VDR Immunohistochemistry Expression Is Down-Regulated in Colorectal Cells of Patients with IBD and Could Rank the Patients According to Their Complications Risk
Abstract
1. Introduction
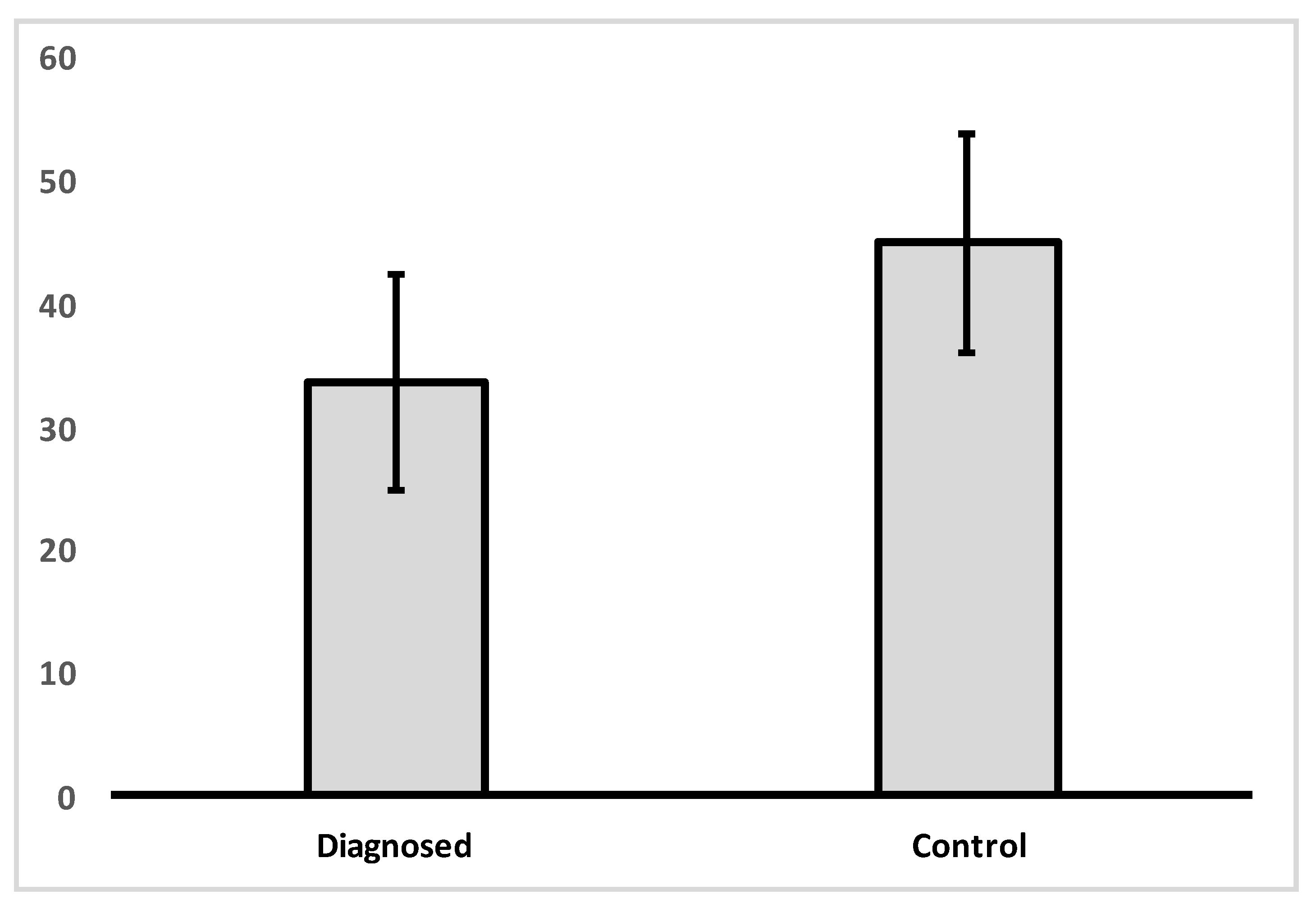
- VDR nuclear expression is significantly reduced in patients with IBD compared to controls;
- VDR down-regulation in patients with inflammatory bowel disease (especially patients with UC) could establish stratifying them into groups, regarding closer surveillance according to their risk for CAC;
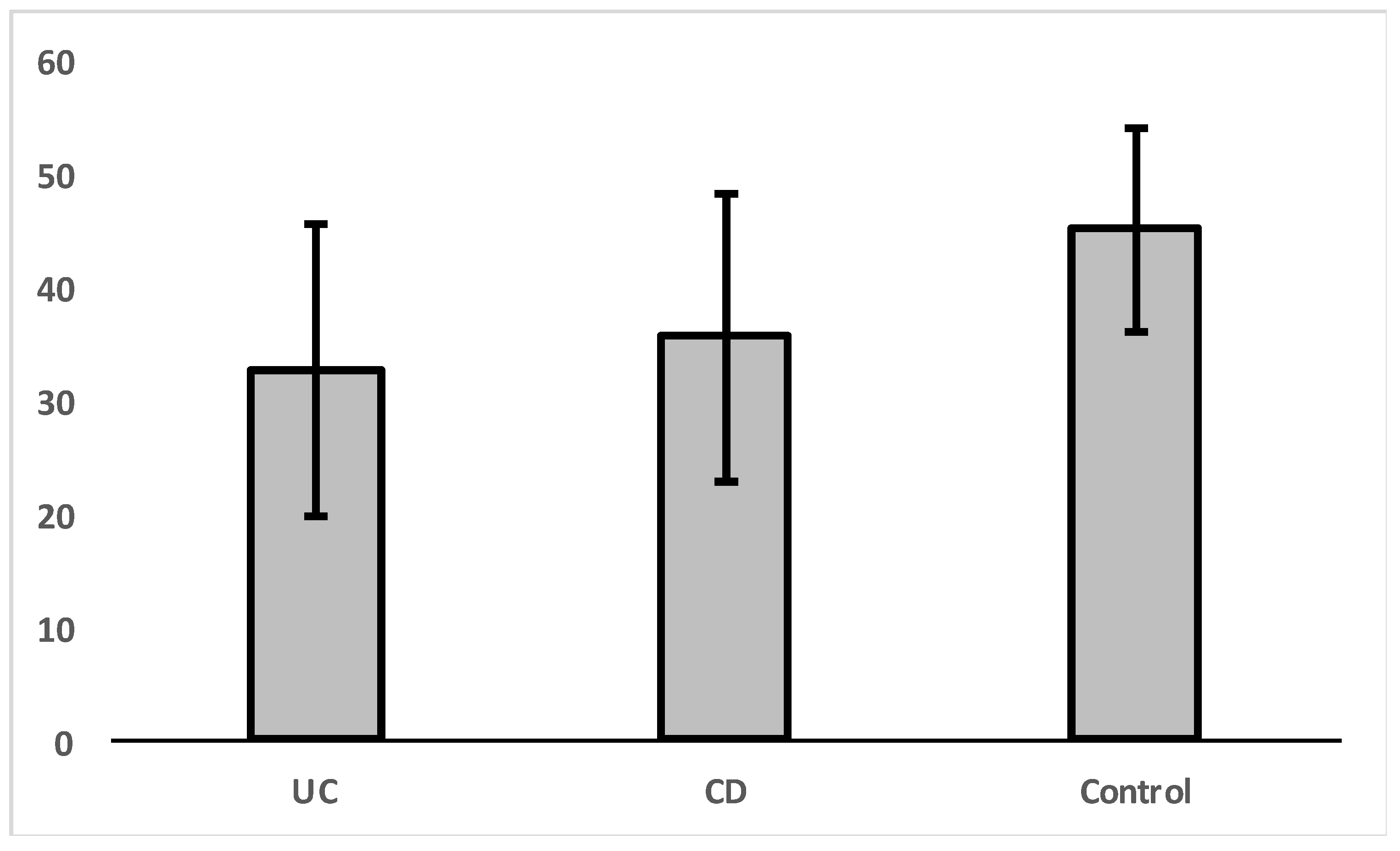
- VDR expression could contribute in differentiating patients with IBD according to their specific diagnosis, into patients with UC and patients with CD, respectively.
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Sample Processing for IHC Examination
2.4. Evaluation of Immunostaining
2.5. Statistical Analysis
2.6. Ethical Considerations
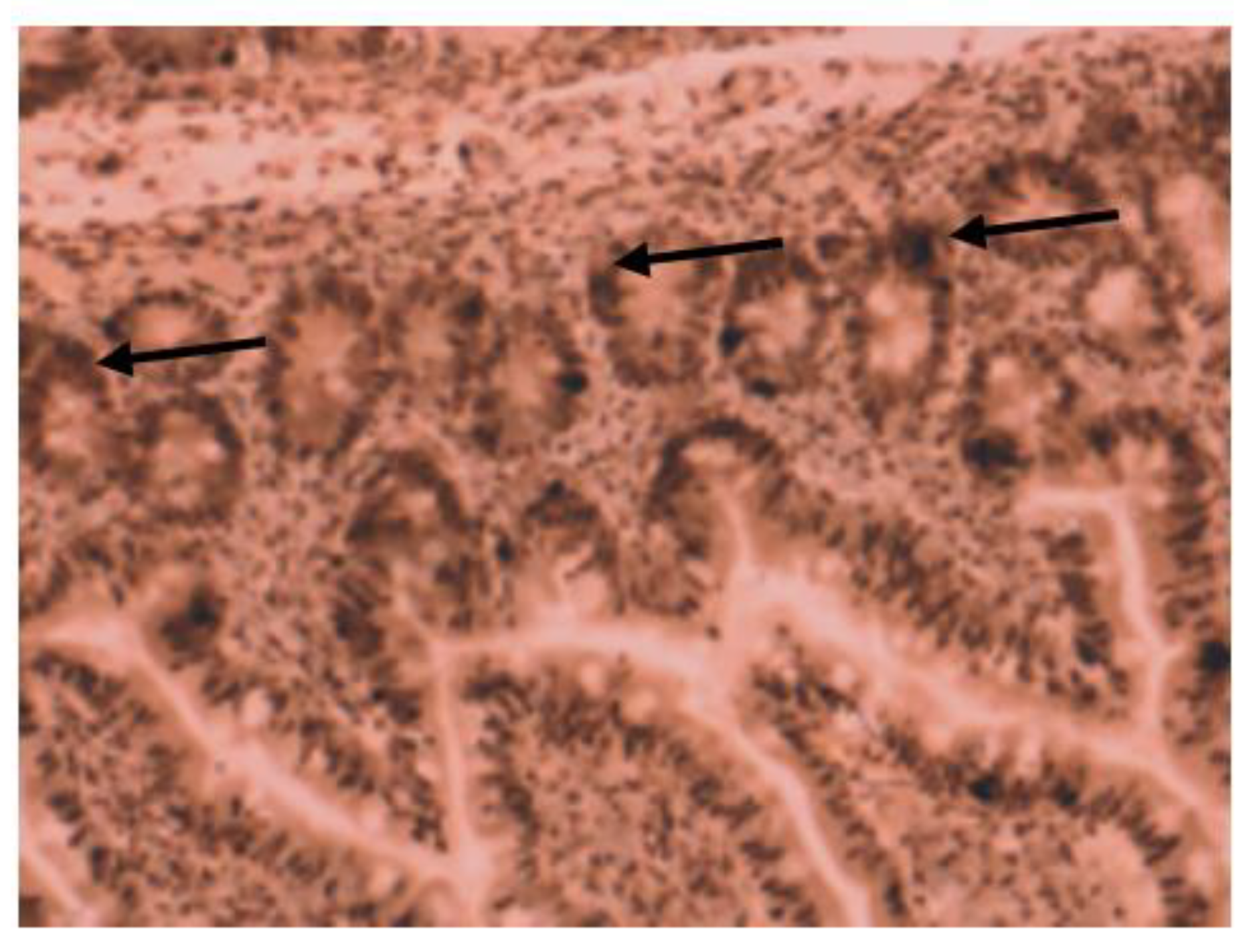
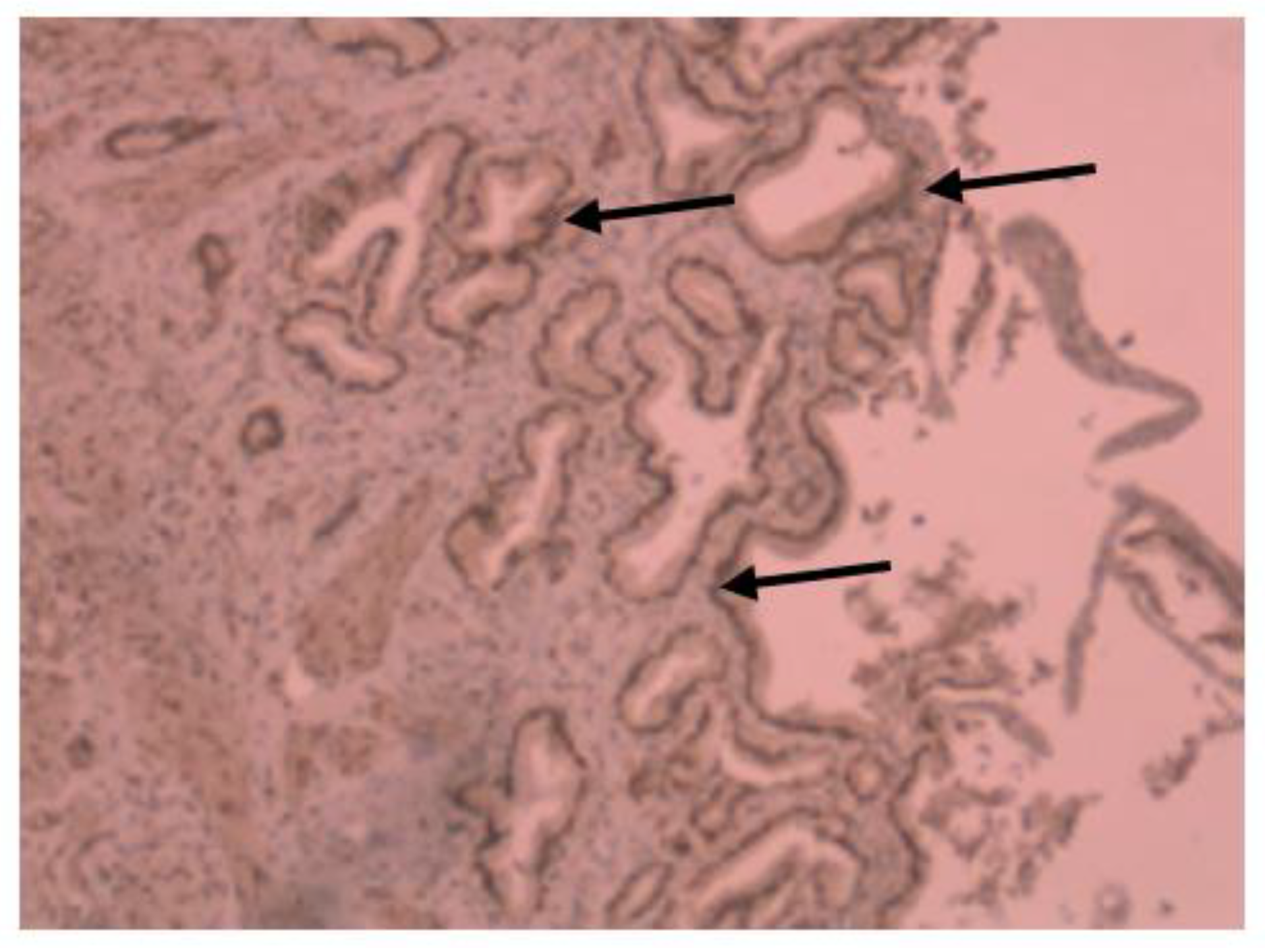
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. Vitamin D Therapy in Adults with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830. [Google Scholar] [CrossRef]
- Kellermann, L.; Jensen, K.B.; Bergenheim, F.; Gubatan, J.; Chou, N.D.; Moss, A.; Nielsen, O.H. Mucosal vitamin D signaling in inflammatory bowel disease. Autoimmun. Rev. 2020, 19, 102672. [Google Scholar] [CrossRef] [PubMed]
- Vaghari-Tabari, M.; Targhazeh, N.; Moein, S.; Qujeq, D.; Alemi, F.; Majidina, M.; Younesi, S.; Asemi, Z.; Yousefi, B. From inflammatory bowel disease to colorectal cancer: What’s the role of miRNAs? Cancer Cell Int. 2022, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Al Bakir, I.; Curtius, K.; Graham, T.A. From Colitis to Cancer: An Evolutionary Trajectory that Merges Maths and Biology. Front. Immunol. 2018, 9, 2368. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Hirayama, D.; Wagatsuma, K.; Yamakawa, T.; Yokoyama, Y.; Nakase, H. Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 3062. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR with New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef]
- Gallone, G.; Haerty, W.; Disanto, G.; Ramagopalan, S.V.; Ponting, C.P.; Berlanga-Taylor, A.J. Identification of genetic variants affecting vitamin D receptor binding and associations with autoimmune disease. Hum. Mol. Genet. 2017, 26, 2164–2176. [Google Scholar] [CrossRef]
- Naderi, N.; Farnood, A.; Habibi, M.; Derakhshan, F.; Balaii, H.; Motahari, Z.; Agah, M.R.; Firouzi, F.; Rad, M.G.; Aghazadeh, R.; et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2008, 23, 1816–1822. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1717S–1720S. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yan, J.; Zhi, C.; Zhou, Q.; Yuan, X. 1, 25(OH)2 D3 deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model. Gut Pathog. 2019, 11, 8. [Google Scholar] [CrossRef]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Tanaka, H.; Maeda, K.; Inoue, T.; Noda, E.; Amano, R.; Kubo, N.; Muguruma, K.; Yamada, N.; Yashiro, M.; et al. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol. Rep. 2009, 22, 1021–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nobile, S.; Tenace, M.A.; Pappa, H.M. The Role of Vitamin D in the Pathogenesis of Inflammatory Bowel Disease. Gastrointest. Disord. 2019, 1, 231–240. [Google Scholar] [CrossRef]
- Sun, J. The Role of Vitamin D and Vitamin D Receptors in Colon Cancer. Clin. Transl. Gastroenterol. 2017, 8, e103. [Google Scholar] [CrossRef]
- Domazetovic, V.; Iantomasi, T.; Bonanomi, A.G.; Stio, M. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int. J. Color. Dis. 2020, 35, 1231–1242. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.; Claud, E.C.; Sun, J. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm. Bowel Dis. 2019, 25, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Sluka, P.; Wardan, H.; Hosking, P.; Monagle, S.; Thomas, M.; Lubel, J.S.; et al. The intestinal vitamin D receptor in inflammatory bowel disease: Inverse correlation with inflammation but no relationship with circulating vitamin D status. Ther. Adv. Gastroenterol. 2019, 12, 1756284818822566. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Delgado, Y.; Isidro, R.A.; Torres, E.A.; González, A.; Cruz, M.L.; Isidro, A.A.; González-Keelan, C.I.; Medero, P.; Appleyard, C.B. Serum vitamin D and colonic vitamin D receptor in inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 3581–3591. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, T.; Liu, Y.; Lyu, L.; Li, X.; Yue, W. How would serum 25(OH) D level change in patients with inflammatory bowel disease depending on intestinal mucosa vitamin D receptor (VDR) and vitamin D1-α hydroxylase (CYP27B1)? Turk. J. Gastroenterol. 2019, 30, 132–138. [Google Scholar] [CrossRef]
- Tanaka, H.; Maeda, K.; Wada, K.; Yashiro, M.; Onoda, N.; Yamada, N.; Sawada, T.; Ohira, M.; Hirakawa, K. Correlation of vitamin D receptor expression with colorectal cancer in ulcerative colitis. Cancer Res. 2008, 68 (Suppl. 9), 5501. [Google Scholar]
- James, J.; Nielsen, B.S.; Christensen, I.; Langholz, E.; Malham, M.; Riis, L.; Høgdall, E. MUCOSAL Expression of genes PI3, ANXA1, and VDR descriminates Crohn’s disease from ulcerative colitis in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2023, 29 (Suppl. 1), S13. [Google Scholar] [CrossRef]
- Isidro, R.A.; Cruz, M.L.; Isidro, A.A.; Baez, A.; Arroyo, A.; González-Marqués, W.A.; González-Keelan, C.; Torres, E.A.; Appleyard, C.B. Immunohistochemical expression of SP-NK-1R-EGFR pathway and VDR in colonic inflammation and neoplasia. World J. Gastroenterol. 2015, 21, 1749–1758. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, H.H.; Zhang, Z.; Gao, J.; Li, J.; Li, X. Vitamin D3 alleviates inflammation in ulcerative colitis by activating the VDR-NLRP6 signaling pathway. Front. Immunol. 2023, 14, 1135930. [Google Scholar] [CrossRef]
- Sharifi, A.; Vahedi, H.; Honarvar, M.R.; Amiriani, T.; Nikniaz, Z.; Rad, E.Y.; Hosseinzadeh-Attar, M.J. Vitamin D decreases CD40L gene expression in ulcerative colitis patients: A randomized, double-blinded, placebo-controlled trial. Turk. J. Gastroenterol. 2020, 31, 99–104. [Google Scholar] [CrossRef]
- Bafutto, M.; Oliveira, E.C.; Rezende Filho, J. Use of Vitamin D with Anti-Tumor Necrosis Factor Therapy for Crohn’s Disease. Gastroenterol. Res. 2020, 13, 101–106. [Google Scholar] [CrossRef]
| Age (Year) | ||
|---|---|---|
| Range | 19–81 | |
| Median | 43.6 | |
| Age at diagnosis (examination) | ||
| Median | 42 | |
| Gender | ||
| Male | 17 | |
| Female | 18 | |
| Diagnosis | ||
| Control | 8 | (5m/3f) |
| Crohn’s disease | 10 | (4m/6f) |
| Ulcerative Colitis | 17 | (8m/9f) |
| IHC Expression (%) | ||
| VDR | 33.7 (12.68) | |
| Grouping | VDR | |
|---|---|---|
| Control | Mean | 45.00 |
| N | 8 | |
| Std. Deviation | 8.86 | |
| Diagnosed with CD or UC | Mean | 33.70 |
| N | 27 | |
| Std. Deviation | 12.68 | |
| Total | Mean | 36.29 |
| N | 35 | |
| Std. Deviation | 12.74 | |
| Group | N | Mean | Std. Deviation | |
|---|---|---|---|---|
| VDR | Control | 8 | 45.00 | 8.86 |
| UC | 17 | 32.65 | 12.88 | |
| CD | 10 | 35.50 | 12.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juniku-Shkololli, A.; Manxhuka-Kërliu, S.; Hamza, V.; Basholli, M. VDR Immunohistochemistry Expression Is Down-Regulated in Colorectal Cells of Patients with IBD and Could Rank the Patients According to Their Complications Risk. Gastroenterol. Insights 2023, 14, 342-351. https://doi.org/10.3390/gastroent14030025
Juniku-Shkololli A, Manxhuka-Kërliu S, Hamza V, Basholli M. VDR Immunohistochemistry Expression Is Down-Regulated in Colorectal Cells of Patients with IBD and Could Rank the Patients According to Their Complications Risk. Gastroenterology Insights. 2023; 14(3):342-351. https://doi.org/10.3390/gastroent14030025
Chicago/Turabian StyleJuniku-Shkololli, Argjira, Suzana Manxhuka-Kërliu, Valon Hamza, and Mimoza Basholli. 2023. "VDR Immunohistochemistry Expression Is Down-Regulated in Colorectal Cells of Patients with IBD and Could Rank the Patients According to Their Complications Risk" Gastroenterology Insights 14, no. 3: 342-351. https://doi.org/10.3390/gastroent14030025
APA StyleJuniku-Shkololli, A., Manxhuka-Kërliu, S., Hamza, V., & Basholli, M. (2023). VDR Immunohistochemistry Expression Is Down-Regulated in Colorectal Cells of Patients with IBD and Could Rank the Patients According to Their Complications Risk. Gastroenterology Insights, 14(3), 342-351. https://doi.org/10.3390/gastroent14030025





