Hepatoprotective Effects of Liv.52 in Chronic Liver Disease Preclinical, Clinical, and Safety Evidence: A Review
Abstract
1. Introduction
2. Liv.52 Formulation
2.1. Hepatoprotective Effect of Individual Component of Liv.52
2.1.1. Cichorium intybus
2.1.2. Capparis spinosa
2.1.3. Solanum nigrum
2.1.4. Cassia occidentalis
2.1.5. Terminalia arjuna
2.1.6. Achillea millefolium
2.1.7. Tamarix gallica
2.1.8. Mandur bhasma
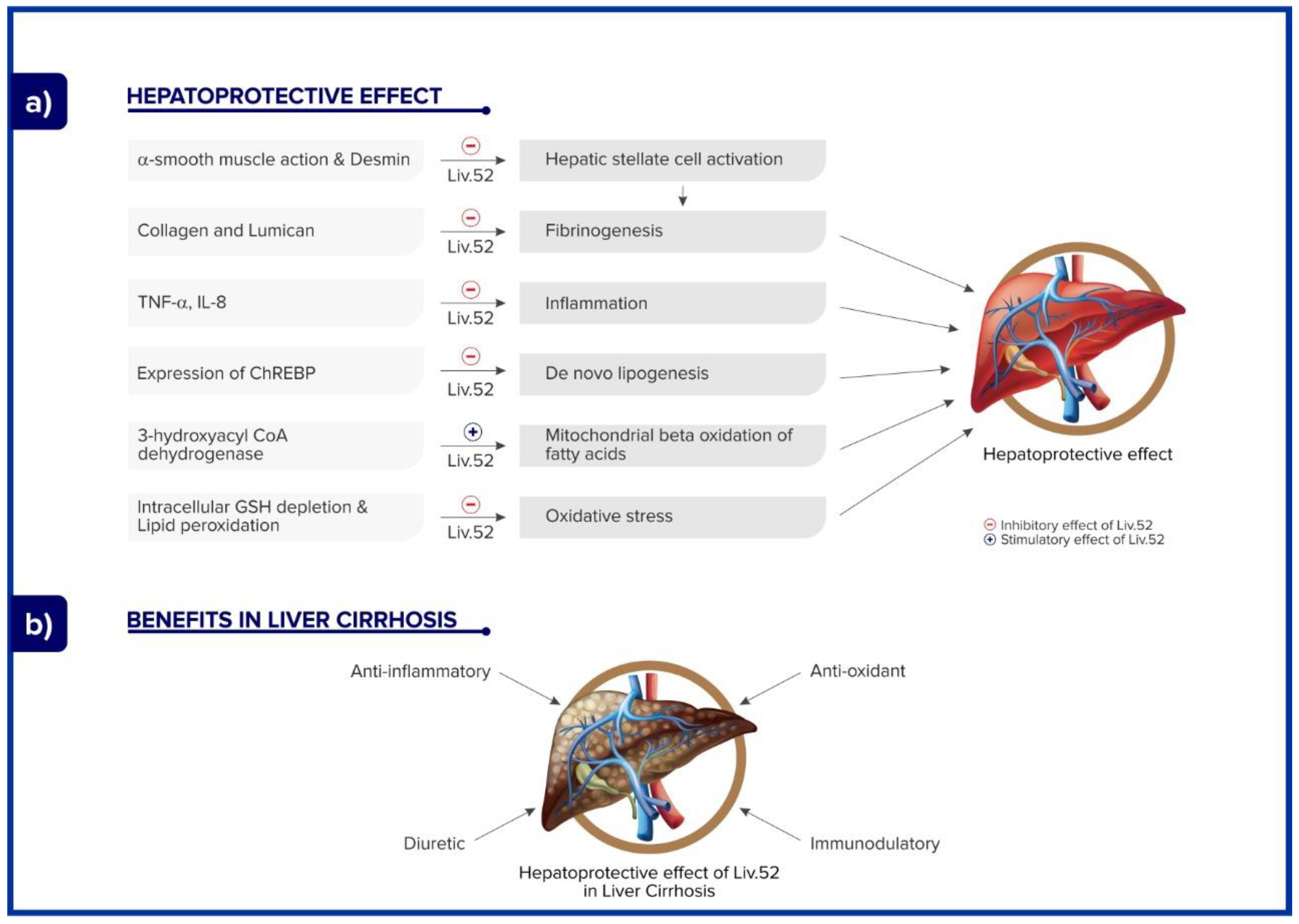
2.2. Mechanism of Action of Liv.52
2.3. Preclinical Studies: Hepatoprotective Effects of Liv.52
2.3.1. Hepatoprotective Effect
2.3.2. Antioxidant Effect and Radiation Hazard
2.4. Clinical Studies: Hepatoprotective Effect of Liv.52 in Different Clinical Conditions
2.4.1. Tuberculosis
2.4.2. Alcoholic Liver Disease
2.4.3. Viral Hepatitis
2.4.4. Non-Infectious Chronic Liver Disease
2.4.5. Liver Function in Pregnancy
2.4.6. Liver Cirrhosis
2.4.7. Non-Alcoholic Steatohepatitis (NASH) and Non-Alcoholic Fatty Liver Disease (NAFLD)
2.4.8. Hepatomegaly Syndrome
2.4.9. Safety
| Sr. No. | Author | Year | Type of Study | Aim | Findings Description | Reference |
|---|---|---|---|---|---|---|
| Hepatoprotective effect | ||||||
| 1 | Vidyashankar et al. | 2012 | In vitro study | Evaluate Liv.52 in oleic acid-induced non-alcoholic fatty liver disease (NAFLD) in HepG2 cells. |
| [38] |
| 2 | Vidyashankar et al. | 2010 | In vitro study | Evaluate the hepatoprotective effect of Liv.52 against oxidative damage induced by tert-butyl hydroperoxide (t-BHP) in HepG2 cells. |
| [36] |
| 3 | Mitra et al. | 2008 | In vitro study | Determine whether ethanol and Liv.52 can modulate PPARc and TNFα induction in human hepatoma cells, HepG2. | Liv.52 is capable of attenuating ethanol-induced expression of TNFα and abrogating ethanol-induced suppression of PPARc in liver cells, suggesting its immunomodulatory and hepatoprotective effect. Liv.52 reverses the effects of carbon-tetrachloride-induced elevated activities of hepatic enzymes and NADPH-dependent lipid peroxidation. | [35] |
| 4 | Cimen et al. | 2020 | In vivo study | Evaluate effect of Liv-52 on liver ischemia-reperfusion damage in rats | Liv-52 is effective in reducing markers of liver damage and improving the histopathological condition of the liver tissue in the context of liver I/R injury. | [9] |
| 5 | Sandhir et al. | 1999 | In vivo study | Evaluate hepatoprotective effects of Liv-52 on ethanol-induced hepatic damage in rats. | Liv-52 prevents ethanol-induced increase in the activity of the enzyme gamma-glutamyl transpeptidase and decreases ethanol-accentuated lipid peroxidation. | [40] |
| 6 | Kataria et al. | 1997 | In vitro study | Evaluate effect of Liv.52 and Kumaryasava on growth and hepatic enzymes of CCl4 treated rats | Liv.52 and Kumaryasva both provide a certain amount of protection and correct liver dysfunction due to CCl4-induced hepatotoxicity. Also, the combination stimulates regeneration of hepatic and microsomal enzyme decreased due to CCl4 toxicity. | [41] |
| Antioxidant effect and radiation hazard | ||||||
| 1 | Jagetia et al. | 2006 | In vivo study | To evaluate radioprotective activity of Liv 52 in mice |
| [46] |
| Sr No. | Study and Origin | Study Design | Treatment Duration | Country | Participant | Sample Size (Commenced, Completed) | Intervention | Outcome Measures | Results | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug-induced hepatotoxicity: tuberculosis | ||||||||||
| 1 | Choijamts et al.; 2018 | Double Blind Placebo Controlled Study | 6 months | Mongolia | Patients aged between 18 years to 60 years under ATT. | Liv 52 DS (47,47), Placebo (43,43) | Liv 52 DS- 2 tablets twice daily. | LFT (SGOT, SGPT, serum alkaline phosphatase, and serum bilirubin), hemoglobin, serum protein | Compared to placebo, significant reduction was observed in LFT (SGOT, SGPT, serum alkaline phosphatase, and serum bilirubin) (p < 0.05). Hemoglobin improved from 13.17 ± 1.70 to 13.29 ± 1.21 with a significance of p < 0.004. | [51] |
| Non-alcoholic fatty liver disease | ||||||||||
| 1 | Siregar et al., 2021 | Prospective, interventional study | 2 months | Indonesia | Patients aged between 18 and 65 years with NAFLD | Liv.52 (60,60) | Liv.52 DS- 2 tablets twice daily for 2 months. | LFT (SGOT, SGPT), ultrasound, NAFLD Fibrosis Score. | Compared to placebo, Liv.52 group showed greater reduction in the fibrosis score for NAFLD. Hepatomegaly decreased to 42% of the participants. SGOT and SGPT levels significantly decreased (p < 0.0339 and p < 0.0022, respectively.) | [6] |
| Non-alcoholic steatohepatitis (NASH) | ||||||||||
| 1 | Maity et al., 2015 | Randomized controlled study | 12 weeks | India | Patients aged between 18 and 65 years with non-alcoholic steatohepatitis | Liv.52 DS group (19,19); UDCA (16,16). | Liv.52 DS -2 tablets twice daily; 600 mg of UDCA thrice daily for 12 weeks | SGPT, SGOT, ALT, serum bilirubin, total protein, albumin and globulin | Compared to UDCA, Liv.52 showed faster clinical and biochemical recovery. Significant decrease was noted in the levels of SGPT(p < 0.004), SGOT (p < 0.033), and ALP (p < 0.008). | [71] |
| 2 | Ghosh et al., 2014 | Open clinical study | 3 months | India | Patients suffering from steatohepatitis. | Liv.52 DS (50) | Liv.52 DS- 2 tablets twice daily for a period of 3 months | ALP, total protein, total cholesterol, random blood sugar, TSH, serum triglyceride | Liv.52 showed improvement (p < 0.0001) in clinical and liver function parameters along with ultraso-nographic and NAFLD scores. | [72] |
| Viral hepatitis | ||||||||||
| 1 | Kar et al., 2009 | Open-labeled clinical trial | 6 months | India | Patients aged 18–60 years with hepatitis B infection | HD-03/ES (51,51) | HD-03/ES- 2 capsules twice daily for 6 months. | LFT, serum HBsAg, HBeAg and HBV DNA | Liv.52 showed significant reduction of ALT values from 71.2 ± 16.3 to 36.4 ± 6.8 (p < 0.01) and a significant HBeAg loss (27.4%) and HBV DNA loss (27.4%) (p < 0.01). | [62] |
| 2 | Rajkumar et al., 2007 | Open prospective controlled clinical trial | 6 months | India | Patients aged 18–60 years with hepatitis B infection | HD-03/ES(25) | HD-03/ES-2 capsules twice day for 6 months | ALT, AST, total bilirubin, serum protein | HD-03/ES showed significant reduction in ALT values from 66.5 ± 11.1 to 39.1 ± 5.2 (p < 0.01), significant HBsAg loss (52%, p < 0.001), HBeAg loss (60%, p < 0.05) and HBV DNA loss (60%, p < 0.05). | [63] |
| 3 | Habibullah et al., 1978 | Double-blind study | Till total biochemical recovery | India | Patients suffering from viral hepatitis | Liv.52 group (25,25); Placebo (25,25) | Liv.52-2 tabs three times daily | SGPT, ALP, serum albumin, serum globulin, serum cholesterol, prothrombin time, serum bilirubin | Compared to placebo, Liv.52 showed faster biochemical recovery (2.4 weeks in Liv.52 group versus 3.8 weeks in placebo group). | [59] |
| 4 | Gupta et al., 1972 | Controlled clinical study | Follow up at intervals of 15 days/one month | India | Patients of infectious hepatitis from infancy to twelve years | Liv.52 (55, ND); control (30, ND) | Liv.52-1 tablet or 10 drops three times a day (up to 2 years); 1 tablet three times a day (up to 2–5 years); 1 tablet four times a day (above 5 years) | LFT, Urine examination; Haemogram, Liver biopsy, Radiological examination | Compared with control, serum bilirubin levels, albumin/globulin ratio, SGOT, and SGPT levels were normal in Liv.52 group. Total serum protein, serum alkaline phosphatase, and prothrombin time values were not affected. Also, biopsies clearly showed receding phase of infectious hepatitis in the group treated with Liv.52 tablets. | [60] |
| 7 | Singh et al., 1977 | Controlled study | 8 weeks | India | Patients with infective hepatitis of varying age groups | Liv.52 (25, 25); Control (25, 25) | Liv.52-6 tablets in divided doses along with B-complex and corticosteroids daily | SGOT, SGPT, Serum bilirubin, serum alkaline phosphatase, thymol turbidity | Compared with placebo, serum bilirubin values reduced by 86%(66% in placebo). The values in the Liv.52 group after 4 weeks and 8 weeks of treatment were reduced by 23% and 42% over the initial values. The percentage decline in thymol turbidity after 4 and 8 weeks of treatment in the control group was 24% and 29% as against 37% and 66% in the Liv.52 group. SGPT and SGOT values also showed reductions. | [61] |
| 8 | Jha et al., 2021 | Comparative study | 18 months | India | Patients with hepatitis B | Tenofovir(35,35); Liv.52 HB (32,32); Tenofovir plus Liv.52 HB (37,37) | - | ALT, AST, Serum bilirubin, ALP, serum creatinine, HbeAg, blood urea, INR, Hb | Tenofovir plus Liv.52 group showed significant reductions in Serum Bilirubin, serum ALT, AST, and ALP levels compared to tenofovir alone or Liv52 HB alone. There was a statistically significant reduction in HbsAg after 12 months within the tenofovir + Liv52Hb group, and also the outcome was statistically better when compared to other two groups. HbeAg positivity was also significantly better in the tenofovir + Liv52 HB group in both inter and intra-group comparisons. | [75] |
| Non-infectious chronic liver disease | ||||||||||
| 1 | Al- Khazraji et al., 2022 | Interventional randomized blind clinical trial | 6 months | Iraq | Patients with liver damage | Liv.52 (100, ND; Control (100, ND) | Liv.52-2 tablets thrice daily | ALT, AST, total serum bilirubin, ALP serum albumin | Compared to control, Liv.52 showed significant reduction in ALT (p = 0.019), AST (p = 0.231), total serum bilirubin (p = 0.148), ALP (p = 0.359), and serum albumin (p < 0.001). | [65] |
| Alcoholic liver disease | ||||||||||
| 1 | Kalab et al., 1997 | Retrospective study | 1 year | Prague | Patients having liver damage caused by alcohol, steatosis and persistent hepatitis. | Liv.52 (19, ND) | Liv.52-2 tablets (b.i.d.) for 1 year | ALT, AST, ALP, serum bilirubin, TZR, GMT | Liv.52 showed significant reduction in ALT, AST, GMT levels. There was no influenced on the value of TZR. | [76] |
| 2 | De Silva et al., (84) | Prospective, double-blind, randomized, placebo-controlled trial | 6 months | Srilanka | Patients with alcoholic liver disease. | Liv.52 (40, 19); Placebo(40, 19) | Liv.52-3 capsules twice daily for 6 months | ALT and AST, gamma-GT, albumin, and bilirubin | Compared to placebo, there were no significant outcome measures observed in Liv.52 treated group. No subject complained of adverse effects attributable to the drug. | [11] |
| Liver dysfunction in pregancy | ||||||||||
| 1 | Mitra et al., 2008 | - | 6 weeks | India | Pregnant women with severe viral hepatitis | Liv.52 (84, ND) | - | Liver biopsy | Liv.52 brought reduced the earlier reported mortality from 26.7% to 1.1% in patients of jaundice with pregnancy. All the patients recovered completely after 6 weeks of treatment except one patient. | [70] |
| Liver cirrhosis | ||||||||||
| 1 | Huseini et al., 2004 | Randomized, double-blind, placebo-controlled | 6 months | Iran | Subjects with liver cirrhosis were selected | Placebo (18,18); Liv.52 (18,18) | Liv.52—3 tablets twice daily for 6 months | ALT, AST, Child–Pugh score, ascites | Significant reduction in ALT (Mean 89 to 38), AST values (mean from 89 to 57) along with reduction in ascites and child-pugh scores. | [37] |
| Hepatomegaly syndrome | ||||||||||
| 1 | Marginean et al., 2002 | Open clinical trial | 6 months | Romania | Children (2–17 years) diagnosed with hepatomegaly syndrome. | Liv.52 (51,51); control (20,20) | Liv.52 syrup-2.5 mL (½ teaspoon), twice daily (children under 12 years of age); 5 mL (1 teaspoon) twice daily (children above 12 years) | AST, ALT, lactic-dehydrogenase, immunoglobulins, and serum proteins. | Compared to control, Liv.52 group showed significant decrease in AST and ALT values. Liv.52 group stimulated the synthesis of gammaglobulin. Liv.52 also improved protein synthesis. | [74] |
| Sr No. | Study and Origin | Study Design | Country | Indication | Study Size (N), Duration | Intervention | Outcome Measures | Results | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ganesh et al. 2022 | Review | India | Alcoholic liver disease | N = 19 to 50, upto 1 year | Liv.52 DS | Liver parameters like ALT, AST, prothrombin, clinical symptoms like ascites, USG | Improvement in liver parameters, clinical symptoms, and USG findings. | [57] |
| 2 | Sharad C et al., 2022 | Cumulative efficacy analysis | India | Non-alcoholic fatty liver disease | N = 35 to 50, up to 3 months | Liv.52 DS | Liver parameters like ALT, AST, clinical symptoms, USG (fatty liver grading, hepatomegaly), NAFLD fibrosis score | Improvement of hepatic parameters and clinical symptoms | [73] |
| 3 | Maji et al., 2013 | Review | India | Hepatitis B infection | N = 14 to 51 (up to 6 months) | Liv.52 HB | LFT paramters, HbsAg, HbeAg, HBV DNA | Improvement in LFT paramters and lss of HbsAg, HbeAg, HBV DNA | [64] |
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Nagalli, S. Chronic Liver Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sun, J.; Tanaka, J.; Valenti, L. The changing epidemiology of liver diseases in Asia. Liver Int. 2022, 42, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.S.; Rockey, D.C. Complications and outcomes in chronic liver disease. Curr. Opin. Gastroenterol. 2011, 27, 204–209. [Google Scholar] [CrossRef]
- Liv.52. Himalaya Wellness (India). Available online: https://himalayawellness.in/products/liv-52 (accessed on 6 June 2023).
- Girish, C.; Koner, B.C.; Jayanthi, S.; Rao, K.R.; Rajesh, B.; Pradhan, S.C. Hepatoprotective activity of six polyherbal formulations in paracetamol induced liver toxicity in mice. Indian J. Med. Res. 2009, 129, 569–578. [Google Scholar]
- Siregar, G.; Paramesh, R.; Kumawat, R.; HA, S. A prospective, interventional clinical study to evaluate the safety and efficacy of Liv.52 DS in the management of non-alcoholic fatty liver disease. Eur. J. Clin. Exp. Med. 2021, 19, 129–136. [Google Scholar] [CrossRef]
- Khetarpal, S.K.; Ramakumar, L.; Ramlubhaya, R. Malnutrition-Hepatic Function and Liv.52 Therapy. Curr. Med. Pract. 1972, 11, 481–488. [Google Scholar]
- Seeff, L.B.; Lindsay, K.L.; Bacon, B.R.; Kresina, T.F.; Hoofnagle, J.H. Complementary and alternative medicine in chronic liver disease. Hepatology 2001, 34, 595–603. [Google Scholar] [CrossRef]
- Cimen, O.; Eken, H.; Keskin Cimen, F.; Cekic, A.B.; Kurt, N.; Ozbek Bilgin, A.; Suleyman, B.; Suleyman, H.; Mammadov, R.; Pehlivanoglu, K.; et al. The effect of Liv-52 on liver ischemia reperfusion damage in rats. BMC Pharmacol. Toxicol. 2020, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Nimesh, S. Pharmacological Strategies on Medicinal Plants as Hepatoprotective Agents. Acta Sci. Pharm. Sci. 2019, 3, 9–14. [Google Scholar] [CrossRef]
- de Silva, H.A.; Saparamadu, P.A.M.; Thabrew, M.I.; Pathmeswaran, A.; Fonseka, M.M.D.; de Silva, H.J. Liv.52 in alcoholic liver disease: A prospective, controlled trial. J. Ethnopharmacol. 2003, 84, 47–50. [Google Scholar] [CrossRef]
- Aktay, G.; Deliorman, D.; Ergun, E.; Ergun, F.; Yeşilada, E.; Cevik, C. Hepatoprotective effects of Turkish folk remedies on experimental liver injury. J. Ethnopharmacol. 2000, 73, 121–129. [Google Scholar] [CrossRef]
- Zafar, R.; Mujahid Ali, S. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J. Ethnopharmacol. 1998, 63, 227–231. [Google Scholar] [CrossRef]
- Ahmed, B.; Al-Howiriny, T.A.; Siddiqui, A.B. Antihepatotoxic activity of seeds of Cichorium intybus. J. Ethnopharmacol. 2003, 87, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, I.; Ustün, O.; Yeşilada, E.; Sezik, E.; Akyürek, N. In vivo gastroprotective effects of five Turkish folk remedies against ethanol-induced lesions. J. Ethnopharmacol. 2002, 83, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mun, Y.-J.; Woo, W.-H.; Jeon, K.-S.; An, N.-H.; Park, J.-S. Effects of the ethanol extract of Cichorium intybus on the immunotoxicity by ethanol in mice. Int. Immunopharmacol. 2002, 2, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Gadgoli, C.; Mishra, S.H. Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J. Ethnopharmacol. 1999, 66, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, M.S.; Abdelsattar, E.A.; Khalifa, S.I.; El-Feraly, F.S. Isolation and identification of an anti-inflammatory principle from Capparis spinosa. Die Pharm. 1988, 43, 640–641. [Google Scholar]
- Ageel, A.M.; Parmar, N.S.; Mossa, J.S.; Al-Yahya, M.A.; Al-Said, M.S.; Tariq, M. Anti-inflammatory activity of some Saudi Arabian medicinal plants. Agents Actions 1986, 17, 383–384. [Google Scholar] [CrossRef]
- Gilani, A.H.; Janbaz, K.H.; Shah, B.H. Esculetin prevents liver damage induced by paracetamol and CCL4. Pharmacol. Res. 1998, 37, 31–35. [Google Scholar] [CrossRef]
- Martin-Aragón, S.; Benedi, J.M.; Villar, A.M. Effects of the antioxidant (6,7-dihydroxycoumarin) esculetin on the glutathione system and lipid peroxidation in mice. Gerontology 1998, 44, 21–25. [Google Scholar] [CrossRef]
- Germano, M.P.; De Pasquale, R.; D’angelo, V.; Catania, S.; Silvari, V.; Costa, C. Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J. Agric. Food Chem. 2002, 50, 1168–1171. [Google Scholar] [CrossRef]
- Raju, K.; Anbuganapathi, G.; Gokulakrishnan, V.; Rajkapoor, B.; Jayakar, B.; Manian, S. Effect of dried fruits of Solanum nigrum LINN against CCl4-induced hepatic damage in rats. Biol. Pharm. Bull. 2003, 26, 1618–1619. [Google Scholar] [CrossRef] [PubMed]
- Moundipa, P.F.; Domngang, F.M. Effect of the leafy vegetable Solanum nigrum on the activities of some liver drug-metabolizing enzymes after aflatoxin B1 treatment in female rats. Br. J. Nutr. 1991, 65, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Perwaiz, S.; Iqbal, M.; Athar, M. Crude extracts of hepatoprotective plants, Solanum nigrum and Cichorium intybus inhibit free radical-mediated DNA damage. J. Ethnopharmacol. 1995, 45, 189–192. [Google Scholar] [CrossRef]
- Jafri, M.A.; Jalis Subhani, M.; Javed, K.; Singh, S. Hepatoprotective activity of leaves of Cassia occidentalis against paracetamol and ethyl alcohol intoxication in rats. J. Ethnopharmacol. 1999, 66, 355–361. [Google Scholar] [CrossRef]
- Yadav, J.P.; Vedpriya, A.; Yadav, S.; Panghal, M.; Kumar, S.; Dhankhar, S. Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile|Request PDF. Fitoterapia 2010, 81, 223–230. [Google Scholar] [CrossRef]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Doorika, P.; Ananthi, T. Antioxidant and Hepatoprotective properties of Terminalia arjuna Bark on Isoniazid Induced Toxicity in Albino rats. Asian J. Pharm. Technol. 2012, 2, 15–18. [Google Scholar]
- Sumitra, M.; Manikandan, P.; Kumar, D.A.; Arutselvan, N.; Balakrishna, K.; Manohar, B.M.; Puvanakrishnan, R. Experimental myocardial necrosis in rats: Role of arjunolic acid on platelet aggregation, coagulation and antioxidant status. Mol. Cell. Biochem. 2001, 224, 135–142. [Google Scholar] [CrossRef]
- Urfi, M.K.; Mujahid, M.; Rahman, M.A.; Rahman, M.A. The Role of Tamarix gallica Leaves Extract in Liver Injury Induced by Rifampicin Plus Isoniazid in Sprague Dawley Rats. J. Diet. Suppl. 2018, 15, 24–33. [Google Scholar] [CrossRef]
- Devarshi, P.; Kanase, A.; Kanase, R.; Mane, S.; Patil, S.; Varute, A.T. Effect of Mandur bhasma on lipolytic activities of liver, kidney and adipose tissue of albino rat during CCl4 induced hepatic injury. J. Biosci. 1986, 10, 227–234. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Pavan, K.B.; Baig, M.R.; Murthy, M.O.; Azeemuddin, M.; Hariprasad, V.R.; Rafiq, M.; Rao, R.P. Treatment with a polyherbal extract improves fat metabolism, attenuates hepatic stellate cell activation and fibrogenesis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mitra, S.K.; Varma, S.R.; Godavarthi, A.; Nandakumar, K.S. Liv.52 regulates ethanol induced PPARγ and TNF α expression in HepG2 cells. Mol. Cell. Biochem. 2008, 315, 9–15. [Google Scholar] [CrossRef]
- Vidyashankar, S.; K Mitra, S.; Nandakumar, K.S. Liv.52 protects HepG2 cells from oxidative damage induced by tert-butyl hydroperoxide. Mol. Cell. Biochem. 2009, 333, 41. [Google Scholar] [CrossRef] [PubMed]
- Fallah Huseini, H.; Alavian, S.M.; Heshmat, R.; Heydari, M.R.; Abolmaali, K. The efficacy of Liv-52 on liver cirrhotic patients: A randomized, double-blind, placebo-controlled first approach. Phytomedicine 2005, 12, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Vidyashankar, S.; Sharath Kumar, L.M.; Barooah, V.; Sandeep Varma, R.; Nandakumar, K.S.; Patki, P.S. Liv.52 up-regulates cellular antioxidants and increase glucose uptake to circumvent oleic acid induced hepatic steatosis in HepG2 cells. Phytomedicine Int. J. Phytother. Phytopharm. 2012, 19, 1156–1165. [Google Scholar] [CrossRef]
- Dhawan, D.; Goel, A. Hepatoprotective effects of Liv-52 and its indirect influence on the regulation of thyroid hormones in rat liver toxicity induced by carbon tetrachloride. Res. Exp. Med. 1994, 194, 203–215. [Google Scholar] [CrossRef]
- Sandhir, R.; Gill, K.D. Hepatoprotective Effects of Liv-52 on Ethanol Induced Liver Damage in Rats; NISCAIR-CSIR: New Delhi, India, 1999; Volume 37, pp. 762–766. [Google Scholar]
- Kataria, M.; Singh, L.N. Hepatoprotective effect of Liv-52 and kumaryasava on carbon tetrachloride induced hepatic damage in rats. Indian J. Exp. Biol. 1997, 35, 655–657. [Google Scholar]
- Chandrashekhar, V.M.; Muchandi, A.A.; Sudi, S.V.; Ganapty, S. Hepatoprotective activity of Stereospermum suaveolens against CCl4-induced liver damage in albino rats. Pharm. Biol. 2010, 48, 524–528. [Google Scholar] [CrossRef]
- Eesha, B.R.; Mohanbabu, A.V.; Meena, K.K.; Vijay, M.; Lalit, M.; Rajput, R. Hepatoprotective activity of Terminalia paniculata against paracetamol induced hepatocellular damage in Wistar albino rats. Asian Pac. J. Trop. Med. 2011, 4, 466–469. [Google Scholar] [CrossRef]
- Azeemuddin, M.; Rafiq, M.; Anturlikar, S.D.; Kumar LM, S.; Patki, P.S.; Babu, U.V.; Shyam, R. Extract of a polyherbal formulation ameliorates experimental nonalcoholic steatohepatitis. J. Tradit. Complement. Med. 2016, 6, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Iroanya, O.O.; Adebesin, O.A.; Okpuzor, J. Evaluation of the hepato and nephron-protective effect of a polyherbal mixture using wistar albino rats. J. Clin. Diagn. Res. JCDR 2014, 8, HC15. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Ganapathi, N.G.; Venkatesh, P.; Rao, N.; Baliga, M.S. Evaluation of the radioprotective effect of Liv 52 in mice. Environ. Mol. Mutagen. 2006, 47, 490–502. [Google Scholar] [CrossRef]
- Mahashur, A.A.; Prabhudesai, P.P. Hepatitis and antitubercular therapy. J. Assoc. Physicians India 1991, 39, 595–596. [Google Scholar] [PubMed]
- Baskaran, U.L.; Sabina, E.P. Clinical and experimental research in antituberculosis drug-induced hepatotoxicity: A review. J. Integr. Med. 2017, 15, 27–36. [Google Scholar] [CrossRef]
- Kolhapure, S. Meta-analysis of 50 Phase III clinical trials in evaluation of efficacy and safety of Liv.52 in infective hepatitis. Med. Update 2004, 12, 51–61. [Google Scholar]
- Gupta, V.K. Experience with Liv.52 as an Adjuvant to Anti-tubercular Treatment. Capsule 1980, 6, 122–123. [Google Scholar]
- Choijamts, G.; Batsyren, C.; Orkhon, B.; Otgontyr, D.N.; Oyunerdene, X.; Zagirjav, T.; Erdenesuvd, E.; Pyrevbazar, O.; Tungalag, D.E.; Enkhtsetseg, M.B.; et al. Role of Liv.52 DS tablets as a hepatoprotective agent in tuberculosis patients receiving antitubercular drugs: A double blind placebo controlled study. J. Liver Clin. Res 2018, 5, 1042–1050. [Google Scholar]
- Dange, S.V. Liv.52 in the Prevention of Hepatotoxicity in Patients Receiving Antitubercular Drugs: A Metaanalysis. Indian J. Clin. Pract. 2010, 21, 81–86. [Google Scholar]
- Kishore, B.; Hazra, D.U.; Sachan, A.S.; Agrawal, B.M.; Bharadwaj, A.K.; Kumar, A.; Mehrotra, M.M.N. The effect of Liv.52 on liver functions of tubercular patients receiving second line anti-tubercular drugs. Probe 1978, 17, 125–131. [Google Scholar]
- Agal, S.; Prasad, S.R.; Mitra, S.K. Liv.52 DS Tablets Evaluation of Efficacy and Safety in Alcoholic Liver Cirrhosis. Med. Update 2007, 15, 25–32. [Google Scholar]
- Kalab, M.; Krechler, T. Effect of the Hepatoprotective Drug LIV 52 on Liver Damage. J. Czech Physicians 1997, 136, 758–760. [Google Scholar]
- Mahto, A.K.; Sailal, M. Hepatitis: Clinical, Biochemical and Ultrasonographic Evaluation. Indian Med. J. 2009, 1, 5. [Google Scholar]
- Ganesh, S.; Joshi, N.; Jain, M.K.; Sharma, L.; Desai, A.; Rafiq, M.; Babu, U.V.; Kumawat, R. Clinical and Safety Evaluation of Liv.52 in Alcoholic Liver Disease: A Review. Gastroenterol. Insights 2022, 13, 377–386. [Google Scholar] [CrossRef]
- Shaw, T.; Locarnini, S. Entecavir for the treatment of chronic hepatitis B. Expert Rev. Anti-Infect. Ther. 2004, 2, 853–871. [Google Scholar] [CrossRef]
- Habibullah, C.M.; Chandra, V.; Padmanaban, C.G.; Ramakrishna, R. Liv.52 in acute viral hepatitis–Results of a double blind study. Antiseptic 1978, 75, 491–493. [Google Scholar]
- Gupta, S.; Khatri, R.L.; Srivastava, G. Therapy of infectious hepatitis and other liver disorders. Probe 1972, 2, 93. [Google Scholar]
- Singh, K.K.; Singh, Y.K.; Sinha, S.K.; Sharma, V.; Mishra, B.N. Observations on the treatment of infective hepatitis with an indigenous drug Liv.52. Ind. Med. J. 1977, 5, 69–74. [Google Scholar]
- Kar, P.; Asim, M.; Sarma, M.P.; Patki, P.S. HD-03/ES: A promising herbal drug for HBV antiviral therapy. Antivir. Res. 2009, 84, 249–253. [Google Scholar] [CrossRef]
- Rajkumar, J.S.; Sekar, M.G.; Mitra, S.K. Safety and efficacy of oral HD-03/ES given for six months in patients with chronic hepatitis B virus infection. World J. Gastroenterol. WJG 2007, 13, 4103–4107. [Google Scholar] [CrossRef]
- Maji, A.; Goutam, D.; Rugvedi, P. Role of Liv.52 HB Capsules in the Management of Hepatitis B Infection: A Review. Indian J. Clin. Pract. 2013, 24, 422–428. [Google Scholar]
- Al-Khazraji, K.A.; Khalid, A.; Hashim, M.K.; Hashim, M.K.; Abdulla, S.K.; Abdulla, M.K.; Khudhair, I.H.; Abbas, W.K. Role of Liv.52 in Non-Infectious Chronic Liver Disease. Glob. J. Health Sci. 2022, 14, 76–92. [Google Scholar]
- Arora, A.; Kumar, A.; Anand, A.C.; Puri, P.; Dhiman, R.K.; Acharya, S.K.; Aggarwal, K.; Aggarwal, N.; Aggarwal, R.; Chawla, Y.K.; et al. Indian National Association for the Study of the Liver—Federation of Obstetric and Gynaecological Societies of India Position Statement on Management of Liver Diseases in Pregnancy. J. Clin. Exp. Hepatol. 2019, 9, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Combes, B. Viral Hepatitis During Pregnancy. JAMA 1965, 192, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.M. Hepatitis in pregnancy. Surg. Gynecol. Obstet. 1962, 114, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.K. Prognosis of infective hepatitis in pregnant women. In Hepatitis; Adoni Printers and Publishers: Bombay, India, 1975; pp. 50–53. [Google Scholar]
- Mitra, A.K.; Patki, P.S.; Mitra, S.K. Liver disorders during pregnancy and their management. Antiseptic 2008, 105, 193–196. [Google Scholar]
- Maity, D.S.G.; Mandal, D.A.K. A clinical comparative study to evaluate the efficacy and safety of Liv.52 DS tablets in non- alcoholic steatohepatitis (NASH). World J. Pharm. Res. 2015, 4, 17. [Google Scholar]
- Ghosh, S.; Singh, D.P.; Mandal, K.D.; Kumar, S. Evaluation of safety and efficacy of a Polyherbal formulation Liv.52 DS in the management of non-alcoholic steatohepatitis (NASH): An open clinical study. Int. J. Curr. Res. Acad. Rev. 2014, 2, 305–316. [Google Scholar]
- Shah, S.C.; Shah, P.S.; Pocha, N.P.; Siregar, G.; Srikrishna, H.A.; Kumawat, R. The Effects of Liv.52 DS Tablets on Various Liver Parameters in Non-Alcoholic Fatty Liver Disease: Preliminary Trend Identified from a Cumulative Efficacy Analysis. Int. J. Pharm. Clin. Res. 2022, 14, 739–751. [Google Scholar]
- Marginean, O.; Micle, I.; Lesovici, M.; Balean, R.; Ioana, B. Use of hepatoprotective agents in hepatomegaly syndrome in children: An experience with Liv.52 syrup. Medicine 2002, 10, 57–60. [Google Scholar]
- Jha, U.C.; Kumar, A.; Yadav, R. Comparative study to evaluate clinical profile and outcome in Hepatitis-B patients receiving tenofovir therapy compared to traditional herbal therapy. MedPulse Int. J. Med. 2021, 19, 106–111. [Google Scholar] [CrossRef]
- Fleig, W.; Morgan, M.Y.; Hölzer, M. The Ayurvedic drug LIV.52 in patients with alcoholic cirrhosis. Results of a prospective, randomized, double-blind, placebo-controlled clinical trial. J. Hepatol. 1997, 26, 127. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantharia, C.; Kumar, M.; Jain, M.K.; Sharma, L.; Jain, L.; Desai, A. Hepatoprotective Effects of Liv.52 in Chronic Liver Disease Preclinical, Clinical, and Safety Evidence: A Review. Gastroenterol. Insights 2023, 14, 293-308. https://doi.org/10.3390/gastroent14030021
Kantharia C, Kumar M, Jain MK, Sharma L, Jain L, Desai A. Hepatoprotective Effects of Liv.52 in Chronic Liver Disease Preclinical, Clinical, and Safety Evidence: A Review. Gastroenterology Insights. 2023; 14(3):293-308. https://doi.org/10.3390/gastroent14030021
Chicago/Turabian StyleKantharia, Chetan, Munesh Kumar, Mukesh Kumar Jain, Lokendra Sharma, Lokesh Jain, and Anish Desai. 2023. "Hepatoprotective Effects of Liv.52 in Chronic Liver Disease Preclinical, Clinical, and Safety Evidence: A Review" Gastroenterology Insights 14, no. 3: 293-308. https://doi.org/10.3390/gastroent14030021
APA StyleKantharia, C., Kumar, M., Jain, M. K., Sharma, L., Jain, L., & Desai, A. (2023). Hepatoprotective Effects of Liv.52 in Chronic Liver Disease Preclinical, Clinical, and Safety Evidence: A Review. Gastroenterology Insights, 14(3), 293-308. https://doi.org/10.3390/gastroent14030021




