onset Heart Failure after Liver Transplantation. Liver Transpl. 2013, 19, 701–710. [Google Scholar] [CrossRef]
- Singh, A.D.; Ford, A.; Lyu, R.; Layoun, H.; Harb, S.C.; Fares, M.; Carey, W.D. Impact of Cirrhotic Cardiomyopathy Diagnosed According to Different Criteria on Patients with Cirrhosis Awaiting Liver Transplantation: A Retrospective Cohort Study. Dig Dis Sci. 2022, 67, 5315–5326. [Google Scholar] [CrossRef]
- Sonny, A.; Ibrahim, A.; Schuster, A.; Jaber, W.A.; Cywinski, J.B. Impact and Persistence of Cirrhotic Cardiomyopathy after Liver Transplantation. Clin. Transplant. 2016, 30, 986–993. [Google Scholar] [CrossRef]
- Vetrugno, L.; Cherchi, V.; Zanini, V.; Cotrozzi, S.; Ventin, M.; Terrosu, G.; Baccarani, U.; Bove, T. Association between Preoperative Diastolic Dysfunction and Early Allograft Dysfunction after Orthotopic Liver Transplantation: An Observational Study. Echocardiography 2022, 39, 561–567. [Google Scholar] [CrossRef]
- Enache, I.; Oswald-Mammosser, M.; Woehl-Jaegle, M.-L.; Habersetzer, F.; Di Marco, P.; Charloux, A.; Doutreleau, S. Cirrhotic Cardiomyopathy and Hepatopulmonary Syndrome: Prevalence and Prognosis in a Series of Patients. Respir. Med. 2013, 107, 1030–1036. [Google Scholar] [CrossRef]
- Ershoff, B.D.; Gordin, J.S.; Vorobiof, G.; Elashoff, D.; Steadman, R.H.; Scovotti, J.C.; Wray, C.L. Improving the Prediction of Mortality in the High Model for End-Stage Liver Disease Score Liver Transplant Recipient: A Role for the Left Atrial Volume Index. Transplant. Proc. 2018, 50, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Kwon, A.; Choi, H.J.; Chung, H.S.; Hong, S.H.; Park, C.S.; Choi, J.H.; Chae, M.S. The 2016 ASE/EACVI Recommendations May Be Able to More Accurately Identify Patients at Risk for Diastolic Dysfunction in Living Donor Liver Transplantation. PLoS ONE 2019, 14, e0215603. [Google Scholar] [CrossRef] [PubMed]
- Spann, A.; Coe, C.; Ajayi, T.; Montgomery, G.; Shwetar, M.; Oje, A.; Annis, J.; Slaughter, J.C.; Alexopoulos, S.; Brittain, E.; et al. Cirrhotic Cardiomyopathy: Appraisal of the Original and Revised Criteria in Predicting Posttransplant Cardiac Outcomes. Liver Transpl. 2022, 28, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Kia, L.; Shah, S.J.; Wang, E.; Sharma, D.; Selvaraj, S.; Medina, C.; Cahan, J.; Mahon, H.; Levitsky, J. Role of Pretransplant Echocardiographic Evaluation in Predicting Outcomes Following Liver Transplantation: Echocardiography and Liver Transplant. Am. J. Transplant. 2013, 13, 2395–2401. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Middleton, K.J.; Kopelen, H.A.; Zoghbi, W.A.; Quiñones, M.A. Doppler Tissue Imaging: A Noninvasive Technique for Evaluation of Left Ventricular Relaxation and Estimation of Filling Pressures. J. Am. Coll. Cardiol. 1997, 30, 1527–1533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).