Abstract
(1) Background: The relationship between non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) is bidirectional: NAFLD increases the risk of T2DM, and T2DM promotes the progression of the disease into non-alcoholic steatohepatitis (NASH). (2) Material and methods: We performed a retrospective, open study that included 59 patients with NAFLD and T2DM who were distributed into two groups: 44 (74.57%) patients were diagnosed with hepatic steatosis (HS) and 15 (25.42%) patients were diagnosed with NASH. (3) Results: Among the non-specific inflammatory biomarkers, serum ferritin (SF) and the neutrophil-percentage-to-albumin ratio (NPAR) showed higher and statistically significant mean values (p = 0.003 respectively p = 0.03) in the group of patients with NASH and T2DM. Conclusions: Consequently, it is essential to identify alternative markers for the inflammatory process, particularly in individuals with diabetes, as it is a key characteristic of NASH. This need arises from the desire to avoid the risks associated with liver biopsy procedures (LBP) and to prevent the unpredictable and unfavorable progression of NAFLD in patients with T2DM.
1. Introduction
The term NAFLD dates back more than 40 years and encompasses a broad spectrum of diseases ranging from simple HS with low mortality risk to NASH with progression to cirrhosis and hepatocellular carcinoma (HCC) [1]. Worldwide, NAFLD represents the most common liver disease, with a prevalence of 25%, and is closely related to T2DM and obesity. The association of this disease with T2DM increases the likelihood of developing steatohepatitis, which is characterized by the presence of lobular inflammation and hepatocellular ballooning. This combination poses an increased risk of progression to advanced stages of the disease [2,3]. Current evidence suggests that the overall burden of NAFLD is closely related to the occurrence of extrahepatic complications, such as cardio-metabolic pathologies including diabetes, dyslipidemia, metabolic syndrome (MS), and cardiovascular disease (CVD) which are the leading causes of death among NAFLD patients [4,5,6]. The relationship between T2DM and NAFLD is supported by numerous studies, and there is a complex bidirectional relationship that pejoratively affects the prognosis and outcome of both pathologies [7,8,9]. Patients with NAFLD and diabetes develop advanced forms of the disease, even in the absence of other risk factors for advanced fibrosis such as age, dyslipidemia, hypertension, and an inherited history of NAFLD-associated cirrhosis [10].
The diagnosis of NAFLD is typically confirmed through LBP, an invasive procedure associated with various complications, including bleeding, pain, and abdominal discomfort, which limit its practical utility. Non-invasive techniques such as computed tomography (CT), nuclear magnetic resonance (NMR), and liver ultrasonography, are commonly included in the diagnosis of NAFLD. Among these techniques, liver ultrasonography is the most frequently used method in medical practice due to its simplicity and non-invasive nature. It can detect HS when it affects around 20–30% of hepatocytes [11]. To facilitate the diagnosis of the disease, experts have introduced a range of blood biomarkers into medical practice. Currently, the fatty liver index (FLI), and hepatic steatosis index (HSI) have been validated for diagnosing HS [12]. HS is generally considered to have a benign clinical course while NASH has the potential to contribute to progressive liver fibrosis, eventually leading to cirrhosis, liver failure, and HCC [13].
Thus, it is necessary to distinguish between fatty liver and NASH (inflammation with or without fibrosis). Given the prevalence of HS, a liver biopsy cannot be considered for all patients. Indeed, in a recent study by an Italian group, fatty liver regressed in 50% of all examined cases and had a benign evolution [12,14]. Therefore, there have been efforts to determine predictive markers of fibrosis and/or steatohepatitis to avoid biopsies [15]. For the diagnosis of NASH, the American Gastroenterological Association (AGA) recommends considering an initial stage of biochemical suspicion, characterized by a moderate increase in transaminase levels, reaching up to five times the upper limit of normal [16]. Additionally, elevated levels of gamma-glutamyltransferase (GGT) have been observed in these patients and are associated with advanced fibrosis and higher mortality rates. GGT is included as a biomarker in several non-invasive tests used to assess liver fibrosis, such as FibroTest and Hepascore [17].
Chronic inflammation plays a crucial role in the development of the disease, involving various cell types such as neutrophils, lymphocytes, monocytes, and platelets. The leukocytes are assessed through a range of biological markers that evaluate the inflammatory status, including the neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and systemic inflammatory index (SII) [18]. Platelets also actively contribute to the inflammatory process by facilitating the recruitment of leukocytes and their morphology changes in patients with T2DM and MS. Therefore, mean platelet volume (MPV) and platelet distribution width (PDW) serve as biological markers for platelet activation and are associated with the presence of insulin resistance (IR) and the severity of its complications [19]. Other inflammatory markers used in current medical practice are serum C-reactive protein (CRP), C-reactive protein/albumin ratio (CAR), NPAR, and SF. NPAR is an easily determined biomarker that evaluates systemic inflammation and was initially used to evaluate the prognosis of cancer patients and CVD [20,21]. Recent studies have demonstrated a significant association between the presence of NAFLD and a high value of NPAR, this inflammatory marker having a great potential to be used in evaluating the prognosis of the disease [20,22,23].
Another widely studied biomarker for the diagnosis and prognosis of NAFLD is the level of SF, which is known as both an acute phase reactant and a pro-inflammatory cytokine in infectious and non-infectious systemic inflammation. In individuals diagnosed with NAFLD, around 30% of them showed an elevation in their serum ferritin levels, which some researchers have suggested could potentially serve as a diagnostic marker for inflammation [24,25]. A recent investigation indicated that serum ferritin might serve as a non-invasive biomarker to assess the progression stage of NAFLD, although more research is necessary for confirmation [26]. Furthermore, serum ferritin levels were examined in patients with both T2DM and NAFLD, revealing significantly higher values. The prevalence of NAFLD in T2DM patients exhibited a gradual increase corresponding to the rise in serum ferritin levels. The study’s authors concluded that serum ferritin is an independent risk factor for liver disease in individuals with T2DM [27].
The present study aimed to determine which biological markers (“surrogate” biomarkers), other than aminotransferases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), differentiate the two forms of liver disease in NAFLD (HS from NASH), with emphasis on inflammatory factors.
2. Materials and Methods
2.1. Selection of Patients
We conducted a retrospective, open study at the Diabetes and Nutritional Diseases Clinic of the Filantropia Municipal Clinical Hospital (Craiova, Romania) during 2020–2023. Initially, 120 patients with T2DM and chronic liver disease were included in the study, of which, after applying the exclusion criteria, 59 patients were subsequently diagnosed, based on the specified inclusion criteria, with NAFLD and T2DM. The medical data of these patients were recorded and analyzed by us in this study (Figure 1).
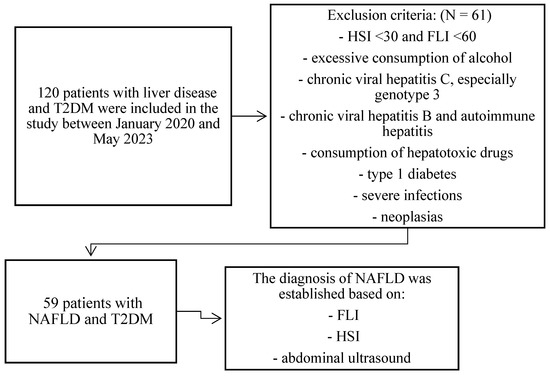
Figure 1.
Study Flow Chart depicts the patient selection process for our study. Abbreviations: T2DM: type 2 diabetes mellitus; HSI: hepatic steatosis index; FLI: fatty liver index; NAFLD: non-alcoholic fatty liver disease.
In order to facilitate the comparative analysis of the outcomes, we divided the patients into two groups: one comprising 44 individuals diagnosed with HS, and the other consisting of 15 patients diagnosed with NASH. The structure of the patient groups under study was determined according to specific criteria. To be included in the study, patients needed to meet certain inclusion criteria, such as having a diagnosis of T2DM, exhibiting ultrasonographic evidence of HS, and demonstrating elevated values of the two diagnostic indicators, namely HSI above 30 and FLI above 60 [28].
The diagnosis of T2DM was established on diabetes symptoms (polyuria, polydipsia, and polyphagia) in combination with a random venous plasma glucose concentration ≥ 11.1 mmol/L (200 mg/dL), a fasting plasma glucose concentration ≥ 7.0 mmol/L (126 mg/dL), two-hour plasma glucose concentration ≥ 11.1 mmol/L (200 mg/dL) two hours after an oral glucose tolerance test (OGTT) with 75 g anhydrous glucose, glycated hemoglobin (HbA1c) greater than 6.4% or ongoing therapy for T2DM. The diagnosis of NAFLD was established based on abdominal ultrasound (AU), FLI, and HSI values [28]. AU was performed by specialist doctors in module B, which allowed the subjective estimation of steatosis. HS was defined according to the criteria of the AGA, such as the increase of hepatic echogenicity compared to renal echogenicity, the presence of intensity as well as the lack of differentiation of periportal and vascular wall intensity as a result of hepatic hyperechogenicity and eventually the presence of hepatomegaly. According to EASL, AU is the first screening imaging investigation performed in patients with NAFLD due to its profitability; however, it does have several limitations [29]. AU exhibits a sensitivity of 90% for detecting liver fat content above 30%, but this sensitivity decreases as the liver fat content decreases as well [30].
FLI was calculated based on the formula:
where y = 0.953 × TG (mg/dL) + 0.139 × BMI (kg/m2) + 0.718 × GGT(U/L) + 0.053 × WC (cm) −15,745. In this formula TG stands for triglycerides (mg/dL), GGT for gamma-glutamyltransferase (U/L), and WC represents the waist circumference (cm) [31].
ey/(1 + ey) × 100
FLI ranges from 0 to 100. In a study conducted by Bedogni et al., a diagnosis of NAFLD was confirmed when the FLI value reached or exceeded 60, while a value below 30 excluded the diagnosis, demonstrating excellent diagnostic accuracy [32].
HSI serves as a screening tool to optimize the management of NAFLD. HSI is calculated based on the formula:
where 2 points more will be added if the patient has diabetes and 2 points more if the individual is a female [33].
8 × ALT/AST + BMI,
The validation of HSI enables clinicians to effectively identify patients who require dietary and lifestyle modifications, as well as those who may need further assessment through AU. A value of HIS < 30 excludes the diagnosis of NAFLD (negative probability of 0.186), while a value > 36 supports the diagnosis. The AUROC value is 0.182 (95% CI, 0.801–0.824) and the sensitivity of AU for a value below 30 was 93.1%, effectively excluding the diagnosis, while a value above 36 demonstrated a specificity of 92.4% to confirm the diagnosis [34]. The diagnostic criteria used to distinguish HS from NASH in diabetic patients with NAFLD were increased values of ALT and AST, with values for ALT > 55 U/L and for AST > 34 U/L.
The exclusion criteria were excessive alcohol consumption (for men more than 30 mL/day and women more than 20 mL/day), the presence of chronic viral hepatitis C, especially genotype 3, chronic viral hepatitis B, autoimmune hepatitis, the utilization of medications known for their hepatotoxic properties (amiodarone, methotrexate, glucocorticoids), type 1 diabetes, severe infections, neoplasias, and patients with HSI < 30 and FLI < 60.
2.2. Characterization of the Studied Variables
2.2.1. Clinical Parameters
Detailed clinical data were collected from all patients included in the study. The anthropometric data obtained from the patients included measurements of height and body weight, which were utilized to evaluate or calculate the body mass index (BMI) and waist circumference (WC). The BMI was calculated based on the formula weight/height2. Based on the resulting value, the degree of obesity was determined. Patients with a BMI between 24 and 29.9 kg/m2 were classified as overweight, while those with a BMI exceeding 30 kg/m2 were categorized as having obesity. WC was measured halfway between the lowest rib and the iliac crest to assess the degree of abdominal adiposity. In women, values greater than 80 cm were associated with abdominal adiposity, while men with abdominal adiposity presented values greater than 94 cm. Comorbidities such as hypertension (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mm Hg, or those on treatment) and dyslipidemia (serum triglyceride concentration ≥ 150 mg/dL or fasting HDL-C < 40 mg/dL in men and <50 mg/dL in women, or patients on treatment) were also recorded. Based on these comorbidities, the diagnosis of MS was established according to the already known diagnostic criteria.
2.2.2. Laboratory Parameters
Measurements of hematological (complete blood count), biochemical parameters (glycemia, total cholesterol, triglycerides, albumin), liver tissue function (ALT, AST, GGT), and inflammatory parameters (erythrocyte sedimentation rate (ESR), CRP, fibrinogen (Fg)), and SF were performed by standard laboratory methods in the Clinical Laboratory of the “Filantropia” Municipal Clinical Hospital, Craiova, with the help of analyzers using the Mindray BC Auto Hematology and Architect Clinical Chemistry Systems.
In order to assess the subclinical systemic inflammation commonly observed in patients with NASH, we conducted a comprehensive analysis of various biological markers, apart from traditional inflammatory markers like white blood cell count (WBC), ESR, CRP, Fg, SF levels, and other hematological markers and biochemical parameters described in the existing literature to evaluate the inflammatory status.
In this context, we calculated the ratio neutrophils%/lymphocytes% (NLR), the ratio monocytes%/lymphocytes% (MLR), the systemic inflammatory index (SII) based on the formula: neutrophils (/mm3) × platelets (/mm3)/ lymphocytes (no /mm3), the CAR as well as the NPAR, which was calculated based on the formula neutrophil count (%) × 100/albumin (g/dL). We also used MPV and PDW, which are closely correlated with IR, considering that platelets are active participants in the liver inflammatory process, as a result of their role in recruiting leukocytes through the liver sinusoids.
2.3. Statistical Analysis
Patient data were initially recorded using Microsoft Excel software, with subsequent data processing conducted using the free statistical and graphical analysis software R-Studio. Descriptive statistics were computed using both Excel and R-Studio and normality testing was performed through the Shapiro–Wilk and Kolmogorov–Smirnov tests. Spearman’s method was employed for correlation and covariance analysis, while the comparison of group differences utilized the Student’s t-test and Mann–Whitney U-test. The interpretation of statistical test results adhered to the following criteria: p < 0.05 indicated a statistically significant difference, p < 0.01 denoted a highly significant difference, and p < 0.001 signified an exceptionally high level of statistical significance.
2.4. Ethical Considerations
The present study was carried out after obtaining the approval of the Scientific Academic Ethics and Deontology Committee of the University of Medicine and Pharmacy in Craiova, number 94/27 April 2023. Each patient signed an informed consent form for inclusion in the study.
3. Results
3.1. Descriptive Analysis of Anthropometric, Clinical, and Ultrasonographic Parameters
The study population comprised 59 individuals diagnosed with both NAFLD and T2DM, with 44 patients (74.57%) having HS and T2DM, while 15 patients (25.42%) had NASH and T2DM. Demographic and epidemiological studies followed the distribution of the studied patients and highlighted the main risk factors that were associated with NAFLD (Table 1).

Table 1.
The incidence and the mean value of the demographic and diagnostic parameters of patients with HS (44 patients) and NASH (15 patients) with T2DM, and the p-value of the comparative analysis of the two groups studied.
Among the patients, there were 25 women (42.37%) and 34 men (57.62%). In both the HS and T2DM subgroup (52.27%) and the NASH and T2DM subgroup (73.33%), men constituted the majority. The mean age of patients with NAFLD was 61.52 ± 10.07 years, with small differences between the values of the mean age of patients with SH and T2DM (62.20 ± 0.70 years) compared to those with NASH and T2DM (59.53 ± 10.06 years). Regarding the patients’ geographical background, 36 (61.01%) patients came from urban areas, while 23 (38.98%) patients were from rural areas. In the studied patients, T2DM presented an equal incidence in both groups of patients. This equality was due to the inclusion criteria of the study, which required patients to have diabetes mellitus. Patients with HS and DM2 presented a higher incidence of obesity (93.18% vs. 86.66%), dyslipidemia (81.81% vs. 86.66%), and MS (95.45% vs. 93.33%), while patients with NASH and T2DM showed a higher incidence of hypertension (93.33% vs. 88.63%).
In these patients, the diagnosis of NAFLD was established based on AU and the calculation of the FLI and HSI diagnostic scores values. The imaging study showed that hyperechoic appearance with inhomogeneous structure was the sonographic appearance encountered in all selected patients. The sagittal diameter of the left lobe recorded a mean value of 7.54 ± 1.14 cm in patients with NAFLD. NASH patients presented a higher mean value (8 ± 1.37 cm) than HS patients (7.38 ± 1.83 cm). The longitudinal diameter of the right liver lobe, measured on the midclavicular line, showed a mean value of 14.3 ± 1.95 cm in patients with NAFLD. Patients with NASH presented a higher mean value of this diameter (15.02 ± 1.48 cm) as compared to patients with HS (14.05 ± 2.03 cm). The diameter of the portal vein assessed by ultrasound exhibited a mean value of 1.084 ± 0.108 cm in patients with NAFLD. This value was higher in patients with NASH (1.12 ± 0.104 cm) as compared to patients with HS (1.072 ± 0.07 cm). The longitudinal diameter of the spleen measured by echography registered a mean value of 10.35 ± 1.09 cm, a higher mean value being observed in patients with NASH (10.7 ± 1.22 cm) compared to patients with HS (10.21 ± 1 cm). Patients with NAFLD recorded a mean value of FLI of 77.76 ± 22.42, while patients with NASH (88.93 ± 10.59) presented a statistically significant higher mean value (p = 0.04), compared to patients with HS (74.40 ± 22.62). The mean value of HSI in patients with NAFLD was 46.58 ± 8.71, with patients with NASH (50.39 ± 10.17) showing a statistically significant higher mean value (p = 0.05), compared to patients with HS (45.31 ± 7.91).
3.2. Descriptive Analysis of the Biochemical Parameters
The biochemical study analyzed the main metabolic and enzymatic parameters used to diagnose HS and NASH in patients with T2DM (Table 2).

Table 2.
The mean values of the metabolic and enzymatic parameters found in patients with HS (44 patients) and NASH (15 patients) with T2DM as well as the values of the statistical test of the comparative analysis of the two studied groups.
“Fasting” blood glucose showed a higher mean value in patients with NASH and T2DM (234.33 ± 89.56 g/dL) compared to patients with HS and T2DM (216.11 ± 107.59 g/dL). Regarding the mean value of HbA1c, patients with NASH and T2DM presented higher mean values (8.57 ± 1.67%), compared to the group of patients with HS and T2DM (8.26 ± 2.74%).
Cholesterolemia showed higher mean values in patients with NASH and T2DM (235.73 ± 145.13 mg/dL) compared to patients with HS and T2DM (193.59 ± 16.26 mg/dL).
Triglyceridaemia also exhibited statistically significant (p = 0.05) higher mean values in patients with NASH and T2DM (384.26 ± 45.90 mg/dL) compared to patients with HS and T2DM (232.79 ± 14.14 mg/dL) (Figure 2).
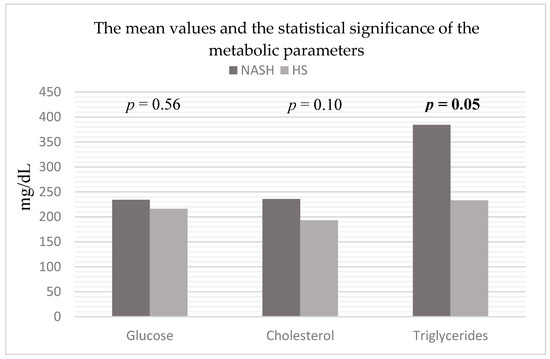
Figure 2.
Mean values and statistical significance of metabolic parameters in the studied patients (44 patients with HS and 15 patients with NASH associated with T2DM). Abbreviations: NASH: non-alcoholic steatohepatitis, HS: hepatic steatosis; p-value was established by comparing the values of patients with HS and T2DM and the values of patients with NASH and T2DM.
The enzymes of the liver cytolysis syndrome (ALT and AST) recorded statistically significant higher mean values (p = 0.0001 respectively p = 0.005) in patients with NASH and T2DM (103.4 ± 54.26 U/L and 56.26 ± 32.80 U/L) compared to the values in patients with HS and T2DM (23.61 ± 9.19 U/L and 18.18 ± 5.11 U/L). Among the enzymes of hepatic cholestasis syndrome, GGT recorded statistically significant higher values (p = 0.002) in patients with NASH and T2DM (99.33 ± 70.17 U/L) in comparison to patients with HS and T2DM (30.15 ± 21.21 U/L) (Figure 3).
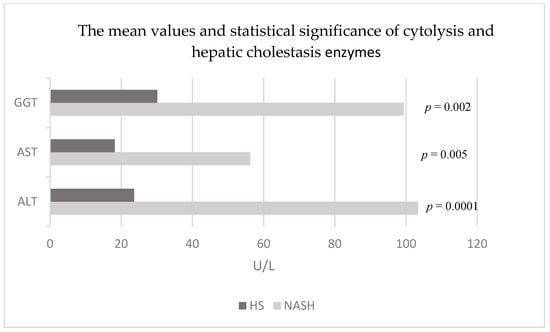
Figure 3.
Mean values and statistical significance of cytolysis and hepatic cholestasis enzymes in the studied patients (44 patients with HS and 15 patients with NASH associated with T2DM). Abbreviations: NASH: non-alcoholic steatohepatitis; HS: hepatic steatosis; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyltranspeptidase; established by comparing the values of patients with HS and T2DM and the values of patients with NASH and T2DM.
Regarding the liver synthesis function, the mean value of albumin was lower in patients with NASH and T2DM (4.42 ± 0.35 g/dL) compared to the group of patients with HS and T2DM (4.66 ± 0.44 g/dL).
3.3. Descriptive Analysis of the Hematological Parameters
The hematological study analyzed the behavior of the hematological parameters in the studied patients (Table 3).

Table 3.
The mean values of the hematological parameters found in patients with HS (44 patients) and NASH (15 patients) with T2DM as well as the value of the statistical test of the comparative analysis of the two studied groups.
Hb recorded a statistically significant lower mean value (p = 0.03) in patients with HS and T2DM (13.09 ± 1.64 g/dL) in comparison with patients with NASH and T2DM (14.1 ± 1.27 g/dL). Regarding the leukocyte series, the patients with HS and T2DM showed higher mean WBC values (8066 ± 1945/mmc vs. 7415 ± 1635/mmc) compared to patients with NASH and T2DM. The behavior of leukocyte subpopulations was different in the two groups of patients. Thus, the percentage of neutrophils (NP) registered a slightly higher but statistically insignificantly increased mean value in patients with HS and T2DM (59.67 ± 0.94%) compared to patients with NASH and T2DM (58.46 ± 6.13%), while the mean value of the percentage of lymphocytes (LP) was higher in NASH and T2DM patients (30.65 ± 6.96%) in comparison to the mean value in HS and T2DM patients (28.65 ± 4.10%). Moreover, the mean value of the percentage of monocytes (MP) was higher in patients with NASH and T2DM (6.72 ± 1.47%) compared to the mean percentage value recorded in patients with HS and T2DM (5.99 ± 1.72%).
Inflammatory biomarkers, NLR and MLR, showed higher mean values in patients with NASH and T2DM (2.53 ± 0.80 respectively 0.23 ± 0.07) compared to patients with HS and T2DM (2.31 ± 0.97 respectively 0.21 ± 0.07). In the case of platelets, patients with HS and T2DM presented a higher mean value compared to patients with NASH and T2DM (251,992 ± 91,923/mmc vs. 226,666 ± 77,524/mmc). Regarding platelet indices, the mean value of PDW in patients with HS and T2DM (41.98 ± 12.62%) was higher than in patients with NASH and T2DM (38.87 ± 18.36%). The same behavior was found in the case of the MPV, which presented a higher value in patients with HS and T2DM (9.92 ± 0.92 fL) compared to patients with NASH and T2DM (9.68 ± 1.28 fL).
3.4. Descriptive Analysis of the Inflammatory Parameters
The study of systemic inflammation parameters that evaluate the subclinical inflammatory process from T2DM, allowed us to identify the parameters that are associated with inflammation in NASH, apart from ALT and AST (Table 4).

Table 4.
The mean values of the inflammatory parameters found in patients with HS (44 patients) and NASH (15 patients) with T2DM as well as the values of the statistical test of the comparative analysis of the two studied groups.
The mean value of the ESR in one hour (ESR/1 h) was higher in patients with NASH and T2DM (41 ± 25.29 mm/1 h) compared to that recorded in HS and T2DM patients (37.54 ± 26.16 mm/1 h). Additionally, the CRP recorded a higher mean value in patients with NASH and T2DM (1.44 ± 2.87 mg/dL) compared to the values found in those with HS and T2DM (0.60 ± 0.21 mg/dL). The same behavior was found in the case of Fg, which showed a higher mean value in patients with NASH and T2DM (391.4 ± 111.27 mg/dL) compared to patients with HS and T2DM (351.76 ± 195.16 mg/dL).
Moreover, there was a statistically significant difference (p = 0.003) in the mean SF levels between patients with NASH and T2DM (266.26 ± 96.74 ng/mL) compared to patients with HS and T2DM (186.70 ± 86.26 ng/mL) (Figure 4).
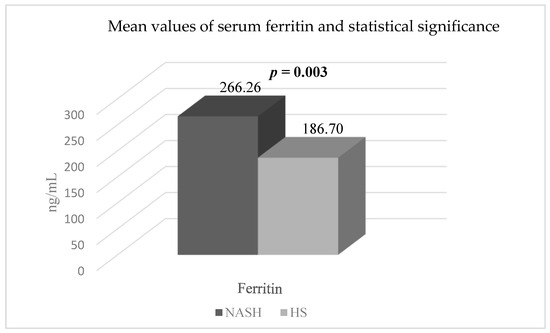
Figure 4.
The mean values and statistical significance of serum ferritin in the two groups of patients (44 patients with HS and T2DM and 15 patients with NASH and T2DM). Abbreviations: NASH: non-alcoholic steatohepatitis; HS: hepatic steatosis; p-value was established by comparing the values of patients with HS and T2DM and the values of patients with NASH and T2DM.
The mean value of the SII was higher in patients with NASH and T2DM (520.97 ± 238.10) compared to patients with HS and T2DM (476.20 ± 36.75). The mean CAR ratio in patients with NASH and T2DM (0.40 ± 0.67) was slightly higher compared to patients with HS and T2DM (0.38 ± 1.77), although this difference was not statistically significant.
Furthermore, the mean value of the NPAR exhibited a statistically significant increase (p = 0.03) in patients with NASH and T2DM (1589.6 ± 713.15) when compared to patients with HS and T2DM (1317.79 ± 235.88) (Figure 5).
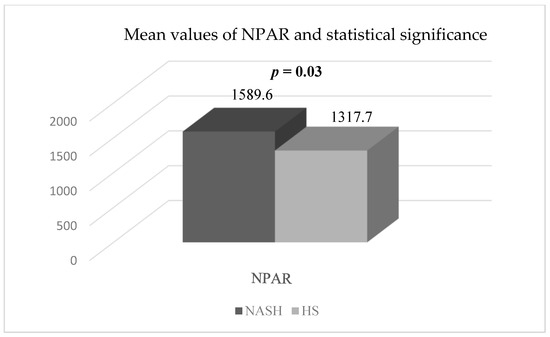
Figure 5.
The mean values and the statistical significance of NPAR in the two groups studied (44 patients with HS and T2DM and 15 patients with NASH and T2DM). Abbreviations: NPAR: neutrophil percentage to albumin ratio; NASH: non-alcoholic steatohepatitis; HS: hepatic steatosis; p-value was established by comparing the values of patients with HS and T2DM and the values of patients with NASH and T2DM.
4. Discussion
Considering the different evolutions of the two pathological entities that define NAFLD, HS, and NASH, as well as the current limited diagnostic methods that differentiate the two forms, through the present study we aimed to identify a series of non-invasive biomarkers that differentiate the two forms. Following the statistical analyses, based on the obtained data, we identified a series of differences between the two groups of patients.
In both groups of patients, the male gender was predominant; in the group of patients with HS and T2DM, the incidence was 52.27%, while in the group of patients with NASH and T2DM, the incidence was higher, 73.33%. NAFLD is known to be a pathology with a higher incidence in men, at least until the woman’s menopause. After the onset of menopause, the incidence is comparable. The same higher incidence for the male gender, but lower compared to our study (51.8%), was present in a Chinese study that investigated the factors of progression to fibrosis in patients with NAFLD and T2DM [35]. Regarding the mean age of the patients, it was higher in patients with HS and T2DM compared to the group of patients with NASH and T2DM. Age is acknowledged to be a factor that contributes to the progression of the disease, an aspect that was also present in our study. Moreover, in the study by Lamonaco et al., it was observed that the mean age of patients with HS and T2DM was higher compared to the mean age of patients with NASH and T2DM [36]. Both patients with HS and those with NASH and T2DM mostly came from urban environments. The incidence of urban patients in the literature is lower (34.1%) compared to our study [37].
The main risk factors of the progression of NAFLD to NASH and subsequently to advanced stages of the disease are represented by MS components. In our study, the inclusion criteria required the presence of diabetes, resulting in a 100% incidence of diabetes in both patient groups. Regarding the comorbidities of the patients included in the study, patients with NASH and T2DM had a higher incidence of arterial hypertension (93.33%), while patients with HS and T2DM presented a higher incidence of obesity (93.18%), dyslipidemia (81.81%), and MS (94.45%). The incidence of arterial hypertension in the study by Bazick et al. [38] was lower (40.7%) compared to our study, while Chen’s study only reported a higher incidence of dyslipidemia (84.44%) in patients with HS and T2DM, the incidence of obesity and MS being lower (68.89% respectively 63.63%) compared to the results of our study [39].
The analysis of carbohydrate metabolism parameters revealed a higher value of both blood glucose and HbA1c in patients with NASH and T2DM compared to the group of patients with HS and T2DM. Currently, the risk factors associated with the development of fibrosis in patients with NAFLD and T2DM are not fully known. To emphasize these factors and their prevalence in patients with T2DM, Julian’s study, which included 930 patients with NAFLD and T2DM, showed lower mean blood glucose values in patients with HS and T2DM (148 ± 45 mg/dL) compared to patients with NASH and T2DM (151 ± 50 mg/dL). Regarding the mean value of HbA1c, patients with HS (7.0 ± 1.2%) had lower values in comparison to patients with NASH (7.1 ± 1.3%), without statistically significant differences [40].
The analysis of lipid metabolism parameters demonstrated the presence of dyslipidemia with statistically substantially higher values (in the case of triglycerides) in patients with NASH and T2DM. The mechanisms of dyslipidemia are related to the appearance of hepatic and peripheral insulin resistance associated with disorders of hepatic glucose metabolism and lipoproteins [41]. In our study, both the mean value of cholesterol and triglycerides were higher and statistically significant in the case of triglycerides (p = 0.05) in patients with NASH and T2DM. The mean values of the two components of lipid metabolism were documented in a study made up of 204 patients with NAFLD and T2DM that examined the prevalence of risk factors in these patients. Both the mean values of cholesterol and triglycerides were higher but statistically insignificant in patients with NASH and T2DM (190.56 ± 44.02 mg/dL respectively 173.51 ± 68.84 md/dL) compared to patients with HS and T2DM (183.97 ± 48.84 md/dL respectively 147.37 ± 74.86 mg/dL) [42]. The analysis of protein metabolism demonstrated a lower mean value of albumin in patients with NASH and T2DM compared to patients with HS and T2DM. Albumin levels can fall in patients with inflammatory disorders and other conditions. This may be due to downregulated production of albumin mRNA by the liver, leading to reduced synthesis, increased albumin catabolism, and increased vascular permeability [43]. In a study made up of 1249 patients that explored the determinants of fibrosis in patients with NAFLD and T2DM, the mean value of albumin was also lower in patients with NASH and T2DM (4.15 ± 0.41 g/dL) compared to patients with HS and T2DM (4.21 ± 0.43 g/dL) [37].
The analysis of the enzymatic parameters of the liver functions highlighted notably higher mean values of liver cytolysis enzymes, ALT and AST (p = 0.0001 respectively p = 0.005), in patients with NASH and T2DM, compared to patients with HS and T2DM. Most patients with NAFLD are asymptomatic and are typically identified when abnormal liver studies are noted on routine laboratory assessment. In particular, the liver enzymes, ALT, and AST levels are elevated. However, these enzymes may not be elevated in all cases of NAFLD, and the level of aminotransferases may not reliably predict the extent of inflammation and cirrhosis [44]. In a study by Mandal et al., an independent association between liver enzymes, particularly ALT, and NAFLD in a population of individuals with T2DM was observed [45]. Mandal et al. also found that 40.4% of the diabetic population had elevated ALT levels, while increases in AST and ALP levels were observed in only 17% and 16% of diabetic individuals, respectively [46]. Similar findings were reported by Shrestha and Bhatt in Nepal, where they also observed a statistically significant elevation in ALT levels among diabetic patients compared to the control groups [47]. Furthermore, a higher prevalence of abnormalities in liver function tests has been linked to individuals with T2DM compared to those without T2DM [48]. Moreover, the enzyme of hepatic cholestasis syndrome, GGT, presented a higher and statistically significant mean value (p = 0.002) in patients with NASH and T2DM compared to patients with HS and T2DM. GGT plays a crucial role in the breakdown of glutathione, facilitating the transfer of the glutamyl component and preserving intracellular balance against oxidative stress [48]. Previous research has established connections between serum GGT levels and various chronic conditions, including NAFLD, cardiovascular diseases (CVD), diabetes, and MS [49]. The mechanisms underlying elevated GGT levels in NAFLD patients involve oxidative stress, inflammatory response, and excessive hepatic fat accumulation, all of which contribute to impaired insulin signaling and IR [50].
The analysis of the mean values of the hematological parameters highlighted a statistically considerably higher mean Hb value (p = 0.03) in patients with NASH and T2DM. In the literature, the mean value of Hb in a study that investigated the association between the glycated albumin (GA) and glycated hemoglobin (GA/HbA1c) ratio and the grade of NAFLD in patients with NAFLD and T2DM, who were ultrasonographical evaluated, was also statistically significantly lower (p < 0.001) in patients with HS and T2DM (12.5 ± 2.2 g/dL) compared to patients with NASH and T2DM (13.8 ± 1.6 g/dL) [51].
The mean value of the parameters of the leukocyte series was different in the two groups of patients, without statistically significant differences being evident. Thus, the mean value of WBC was higher in patients with HS and T2DM compared to patients with NASH and T2DM. In Bazick’s study, which investigated the determinants of fibrosis in 1249 patients with NAFLD, of which 435 (34.8%) had been diagnosed with diabetes, the mean value of leukocytes in patients with HS and T2DM (7180 ± 230 mm3) was statistically insignificant lower compared to the group of patients with NASH and T2DM (7230 ± 210 mm3) [38]. Regarding the NP, their mean value was higher in patients with HS and T2DM compared to patients with NASH and T2DM. Instead, the mean value of the LP was lower in patients with HS and T2DM compared to patients with NASH and T2DM. The mean values of NP and LP have been studied by many authors, as well as the relationship between the value of the NLR ratio and the severity of the degree of steatosis in patients with NAFLD and T2DM. The mean value of the NP in Kahraman’s study, which included 143 patients with NAFLD and T2DM, evaluated by liver ultrasonography, reported a lower value in patients with NAFLD and mild fatty liver (59.2 ± 8.2%) compared to patients with NAFLD and severe fattening (68.6 ± 7.9%). The mean LP varied significantly, with patients diagnosed with NAFLD and mild fatty liver (30.8 ± 8.6%) exhibiting a higher mean value compared to patients with NAFLD and severe fatty liver (22.3 ± 6.4%) [52].
The mean value of the inflammatory indices derived from the leukocyte series in our study was lower in patients with HS and T2DM compared to patients with NASH and T2DM. NLR is known as an easily obtained blood biomarker that evaluates both the inflammatory state and the severity of liver fibrosis in patients with NAFLD. This aspect was suggested in Kahraman’s study, which concluded that NLR increased with the degree of obesity in patients with T2DM [52]. To determine the severity of fibrosis in patients with NAFLD and T2DM, a Chinese study investigated the association between NLR, NPAR, and the presence of NAFLD or advanced liver fibrosis in a group of 3991 patients with T2DM, of which 1355 (33.95%) had NAFLD and T2DM, while 162 (4.05%) patients had T2DM and advanced liver fibrosis. In this study, it was concluded that the NLR is an easily measurable inflammatory marker, that correlated significantly and positively with the degree of steatosis and fibrosis [20]. Another blood biomarker used to assess the inflammatory state in patients with NAFLD is MLR, which has not yet been studied in patients with T2DM. It has been used as a blood biomarker in several studies in patients with different degrees of steatosis. In this regard, a recent study that consisted of 81 patients with NAFLD and associated comorbidities, including diabetes and obesity, reported a higher mean value of MLR in patients with mild steatosis (0.25) compared to patients with severe steatosis (0.19), results that differ compared to our study [53]. The platelets count and their characteristics were also investigated as another hematological parameter in the study. In our study, the average value of the PLT was higher in patients with HS and T2DM compared to the group of patients with NASH and T2DM. The role of platelets in the inflammatory process in NAFLD is well known, as they are included in numerous scores that evaluate the process of non-invasive fibrosis. Thus, a study that included 565 patients with NAFLD and diabetes, and aimed to highlight the factors that are associated with liver fibrosis in these patients, showed a statistically significant (p < 0.01) higher average value of platelets in NAFLD without liver fibrosis patients (a mean value of 226.000/mmc, with limits of 211.000–279.000/mm3) compared to those with NAFLD and liver fibrosis (a mean value of 201.000/mmc, with limits of 173.500–238.000/mmc) [54]. Related to the characteristics of platelets, in our study, the average value of PDW was higher in the group of patients with HS and T2DM, compared to the group of patients with NASH and T2DM. The same behavior was found in the case of MPV, where the average value in the group of patients with HS and T2DM was higher compared to the group of patients with NASH and T2DM. To demonstrate the diagnostic accuracy of platelets and platelet indices, a study by Alempijevic et al. consisting of 98 patients with NAFLD and associated risk factors (diabetes, MS), divided according to the presence or absence of liver fibrosis proven using non-invasive tests, showed a statistically considerably (p < 0.01) higher average PDW distribution in patients with fibrosis (16.91 ± 0.35) compared to patients without fibrosis (16.55 ± 0.77). In another study, a different behavior was found in the case of MPV, where the average value in patients with liver fibrosis (9.21 ± 1.05 fL) was higher but statistically insignificant compared to the group of patients without liver fibrosis (9.04 ± 1.46 fL) [19].
In our study, we observed that patients diagnosed with both NASH and T2DM had higher levels of all inflammatory markers compared to those with HS and T2DM. Particularly noteworthy was the mean NPAR, which exhibited significantly elevated values (p = 0.03) in patients with NASH and T2DM, in contrast to patients with HS and T2DM. Chi-Feng Liu et al. also conducted a study that revealed significant connections between the NPAR, NLR, and the presence of NAFLD or advanced liver fibrosis. They found that incremental increases in both the NLR and NPAR were linked to a higher risk of developing NAFLD. Furthermore, among individuals with T2DM, a notable and independent correlation existed between higher NPAR levels and the likelihood of having NAFLD. Based on the findings, the NPAR demonstrates promise as a reliable predictive biomarker for NAFLD. As far as we know, this is the only study besides ours that has looked at the association between NPAR and NAFLD [20]. The NPAR has also been employed as a predictive tool for systemic inflammation in various medical conditions, including unresectable pancreatic cancer [55]. Furthermore, NPAR has been linked to all-cause mortality in critically ill patients with acute kidney injury [56] and septic shock [57]. It is well known that chronic inflammation plays a crucial role in the progression of NAFLD, which encompasses a spectrum ranging from simple steatosis to NASH, advanced liver fibrosis, cirrhosis, and ultimately end-stage liver disease and hepatocellular carcinoma [58]. Consequently, NAFLD represents a significant contributor to chronic and progressive liver damage, emphasizing the importance of noninvasive diagnostic and monitoring methods for progressive liver disease. The findings of Chi-Feng Liu et al. indicate that an elevated NPAR level is significantly linked to an increased risk of NAFLD, surpassing the effectiveness of albumin, neutrophil percentage, and NLR as biomarkers for predicting NAFLD [20]. Additionally, the relationship between the NPAR and NAFLD was influenced by the presence of T2DM, a recognized risk factor for NAFLD development and progression [59]. Furthermore, T2DM itself is associated with systemic inflammation [60]. Hence, it is plausible that the presence of concurrent T2DM might conceal the correlation between the NPAR and NAFLD [20].
To date, not all factors participating in the progression of NAFLD are known. Therefore, the anticipation of clinical outcomes represents a huge challenge, considering the need for identifying serum biomarkers that facilitate the diagnosis and prognosis of the disease. In this sense, a recent study by Cocuranu et al., who evaluated the relationship between NAFLD, NPAR, and NLR in a group of 115 patients diagnosed with NAFLD by means of CT, demonstrated that patients with HS seen in CT had associated higher and statistically significant values regarding the values of serum activities of GGT, triglycerides, albumin, and NPAR (p = 0.001), compared to patients without steatosis. [61].
In our study, we also observed a statistically significant rise (p = 0.003) in the average SF level among patients diagnosed with NASH and T2DM compared with patients with HS and T2DM. Previous studies have revealed that the level of SF, which is the main storage form of iron in the body, is correlated with T2DM and NAFLD [62,63,64]. A study by Yan et al. revealed that individuals with T2DM and NAFLD displayed notably higher levels of SF when compared to T2DM patients without NAFLD. Moreover, as SF levels increased, the proportions of NAFLD cases also showed a significant rise. Thus, SF was identified as an independent risk factor for NAFLD among patients with T2DM [27]. Research has indicated that elevated levels of SF without corresponding high transferrin saturation can serve as an indicator of glucose or lipid metabolism dysfunction [65]. Increased iron levels may contribute to the development of diabetes by triggering oxidative stress, inflammation, IR, insulin insufficiency, liver impairment, and other factors [66]. Conversely, diabetes itself can disrupt the balance of iron metabolism and lead to iron overload, thereby establishing a detrimental cycle [67]. IR stands as a prominent characteristic of NAFLD [68]. Under conditions of IR, the β-oxidation process of free fatty acids becomes impeded, which subsequently fosters the accumulation of fat within the liver [69] Mayneris-Perxachs et al. also documented a positive association between SF levels and the extent of hepatic fat accumulation [70].
Recently, studies have also demonstrated that the serum level of ferritin may be involved in the occurrence of liver disease, playing the role of an inflammatory cytokine, but the source of the high level remains unknown. The study by Ryan et al. considers that the iron stored in the liver is the main source of SF that is associated with a negative prognosis of NAFLD [71]. A Chinese study studied the correlations of SF levels in patients with NAFLD and T2DM compared to patients without NAFLD to demonstrate whether SF levels can be used as a biological marker for disease diagnosis. Thus, the results of the study demonstrated that the serum level of ferritin was higher in patients with NAFLD and T2DM compared to non-NAFLD diabetic patients and was also associated with an advanced stage of the disease, the highest values being found in patients with F4 fibrosis. Moreover, the authors demonstrated that the SF level was an independent risk factor for the progression of NAFLD in patients with diabetes [27]. Another study, that investigated the relationship between the severity of steatosis and the serum level of ferritin in overweight diabetic patients, under treatment with Metformin and Sulfonylurea, showed that HS was positively correlated with an increased level of ferritin, but without correlation with transferrin. In conclusion, the study demonstrated that the evolution of steatosis correlates directly proportionally with the serum level of ferritin, but other studies are needed to evaluate this relationship [72].
Furthermore, we assessed the clinical utility of CRP as a diagnostic tool for NAFLD. CRP displayed a higher mean value in our study in patients with NASH and DM2, although statistically insignificant, compared to patients with HS and DM2. Several studies have suggested a potential association between elevated serum CRP levels and NAFLD [73,74,75]. Since CRP has a short half-life of approximately 18 h, an increase in its serum levels typically indicates its synthesis in response to a pathological process. As a result, CRP is regarded as a valuable non-specific biochemical marker for chronic inflammation [76]. Oruc et al. further validated that elevated circulating CRP levels can serve as an indicative marker for the presence of NAFLD. However, it is important to note that, due to its limited specificity, interpreting CRP values accurately requires consideration of all other clinical and laboratory information. Consequently, it was proposed that serial CRP measurements could prove beneficial in the clinical management and ongoing monitoring of NAFLD patients [77]. Ndumele et al. conducted a study involving 2388 individuals, both diabetic and non-diabetic, without known coronary heart disease, which revealed a significant correlation between HS, as detected by ultrasound, and elevated levels of high-sensitivity C-reactive protein (hs-CRP). HS was associated with higher hs-CRP levels, irrespective of obesity status, presence of MS, or absence of MS. Notably, obesity and MS were independently linked to increased hs-CRP levels, as expected [78]. It is important to mention that the liver plays a crucial role as the main producer of CRP. Previous research suggests that the level of HS and inflammation, as determined by histology, is linked to the systemic levels of inflammatory biomarkers. In a study involving 85 patients, it was observed that higher grades of HS, necroinflammation, and fibrosis in biopsy samples were associated with progressively elevated hs-CRP levels, reaching the “high risk” range [79]. Additional studies have discovered a direct correlation between the severity of NAFLD and the expression of inflammatory mediators in hepatocytes [78,80].
The results of our study provide evidence supporting an independent association between NASH and systemic inflammation in patients with T2DM. Nevertheless, further research is required in the future to explore the link between inflammatory factors and NAFLD in patients with diabetes. This is crucial since the existing studies on this subject are limited in number.
5. Limitations
Our study has certain limitations that should be taken into account. The study design was retrospective, and it was conducted at a single center. The number of patients studied was reduced, because the time period in which they were analyzed overlapped with the COVID-19 pandemic. Understanding these limits, we propose that, in the future, by carrying out prospective, multicenter, longitudinal studies, with a larger population, we will validate the results of this study and identify new non-invasive biological markers, which can later be included in analysis panels with diagnosis and prognosis for NAFLD in diabetic patients.
6. Conclusions
Given the current high rates of NAFLD and T2DM, along with the subclinical inflammatory mechanism contributing to the histological advancement of steatohepatitis in diabetic patients, an urgent necessity arises to identify surrogate biomarkers for the inflammatory process, the key characteristic of NASH in individuals with diabetes. The essential objective is to avoid the risks associated with LBP and prevent the unpredictable and unfavorable progression of NAFLD in T2DM patients. In our study, the inflammatory biomarkers that recorded statistically significantly higher serum values in NASH patients compared to HS patients were SF and NPAR.
Author Contributions
Conceptualization: S.I.S., T.B. and V.B.; Methodology: S.I.S.; Software: T.B.; Validation: V.B., S.I.S., T.B. and S.D.; formal analysis: S.I.S., T.B. and V.B.; investigation: S.I.S., T.B. and V.B.; resources: S.I.S., T.B., A.M., D.C., R.C., A.E.G. and V.B.; data curation: S.I.S. and T.B.; writing—original draft preparation: S.I.S., T.B. and V.B; writing—review and editing: S.I.S., T.B. and V.B.; visualization: S.I.S., T.B., S.D. and V.B.; supervision: V.B.; project administration: S.I.S., T.B., V.B. and S.D.; funding acquisition: S.I.S., T.B., V.B. and S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Scientific Academic Ethics and Deontology Committee of the University of Medicine and Pharmacy in Craiova, number 94/27 April 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kosmalski, M.; Ziółkowska, S.; Czarny, P.; Szemraj, J.; Pietras, T. The Coexistence of Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. J. Clin. Med. 2022, 11, 1375. [Google Scholar] [CrossRef]
- Spiers, J.; Brindley, J.H.; Li, W.; Alazawi, Q. What’s new in non-alcoholic fatty liver disease? Frontline Gastroenterol. 2022, 13, e102–e108. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017, 67, 328–357. [Google Scholar] [CrossRef]
- Loomba, R.; Wong, R.; Fraysse, J.; Shreay, S.; Li, S.; Harrison, S.; Gordon, S.C. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: A real-world analysis of Medicare data. Aliment. Pharmacol. Ther. 2020, 51, 1149–1159. [Google Scholar] [CrossRef]
- Long, M.T.; Zhang, X.; Xu, H.; Liu, C.; Corey, K.E.; Chung, R.T.; Loomba, R.; Benjamin, E.J. Hepatic Fibrosis Associates With Multiple Cardiometabolic Disease Risk Factors: The Framingham Heart Study. Hepatology 2021, 73, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.P.; De La Cerda, I.; Pan, J.; Fallon, M.B.; Beretta, L.; Loomba, R.; Lee, M.; McCormick, J.B.; Fisher-Hoch, S.P. Elevated Glycated Hemoglobin Is Associated With Liver Fibrosis, as Assessed by Elastography, in a Population-Based Study of Mexican Americans. Hepatol. Commun. 2020, 4, 1793–1801. [Google Scholar] [CrossRef]
- Caussy, C.; Aubin, A.; Loomba, R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr. Diabetes Rep. 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Zarghamravanbakhsh, P.; Frenkel, M.; Poretsky, L. Metabolic causes and consequences of nonalcoholic fatty liver disease (NAFLD). Metab. Open 2021, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Soni, M.; Cui, J.; Bettencourt, R.; Schork, N.; Chen, C.-H.; Al Ikhwan, M.; Bassirian, S.; Cepin, S.; Gonzalez, M.P.; et al. Nonalcoholic fatty liver disease with cirrhosis increases the familial risk for advanced fibrosis. J. Clin. Investig. 2017, 127, 2697–2704. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Cholongitas, E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. Curr. Pharm. Des. 2019, 24, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Manousou, P.; Kalambokis, G.; Grillo, F.; Watkins, J.; Xirouchakis, E.; Pleguezuelo, M.; Leandro, G.; Arvaniti, V.; Germani, G.; Patch, D.; et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011, 31, 730–739. [Google Scholar] [CrossRef]
- Bedogni, G.; Miglioli, L.; Masutti, F.; Castiglione, A.; Crocè, L.S.; Tiribelli, C.; Bellentani, S. Incidence and natural course of fatty liver in the general population: The Dionysos study. Hepatology 2007, 46, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; McCullough, A.J.; Feldstein, A.E. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and future. Hepatology 2007, 46, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; George, J. Reply to: Correspondence regarding “A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement”. J. Hepatol. 2020, 73, 1575. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Shubrook, J.H.; Adams, L.A.; Pfotenhauer, K.; Wai-Sun Wong, V.; Wright, E.; Abdelmalek, M.F.; Harrison, S.A.; Loomba, R.; Mantzoros, C.S.; et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2021, 161, 1657–1669. [Google Scholar] [CrossRef]
- Martinou, E.; Pericleous, M.; Stefanova, I.; Kaur, V.; Angelidi, A.M. Diagnostic Modalities of Non-Alcoholic Fatty Liver Disease: From Biochemical Biomarkers to Multi-Omics Non-Invasive Approaches. Diagnostics 2022, 12, 407. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Milovanovic Alempijevic, T.; Stojkovic Lalosevic, M.; Dumic, I.; Jocic, N.; Pavlovic Markovic, A.; Dragasevic, S.; Jovicic, I.; Lukic, S.; Popovic, D.; Milosavljevic, T. Diagnostic Accuracy of Platelet Count and Platelet Indices in Noninvasive Assessment of Fibrosis in Nonalcoholic Fatty Liver Disease Patients. Can. J. Gastroenterol. Hepatol. 2017, 2017, 6070135. [Google Scholar] [CrossRef]
- Liu, C.F.; Chien, L.W. Predictive Role of Neutrophil-Percentage-to-Albumin Ratio (NPAR) in Nonalcoholic Fatty Liver Disease and Advanced Liver Fibrosis in Nondiabetic US Adults: Evidence from NHANES 2017–2018. Nutrients 2023, 15, 1892. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, D.; Ma, H.; Qian, C.; You, H.; Bu, L.; Qu, S. High-Sensitive CRP Correlates With the Severity of Liver Steatosis and Fibrosis in Obese Patients With Metabolic Dysfunction Associated Fatty Liver Disease. Front. Endocrinol. 2022, 13, 848937. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-T.; Kuo, R.; Sun, J.-Y.; Hung, T.-C.; Chang, S.-C.; Liu, C.-C.; Yun, C.-H.; Wu, T.-H.; Hung, C.-L.; Yeh, H.-I.; et al. Associations between CT-determined visceral fat burden, hepatic steatosis, circulating white blood cell counts and neutrophil-to-lymphocyte ratio. PLoS ONE 2018, 13, e0207284. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, J.; He, H.; Liang, S.; Zhang, H.; Gan, W. Diagnostic performance of novel inflammatory biomarkers based on ratios of laboratory indicators for nonalcoholic fatty liver disease. Front. Endocrinol. 2022, 13, 981196. [Google Scholar] [CrossRef]
- Moris, W.; Verhaegh, P.; Jonkers, D.; Deursen, C.V.; Koek, G. Hyperferritinemia in nonalcoholic fatty liver disease: Iron accumulation or inflammation? Semin. Liver Dis. 2019, 39, 476–482. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.H.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, R.; Yang, S.; Ma, X.; Yu, C. Association between serum ferritin level and the various stages of non-alcoholic fatty liver disease: A systematic review. Front. Med. 2022, 9, 934989. [Google Scholar] [CrossRef]
- Yan, J.X.; Pan, B.J.; Zhao, P.P.; Wang, L.T.; Liu, J.F.; Fu, S.B. Serum ferritin is correlated with non-alcoholic fatty liver disease in middle-aged and older patients with type 2 diabetes. Endocr. Connect. 2021, 10, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Cai, J.J.; Yu, Y.; She, Z.G.; Li, H. Nonalcoholic Fatty Liver Disease: An Update on the Diagnosis. Gene Expr. 2019, 19, 187–198. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Zhou, J.H.; Cai, J.J.; She, Z.G.; Li, H.L. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J. Gastroenterol. 2019, 25, 1307–1326. [Google Scholar] [CrossRef]
- Fatty Liver Index (FLI) MD App. Available online: https://www.mdcalc.com/calc/10001/fatty-liver-index#next-steps (accessed on 22 June 2023).
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Hepatic Steatosis Index (HSI) MD App. Available online: https://www.mdapp.co/hepatic-steatosis-index-hsi-calculator-357 (accessed on 23 June 2023).
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Dai, C.Y.; Fang, T.J.; Hung, W.W.; Tsai, H.J.; Tsai, Y.C. The Determinants of Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Biomedicines 2022, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, R.; Bril, F.; Portillo-Sanchez, P.; Ortiz-Lopez, C.; Orsak, B.; Biernacki, D.; Lo, M.; Suman, A.; Weber, M.; Cusi, K. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients With Type 2 Diabetes. Diabetes Care 2016, 39, 632–638. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, Y.; Wang, Y.; Chen, Y.; Wang, N.; Wang, B.; Lu, Y. Nonalcoholic fatty liver disease and type 2 diabetes: An observational and Mendelian randomization study. Front. Endocrinol. 2023, 14, 1156381. [Google Scholar] [CrossRef] [PubMed]
- Bazick, J.; Donithan, M.; Neuschwander-Tetri, B.A.; Kleiner, D.; Brunt, E.M.; Wilson, L.; Doo, E.; Lavine, J.; Tonascia, J.; Loomba, R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care 2015, 38, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sng, W.K.; Quah, J.H.-M.; Liu, J.; Chong, B.Y.; Lee, H.K.; Wang, X.F.; Tan, N.C.; Chang, P.-E.; Tan, H.C.; et al. Clinical spectrum of non-alcoholic fatty liver disease in patients with diabetes mellitus. PLoS ONE 2020, 15, e0236977. [Google Scholar] [CrossRef]
- Julián, M.T.; Pera, G.; Soldevila, B.; Caballería, L.; Julve, J.; Puig-Jové, C.; Morillas, R.; Torán, P.; Expósito, C.; Puig-Domingo, M.; et al. Atherogenic dyslipidemia, but not hyperglycemia, is an independent factor associated with liver fibrosis in subjects with type 2 diabetes and NAFLD: A population-based study. Eur. J. Endocrinol. 2021, 184, 587–596. [Google Scholar] [CrossRef]
- Martin, A.; Lang, S.; Goeser, T.; Demir, M.; Steffen, H.M.; Kasper, F. Management of Dyslipidemia in Patients with Non-Alcoholic Fatty Liver Disease. Curr. Atheroscler. Rep. 2022, 24, 533–546. [Google Scholar] [CrossRef]
- Prashanth, M.; Ganesh, H.K.; Vima, M.V.; John, M.; Bandgar, T.; Joshi, S.R.; Shah, S.R.; Rathi, P.M.; Joshi, A.S.; Thakkar, H.; et al. Prevalence of Nonalcoholic fatty Liver Disease in Patients with Type 2 Diabetes Mellitus. JAPI 2009, 57, 205–210. [Google Scholar]
- Friedman, A.N.; Fadem, S.Z. Reassessment of albumin as a nutritional marker in kidney disease. J. Am. Soc. Nephrol. 2010, 21, 223–230. [Google Scholar] [CrossRef]
- Mofrad, P.; Contos, M.J.; Haque, M.; Sargeant, C.; Fisher, R.A.; Luketic, V.A.; Sterling, R.K.; Shiffman, M.L.; Stravitz, R.T.; Sanyal, A.J. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatolog 2003, 37, 1286–1292. [Google Scholar] [CrossRef]
- Mandal, A.; Bhattarai, B.; Kafle, P.; Khalid, M.; Jonnadula, S.K.; Lamicchane, J.; Kanth, R.; Gayam, V. Elevated Liver Enzymes in Patients with Type 2 Diabetes Mellitus and Non-alcoholic Fatty Liver Disease. Cureus 2018, 10, e3626. [Google Scholar] [CrossRef]
- Harris, H.E. Elevated Liver Function Tests in Type 2 Diabetes. Clin. Diabetes 2005, 23, 115–119. [Google Scholar] [CrossRef]
- Shrestha, N.; Bhatt, N.P.; Neopane, P.; Dahal, S.; Regmi, P.; Khanal, M.; Shrestha, R. Hepatic involvement with elevated liver enzymes in Nepalese subjects with type 2 diabetes mellitus. Int. J. Biochem. Res. Rev. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Dominici, S.; Paolicchi, A.; Corti, A.; Maellaro, E.; Pompella, A. Prooxidant reactions promoted by soluble and cell-bound g-glutamyltransferase activity. Methods Enzym. 2005, 401, 484–501. [Google Scholar] [CrossRef]
- Kunutsor, S.K. Gamma-glutamyltransferase-friend or foe within? Liver Int. 2016, 36, 1723–1734. [Google Scholar] [CrossRef]
- Chen, L.W.; Huang, M.S.; Shyu, Y.C.; Chien, R.N. Gamma-glutamyl transpeptidase elevation is associated with metabolic syndrome, hepatic steatosis, and fibrosis in patients with nonalcoholic fatty liver disease: A community-based cross-sectional study. Kaohsiung J. Med. Sci. 2021, 37, 819–827. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, B.; Choi, D.H.; Jung, S.H.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Mok, J.O. Association of grade of non-alcoholic fatty liver disease and glycated albumin to glycated hemoglobin ratio in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2017, 125, 53–61. [Google Scholar] [CrossRef]
- Kahraman, N.K.; Kahraman, C.; Kocak, F.E.; Cosgun, S.; Sanal, B.; Korkmaz, M.; Bayham, Z.; Zeren, S. Predictive value of neutrophiltolymphocyte ratio in the severity of non-alcoholic fatty liver disease among type 2 diabetes patients. Acta Gastroenterol. Belg. 2016, 79, 295–300. [Google Scholar]
- Kapici, O.B.; Abus, S.; Ayhan, S.; Karaagac, M.; Sirik, M. Investigation of hemogram and biochemistry parameters in non-alcoholic fatty liver disease. Med. Sci. Int. Med. J. 2023, 12, 63. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.; Zhang, T.; He, X.; Hao, J.; Shen, A.; Zhao, H.; Chen, S.; Ren, L. Factors Associated with Liver Fibrosis in Chinese Patients with Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease. Int. J. Gen. Med. 2023, 16, 293–302. [Google Scholar] [CrossRef]
- Tingle, S.J.; Ma, G.R.S.; Goodfellow, M.; Moir, J.A.; White, S.A. NARCA: A novel prognostic scoring system using neutrophil albumin ratio and Ca19-9 to predict overall survival in palliative pancreatic cancer. J. Surg. Oncol. 2018, 118, 680–686. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Cheng, B.; Ying, B.; Gong, Y. The Neutrophil Percentage-to-Albumin Ratio Is Associated with All-Cause Mortality in Critically Ill Patients with Acute Kidney Injury. Biomed. Res. Int. 2020, 18, 2020–5687672. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Cheng, B.; Ying, B.; Wang, B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol. Infect. 2020, 148, e87. [Google Scholar] [CrossRef]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef]
- Loosen, S.H.; Demir, M.; Kunstein, A.; Jördens, M.; Qvarskhava, N.; Luedde, M.; Luedde, T.; Roderburg, C.; Kostev, K. Variables associated with increased incidence of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e002243. [Google Scholar] [CrossRef]
- Okdahl, T.; Wegeberg, A.M.; Pociot, F.; Brock, B.; Størling, J.; Brock, C. Low-grade inflammation in type 2 diabetes: A crosssectional study from a Danish diabetes outpatient clinic. BMJ Open 2022, 12, e062188. [Google Scholar] [CrossRef]
- Cucoranu, D.C.; Pop, M.; Niculescu, R.; Kosovski, I.B.; Toganel, R.O.; Licu, R.A.; Bacârea, A. The Association of Nonalcoholic Fatty Liver Disease With Neutrophil-to-Lymphocyte Ratio and Neutrophil-Percentage-to-Albumin Ratio. Cureus 2023, 15, e41197. [Google Scholar] [CrossRef]
- You, G.; Ding, J.; Shen, J.; Wang, Y.; Sun, Y. Association between serum ferritin and non-alcoholic fatty liver disease among middle-aged and elderly Chinese with normal weight. Asia Pac. J. Clin. Nutr. 2019, 28, 747–753. [Google Scholar]
- Kunutsor, S.K.; Apekey, T.A.; Walley, J.; Kain, K. Ferritin levels and risk of type 2 diabetes mellitus: An updated systematic review and meta-analysis of prospective evidence. Diabetes/Metab. Res. Rev. 2013, 29, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.F.; El Bendary, A.S.; Ezzat, S.E.; Mohamed, W.S. Serum ferritin level, microalbuminuria and non-alcoholic fatty liver disease in type 2diabetic patients. Diabetes Metab. Syndr. 2019, 13, 2226–2229. [Google Scholar] [CrossRef]
- Lombardi, R.; Pisano, G.; Fargion, S. Role of serum uric acid and ferritin in the development and progression of NAFLD. Int. J. Mol. Sci. 2016, 17, 548. [Google Scholar] [CrossRef]
- Sachinidis, A.; Doumas, M.; Imprialos, K.; Stavropoulos, K.; Katsimardou, A.; Athyros, V.G. Dysmetabolic iron overload in metabolic syndrome. Curr. Pharm. Des. 2020, 26, 1019–1024. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, J.S.; Lee, H.J.; Kim, W.H.; Park, S.I.; Song, J. Effect of excess iron on oxidative stress and gluconeogenesis through hepcidin during mitochondrial dysfunction. J. Nutr. Biochem. 2015, 26, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, B.I.; Yun, J.W.; Kim, J.W.; Park, D.I.; Cho, Y.K.; Sung, I.K.; Park, C.Y.; Sohn, C.I.; Jeon, W.K.; et al. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J. Gastroenterol. Hepatol. 2004, 19, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Hui, A.Y.; Tsang, S.W.; Chan, J.L.; Tse, A.M.; Chan, K.F.; So, W.Y.; Cheng, A.Y.; Ng, W.F.; Wong, G.L.; et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1154–1161. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Cardellini, M.; Hoyles, L.; Latorre, J.; Davato, F.; Moreno-Navarrete, J.M.; Arnoriaga-Rodríguez, M.; Serino, M.; Abbott, J.; Barton, R.H.; et al. Iron status influences non-alcoholic fatty liver disease in obesity through the gut microbiome. Microbiome 2021, 9, 104. [Google Scholar] [CrossRef]
- Ryan, J.D.; Armitage, A.E.; Cobbold, J.F.; Banerjee, R.; Borsani, O.; Dongiovanni, P.; Neubauer, S.; Morovat, R.; Wang, L.M.; Pasricha, S.; et al. Hepatic iron is the major determinant of serum ferritin in NAFLD patients. Liver Int. 2018, 38, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, B.; Samardzic, V.; Gluvic, Z.; Tomasevic, R.; Obradovic, M.; Sudar-Milovanovic, E.; Isenovic, E.R. Serum ferritin levels correlate with ultrasonography-determined liver steatosis severity in type 2 diabetes patients with NAFLD. Endocr. Abstr. 2021, 73, AEP294. [Google Scholar] [CrossRef]
- Haukeland, J.W.; Damås, J.K.; Konopski, Z.; Løberg, E.M.; Haaland, T.; Goverud, I.; Torjesen, P.A.; Birkeland, K.; Bjøro, K.; Aukrust, P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J. Hepatol. 2006, 44, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Iida, H.; Yonemitsu, K.; Kato, S.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; Nozaki, Y.; et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J. Gastroenterol. 2007, 42, 573–582. [Google Scholar] [CrossRef]
- Riquelme, A.; Arrese, M.; Soza, A.; Morales, A.; Baudrand, R.; Pérez-Ayuso, R.M.; González, R.; Alvarez, M.; Hernández, V.; García-Zattera, M.J.; et al. Non-alcoholic fatty liver disease and its association with obesity, insulin resistance and increased serum levels of C-reactive protein in Hispanics. Liver Int. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Kao, P.C.; Shiesh, S.C.; Wu, T.J. Serum C-reactive protein as a marker for wellness assessment. Ann. Clin. Lab. Sci. 2006, 36, 163–169. [Google Scholar]
- Oruc, N.; Ozutemiz, O.; Yuce, G.; Akarca, U.S.; Ersoz, G.; Gunsar, F.; Batur, Y. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: A case control study. BMC Gastroenterol. 2009, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Nasir, K.; Conceiçao, R.D.; Carvalho, J.A.; Blumenthal, R.S.; Santos, R.D. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1927–1932. [Google Scholar] [CrossRef]
- Viallon, A.; Zeni, F.; Pouzet, V.; Lambert, C.; Quenet, S.; Aubert, G.; Guyomarch, S.; Tardy, B.; Bertrand, J.C. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: Diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000, 26, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).