Radiological Features of Microvascular Invasion of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
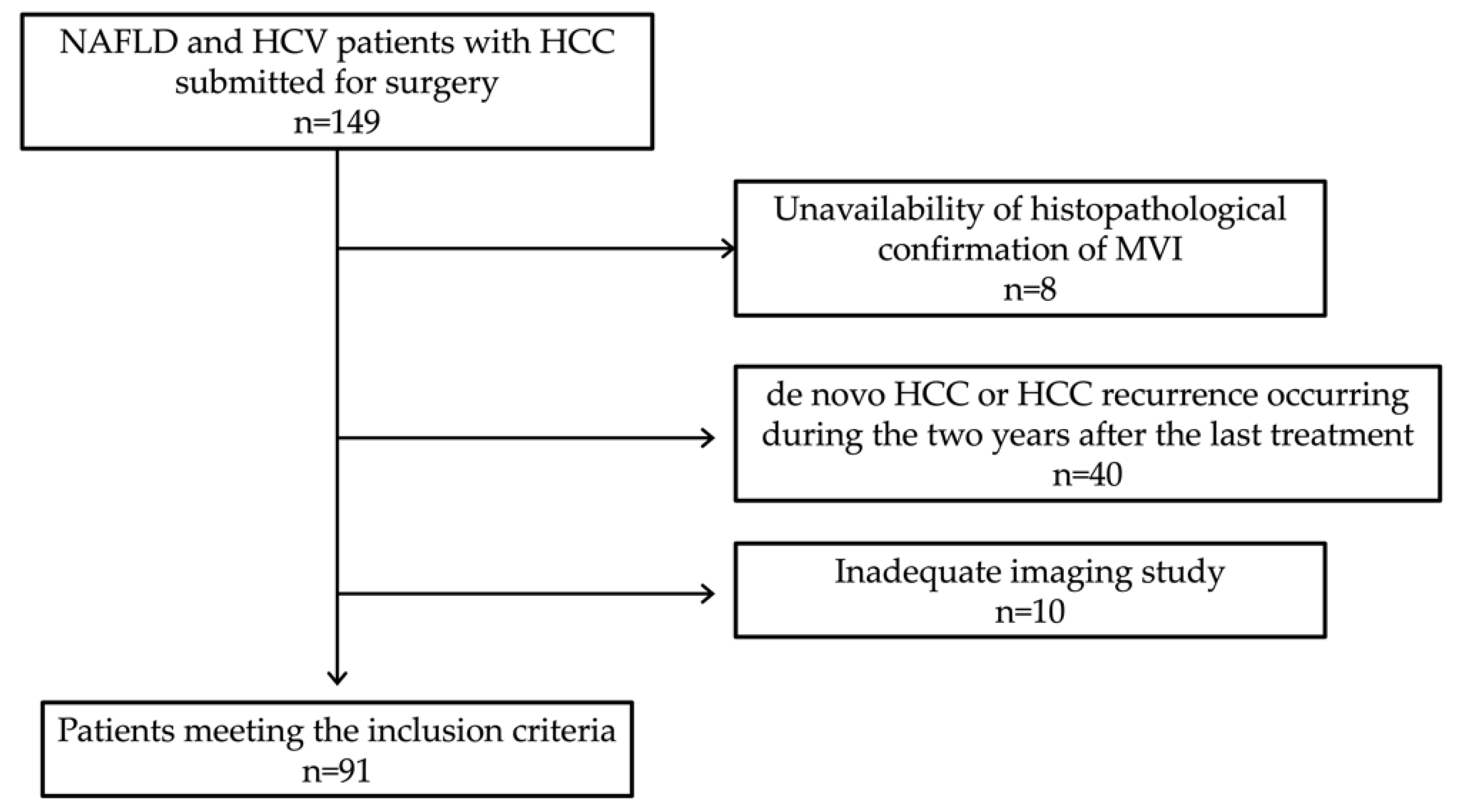
2. Materials and Methods
2.1. Imaging Analysis
2.2. Statistical Analysis
3. Results
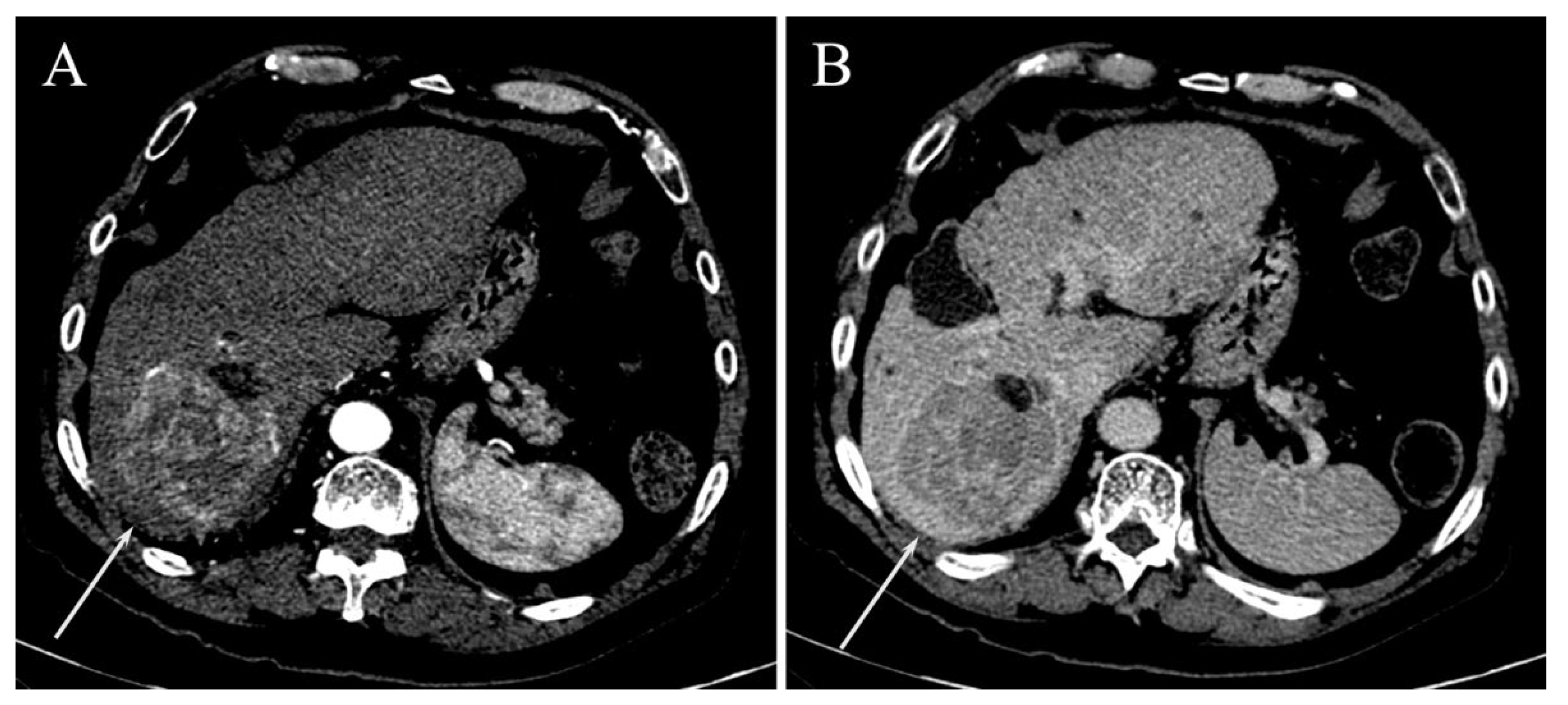
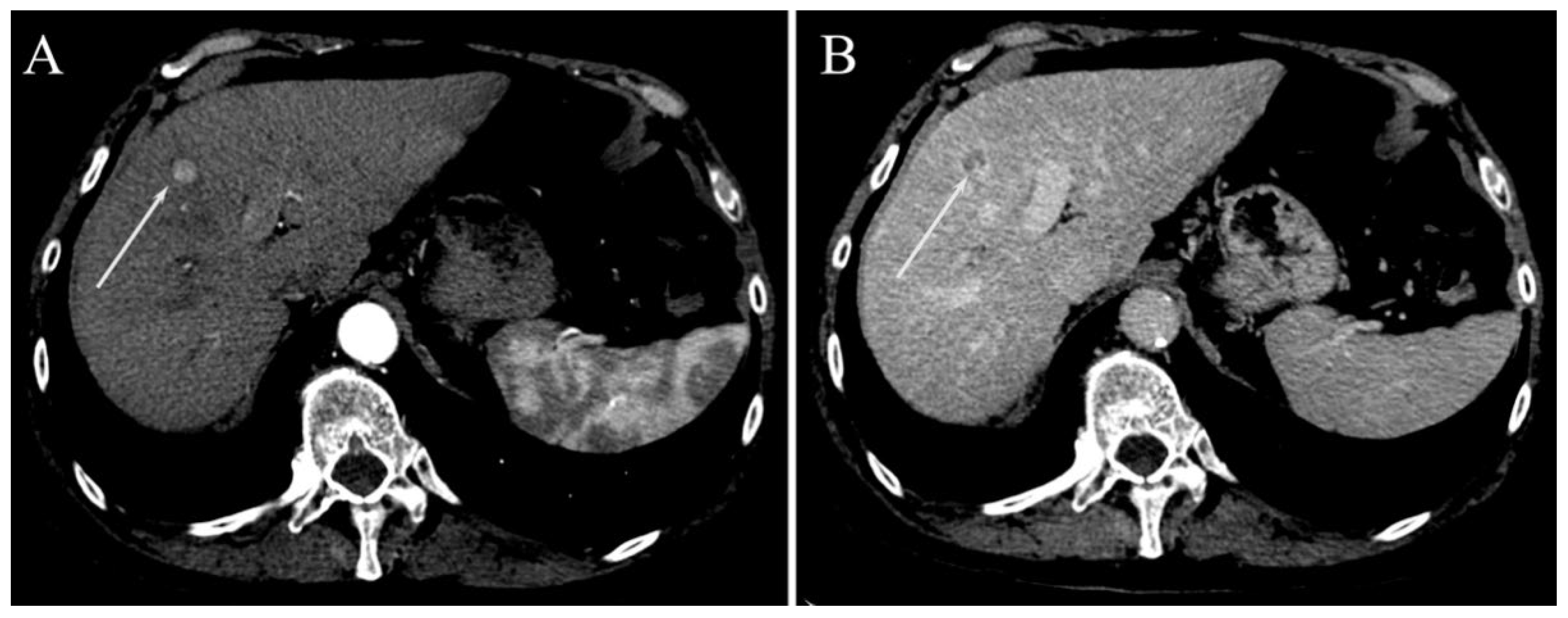
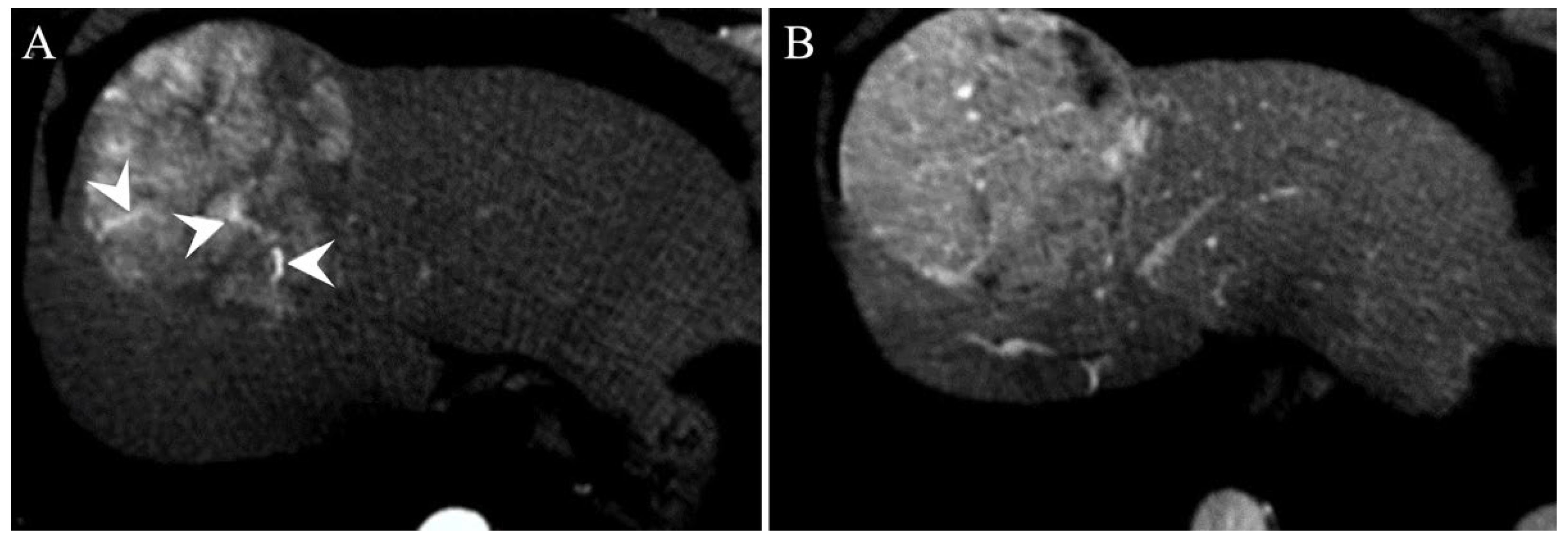
Surrogated Radiological Signs of MVI in NAFLD–HCC Patients and in HCV–HCC Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.A.; Morgan, R.O.; Shaib, Y.; McGlynn, K.A.; El-Serag, H.B. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology 2004, 127, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Harmsen, W.S.; Slettedahl, S.W.; Chaiteerakij, R.; Enders, F.T.; Therneau, T.M.; Orsini, L.; Kim, W.R.; Roberts, L.R. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin. Gastroenterol. Hepatol. 2011, 9, 617–623. [Google Scholar] [CrossRef]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; Giannini, E.G.B. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Sheth, S.G.; Gordon, F.D.; Chopra, S. Nonalcoholic steatohepatitis. Ann. Intern. Med. 1997, 126, 137–145. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef]
- Caldwell, S.; Marchesini, G. Cryptogenic vs. NASH-cirrhosis: The rose exists well before its name. J. Hepatol. 2018, 68, 391–392. [Google Scholar] [CrossRef]
- Davila, J.A.; Morgan, R.O.; Shaib, Y.; McGlynn, K.A.; El-Serag, H.B. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut 2005, 54, 533–539. [Google Scholar] [CrossRef]
- Rosmorduc, O.; Fartoux, L. HCC and NASH: How strong is the clinical demonstration? Clin. Res. Hepatol. Gastroenterol. 2012, 36, 202–208. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, P.L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Baffy, G.; Brunt, E.M.; Caldwell, S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J. Hepatol. 2012, 56, 1384–1391. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Renzulli, M.; Golfieri, R.; Bologna Liver Oncology Group. Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the Western world for patients under surveillance for chronic liver disease. J. Gastroenterol. Hepatol. 2016, 31, 69–80. [Google Scholar] [CrossRef]
- Tovoli, F.; Renzulli, M.; Negrini, G.; Brocchi, S.; Ferrarini, A.; Andreone, A.; Benevento, F.; Golfieri, R.; Morselli-Labate, A.M.; Mastroroberto, M.; et al. Inter-operator variability and source of errors in tumour response assessment for hepatocellular carcinoma treated with sorafenib. Eur. Radiol. 2018, 28, 3611–3620. [Google Scholar] [CrossRef]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol. Med. 2022, 127, 129–144. [Google Scholar] [CrossRef]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Shen, F.; Lau, W.Y. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2018, 33, 347–354. [Google Scholar] [CrossRef]
- Miyata, R.; Tanimoto, A.; Wakabayashi, G.; Shimazu, M.; Nakatsuka, S.; Mukai, M.; Kitajima, M. Accuracy of preoperative prediction of microinvasion of portal vein in hepatocellular carcinoma using superparamagnetic iron oxide-enhanced magnetic resonance imaging and computed tomography during hepatic angiography. J. Gastroenterol. 2006, 41, 987–995. [Google Scholar] [CrossRef]
- Gouw, A.S.; Balabaud, C.; Kusano, H.; Todo, S.; Ichida, T.; Kojiro, M. Markers for microvascular invasion in hepatocellular carcinoma: Where do we stand? Liver Transpl. 2011, 17 (Suppl. 2), S72–S80. [Google Scholar] [CrossRef]
- Hirokawa, F.; Hayashi, M.; Miyamoto, Y.; Asakuma, M.; Shimizu, T.; Komeda, K.; Inoue, Y.; Uchiyama, K. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol. Res. 2014, 44, 846–853. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Anders, R.A.; Assumpcao, L.; Maley, W.; Choti, M.A. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: Implications for transplant eligibility. Ann. Surg. 2007, 245, 435–442. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Llovet, J.M.; Schwartz, M.; Mazzaferro, V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 2005, 25, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Brocchi, S.; Cucchetti, A.; Mazzotti, F.; Mosconi, C.; Sportoletti, C.; Brandi, G.; Pinna, A.D.; Golfieri, R. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology 2016, 279, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Roayaie, S.; Blume, I.N.; Thung, S.N.; Guido, M.; Fiel, M.I.; Hiotis, S.; Labow, D.M.; Llovet, J.M.; Schwartz, M.E. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009, 137, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Dajti, E.; Ierardi, A.M.; Brandi, N.; Berzigotti, A.; Milandri, M.; Rossini, B.; Clemente, A.; Ravaioli, F.; Marasco, G.; et al. Validation of a standardized CT protocol for the evaluation of varices and porto-systemic shunts in cirrhotic patients. Eur. J. Radiol. 2022, 147, 110010. [Google Scholar] [CrossRef]
- Pecorelli, A.; Lenzi, B.; Gramenzi, A.; Garuti, F.; Farinati, F.; Glannini, E.G.; Ciccarese, F.; Piscaglia, F.; Rapaccini, G.L.; Marco, M.D.; et al. Curative therapies are superior to standard of care (transarterial chemoembolization) for intermediate stage hepatocellular carcinoma. Liver Int. 2017, 37, 423–433. [Google Scholar] [CrossRef]
- Chou, C.T.; Chen, R.C.; Lee, C.W.; Ko, C.J.; Wu, H.K.; Chen, Y.L. Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br. J. Radiol. 2012, 85, 778–783. [Google Scholar] [CrossRef]
- Hu, H.; Qi, S.; Zeng, S.; Zhang, P.; He, L.; Wen, S.; Zeng, N.; Yang, J.; Zhang, W.; Zhu, W.; et al. Importance of Microvascular Invasion Risk and Tumor Size on Recurrence and Survival of Hepatocellular Carcinoma After Anatomical Resection and Non-anatomical Resection. Front. Oncol. 2021, 11, 621622. [Google Scholar] [CrossRef]
- Compagnone, G.; Giampalma, E.; Domenichelli, S.; Renzulli, M.; Golfieri, R. Calculation of conversion factors for effective dose for various interventional radiology procedures. Med. Phys. 2012, 39, 2491–2498. [Google Scholar] [CrossRef]
- Terzi, E.; Terenzi, L.; Venerandi, L.; Croci, L.; Renzulli, M.; Mosconi, C.; Allegretti, G.; Granito, A.; Golfieri, R.; Bolondi, L.; et al. The ART score is not effective to select patients for transarterial chemoembolization retreatment in an Italian series. Dig. Dis. 2014, 32, 711–716. [Google Scholar] [CrossRef]
- Tovoli, F.; Ielasi, L.; Casadei-Gardini, A.; Granito, A.; Foschi, F.G.; Rovesti, G.; Negrini, G.; Renzulli, M.; Piscaglia, F. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J. Hepatol. 2019, 71, 1175–1183. [Google Scholar] [CrossRef]
- Yang, L.; Gu, D.; Wei, J.; Yang, C.; Rao, S.; Wang, W.; Chen, C.; Ding, Y.; Tian, J.; Zeng, M.; et al. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 373–386. [Google Scholar] [CrossRef]
- Hong, S.B.; Choi, S.H.; Kim, S.Y.; Shim, J.H.; Lee, S.S.; Byun, J.H.; Park, S.H.; Kim, K.W.; Kim, S.; Lee, N.K. MRI Features for Predicting Microvascular Invasion of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2021, 10, 94–106. [Google Scholar] [CrossRef]
- Min, J.H.; Lee, M.W.; Park, H.S.; Lee, D.H.; Park, H.J.; Lim, S.; Choi, S.Y.; Lee, J.; Lee, J.E.; Ha, S.Y.; et al. Interobserver Variability and Diagnostic Performance of Gadoxetic Acid-enhanced MRI for Predicting Microvascular Invasion in Hepatocellular Carcinoma. Radiology 2020, 297, 573–581. [Google Scholar] [CrossRef]
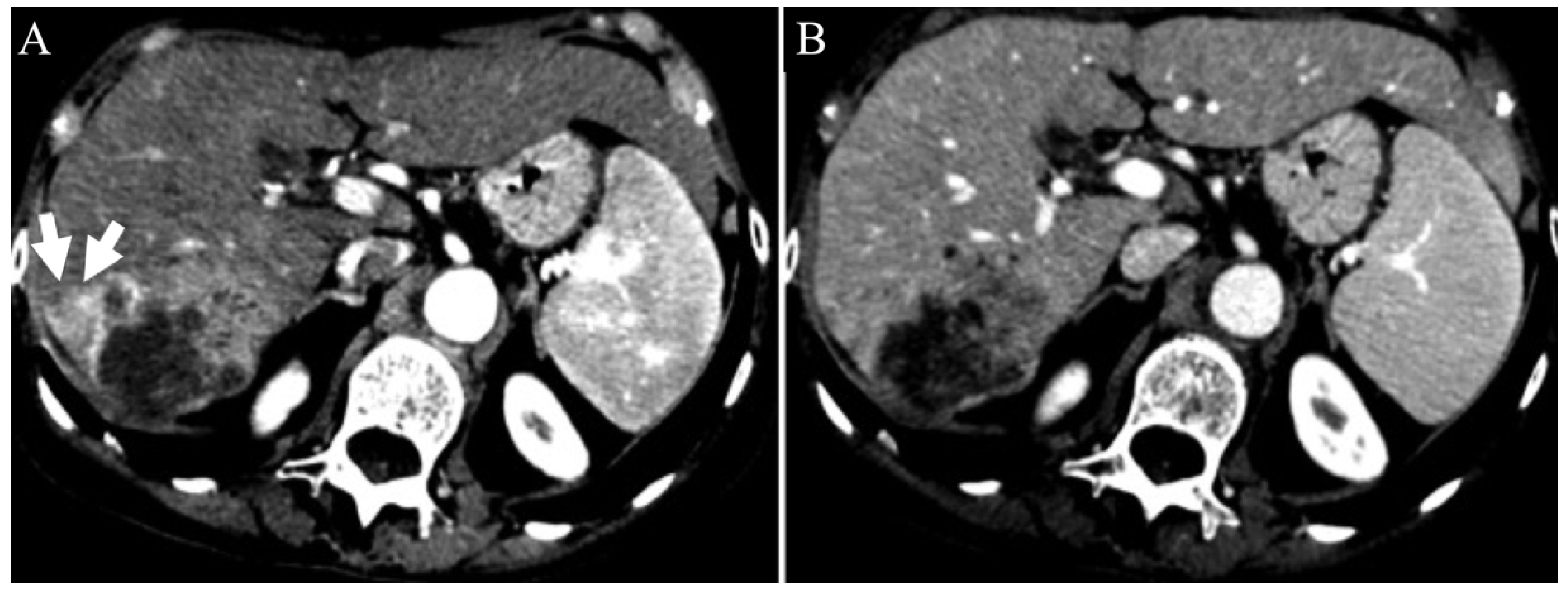
| Variable | NAFLD (n = 36) | HCV (n = 55) | p |
|---|---|---|---|
| Males | 31 (86.8%) | 41 (74.1%) | 0.749 |
| Age (years) | 69 (50–86) | 70 (40–87) | 1.000 |
| BMI (kg/m2): mean ± SD | 28.6 (±5) | 27.1 (±4.1) | 0.871 |
| Diameter (mm): mean ± SD | 51 (±23) | 26 (±16) | <0.0001 |
| Multinodularity | 14 (38.8%) | 32 (58.0%) | 0.349 |
| BCLC | |||
| Very early (0) | 14 (38.8%) | 22 (40%) | 0.882 |
| Early (A) | 22 (61.2%) | 33 (60%) | |
| Child–Pugh | |||
| Class A5 | 15 (41.7%) | 24 (43.6%) | 0.798 |
| Class A6 | 21 (58.3%) | 31 (56.4%) | |
| Milan in | 30 (83.3%) | 55 (100%) | 0.641 |
| MVI Parameter | NAFLD (n = 36) | HCV (n = 55) | p |
|---|---|---|---|
| Diameter (mm): mean ± SD | 51 (±23) | 26 (±16) | 0.004 |
| Irregular margins | 21 (58.3%) | 26 (47.3%) | 0.391 |
| TTPVI | 14 (38.9%) | 11 (20%) | 0.058 |
| Peritumoral enhancement | 4 (11.1%) | 6 (10.9%) | 1.000 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | B (95% CI) | p | B (95% CI) | p |
| Diameter > 50 mm | 21.300 (4.200–107.700) | <0.001 | 21.300 (4.200–107.700) | <0.001 |
| Multinodularity | 1.365 (0.492–3.791) | 0.671 | ||
| Irregular margins | 1.428 (0.758–2.690) | 0.447 | ||
| Peritumoral enhancement | 1.119 (0.939–2.784) | 0.340 | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Age | 1.010 (0.989–1.018) | 0.399 | ||
| Male sex | 1.005 (0.993–1.759) | 0.121 | ||
| Size of the largest nodule | 1.020 (1.020–1.080) | 0.021 | 1.117 (0.787–1.585) | 0.352 |
| Multinodularity | 1.287 (0.959–1.727) | 1.333 | ||
| Irregular margins | 1.000 (1.000–1.000) | 0.318 | ||
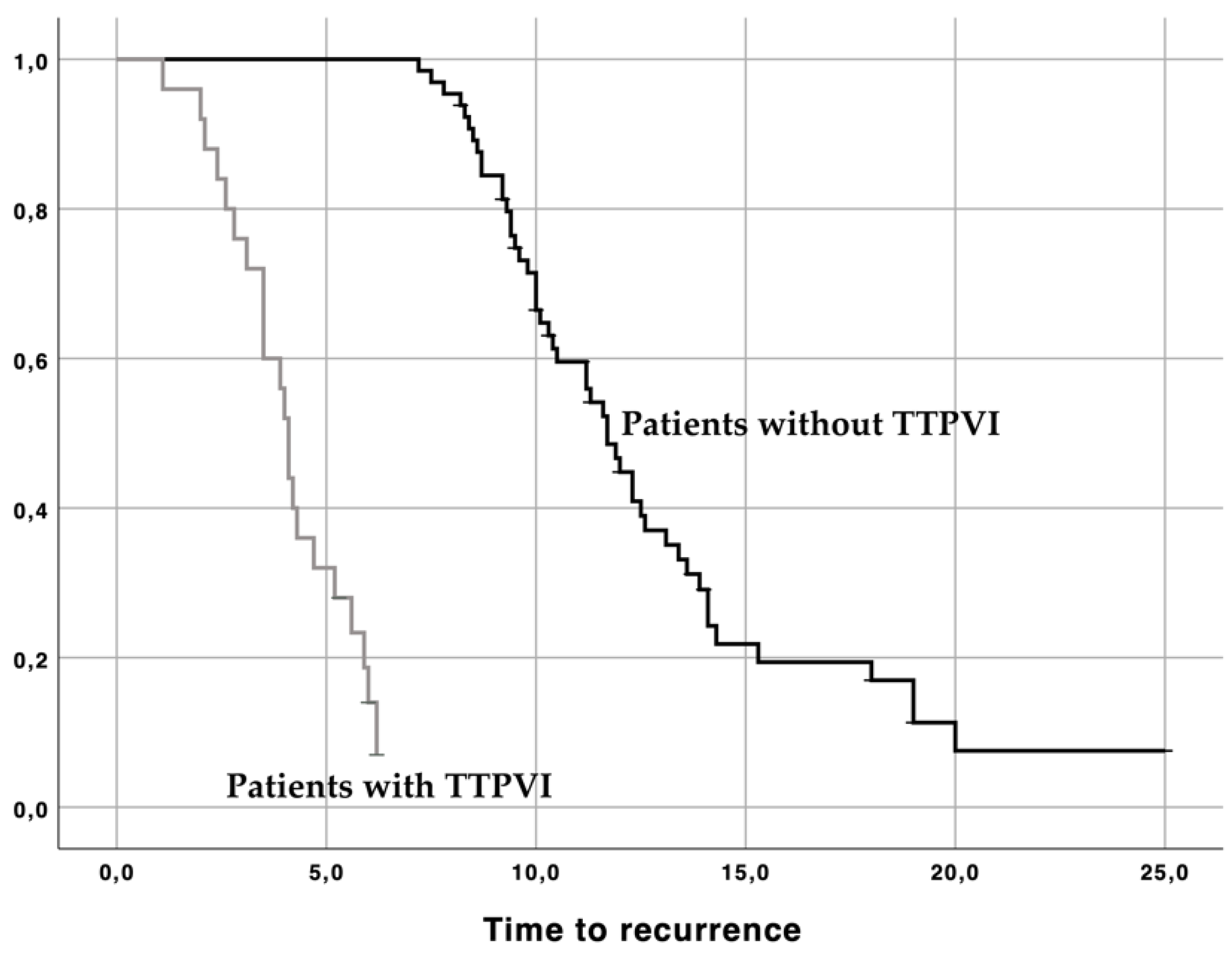
| TTPVI | 2.058 (1.103–3.124) | 0.015 | 2.349 (1.369–4.032) | 0.002 |
| Peritumoral enhancement | 1.152 (0.865–1.534) | 0.334 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzulli, M.; Pecorelli, A.; Brandi, N.; Marasco, G.; Adduci, F.; Tovoli, F.; Stefanini, B.; Granito, A.; Golfieri, R. Radiological Features of Microvascular Invasion of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterol. Insights 2022, 13, 275-285. https://doi.org/10.3390/gastroent13030028
Renzulli M, Pecorelli A, Brandi N, Marasco G, Adduci F, Tovoli F, Stefanini B, Granito A, Golfieri R. Radiological Features of Microvascular Invasion of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology Insights. 2022; 13(3):275-285. https://doi.org/10.3390/gastroent13030028
Chicago/Turabian StyleRenzulli, Matteo, Anna Pecorelli, Nicolò Brandi, Giovanni Marasco, Francesco Adduci, Francesco Tovoli, Bernardo Stefanini, Alessandro Granito, and Rita Golfieri. 2022. "Radiological Features of Microvascular Invasion of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease" Gastroenterology Insights 13, no. 3: 275-285. https://doi.org/10.3390/gastroent13030028
APA StyleRenzulli, M., Pecorelli, A., Brandi, N., Marasco, G., Adduci, F., Tovoli, F., Stefanini, B., Granito, A., & Golfieri, R. (2022). Radiological Features of Microvascular Invasion of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology Insights, 13(3), 275-285. https://doi.org/10.3390/gastroent13030028












