An Extreme Case of Liver Adenomatosis: Are They All the Same?
Abstract
:1. Introduction
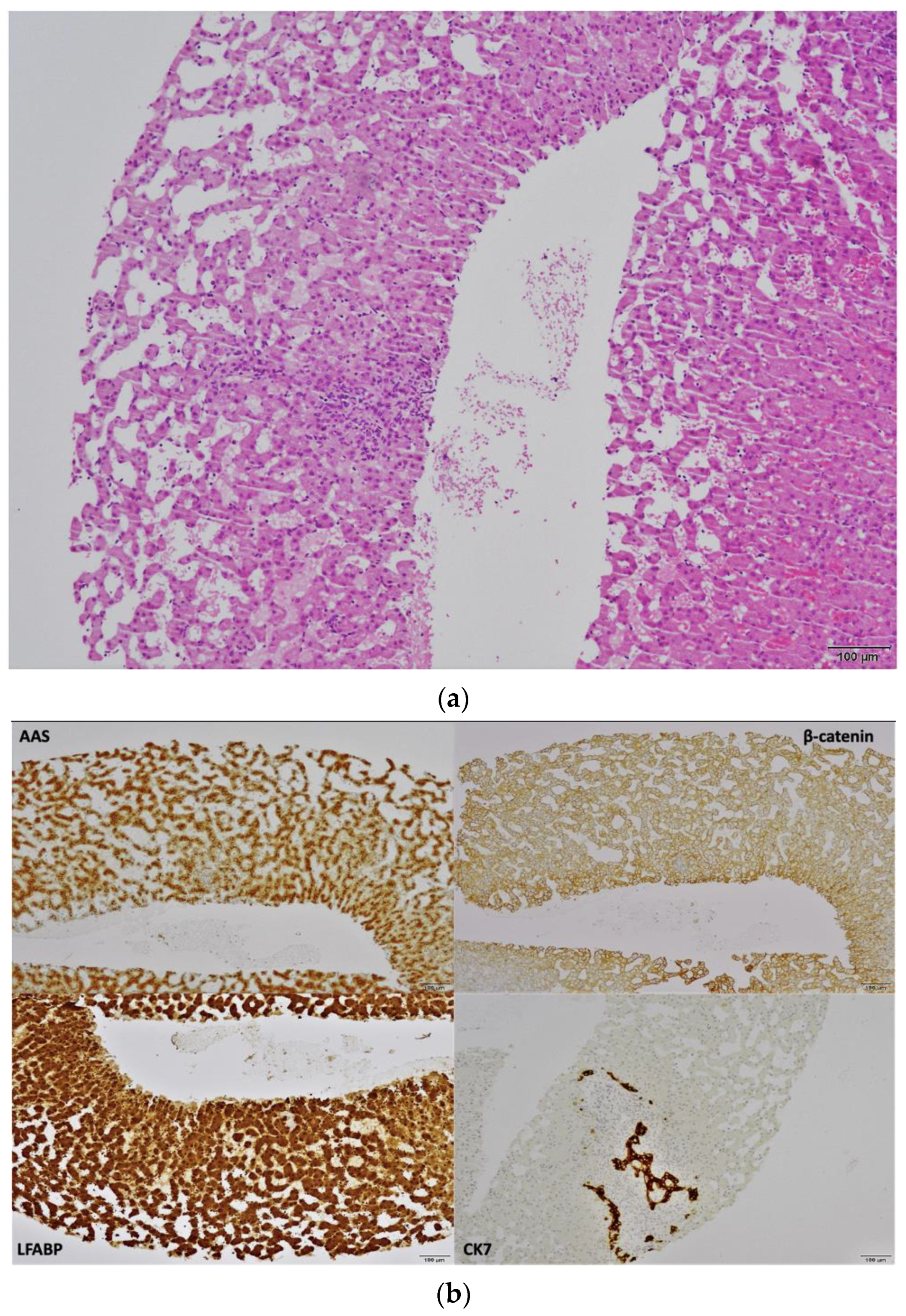
2. Case Report
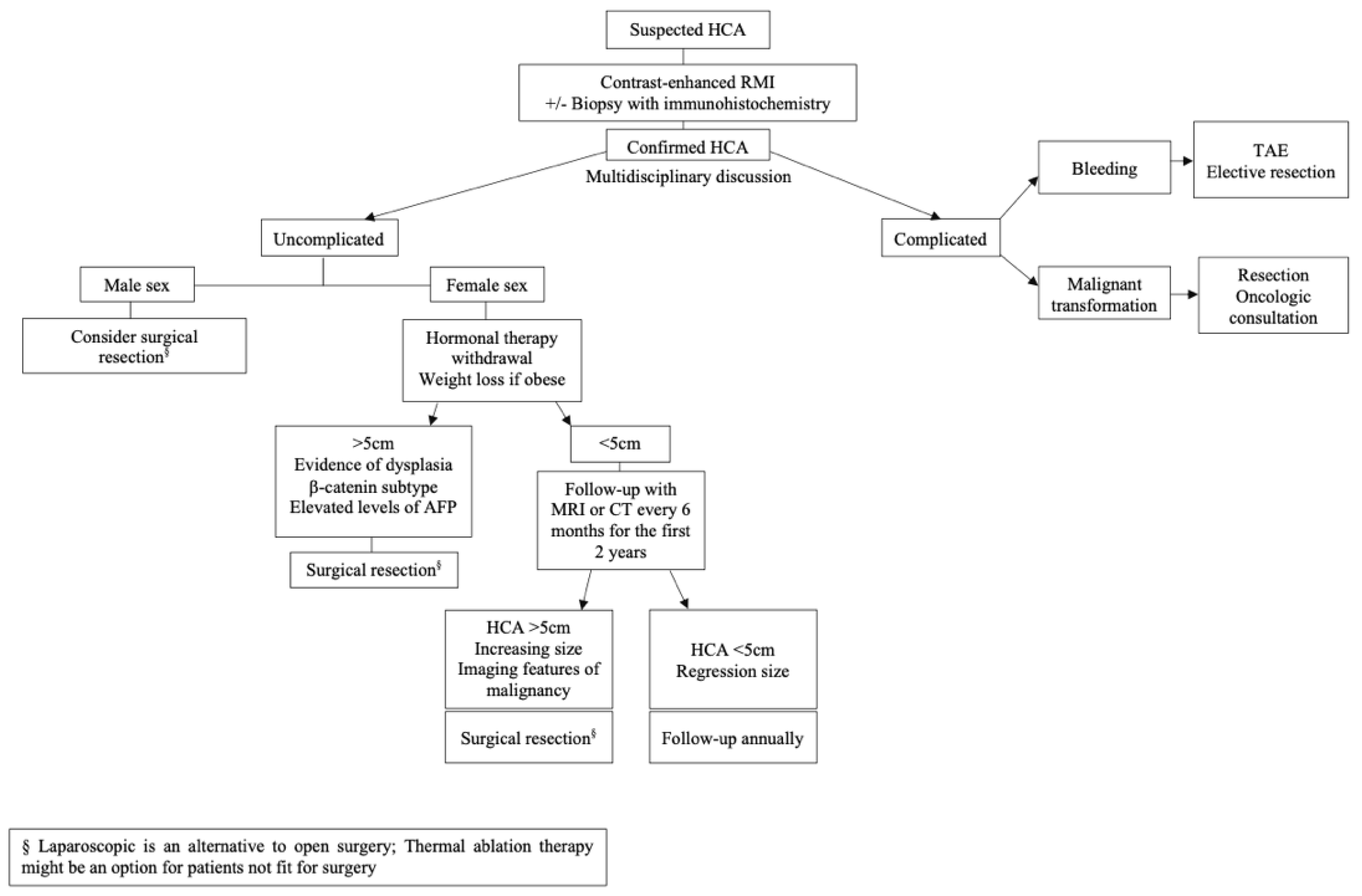
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of benign liver tumours. J. Hepatol. 2015, 65, 386–398. [Google Scholar]
- Rooks, J.B.; Ory, H.W.; Ishak, K.G.; Strauss, L.T.; Greenspan, J.R.; Hill, A.P.; Tyler, C.W., Jr. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 1979, 242, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Nault, J.C.; Dujardin, F.; Scotto, B.; Besson, M.; de Muret, A.; Bourlier, P.; Zucman-Rossi, J.; Salamé, E.; Bacq, Y. Natural history of liver adenomatosis: A long-term observational study. J. Hepatol. 2019, 71, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Sundar Alagusundaramoorthy, S.; Vilchez, V.; Zanni, A.; Sourianarayanane, A.; Maynard, E.; Shah, M.; Daily, M.F.; Pena, L.R.; Gedaly, R. Role of Transplantation in the Treatment of Benign Solid Tumors of the Liver: A Review of the United Network of Organ Sharing Data Set. JAMA Surg. 2015, 150, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karhunen, P.J. Benign hepatic tumours and tumour like conditions in men. J. Clin. Pathol. 1986, 39, 183–188. Available online: https://pubmed.ncbi.nlm.nih.gov/3950039 (accessed on 8 January 2022). [CrossRef] [PubMed] [Green Version]
- Bonder, A.; Afdhal, N. Evaluation of liver lesions. Clin. Liver Dis. 2012, 16, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Giannitrapani, L.; Soresi, M.; La Spada, E.; Cervello, M.; D’Alessandro, N.; Montalto, G. Sex hormones and risk of liver tumor. Ann. N. Y. Acad. Sci. 2006, 1089, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Dokmak, S.; Belghiti, J. Will weight loss become a future treatment of hepatocellular adenoma in obese patients? Liver Int. Off. J. Int. Assoc. Study Liver 2015, 35, 2228–2232. [Google Scholar] [CrossRef]
- Grangé, J.D.; Guéchot, J.; Legendre, C.; Giboudeau, J.; Darnis, F.; Poupon, R. Liver adenoma and focal nodular hyperplasia in a man with high endogenous sex steroids. Gastroenterology 1987, 93, 1409–1413. [Google Scholar] [CrossRef]
- Triantafyllopoulou, M.; Whitington, P.F.; Melin-Aldana, H.; Benya, E.C.; Brickman, W. Hepatic adenoma in an adolescent with elevated androgen levels. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 640–642. [Google Scholar] [CrossRef]
- Renzulli, M.; Clemente, A.; Tovoli, F.; Cappabianca, S.; Bolondi, L.; Golfieri, R. Hepatocellular adenoma: An unsolved diagnostic enigma. World J. Gastroenterol. 2019, 25, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.A.; Zeina, A.R.; Mouch, S.A.; Mari, A. Benign Hepatic Tumors: From Incidental Imaging Finding to Clinical Management. Korean J. Fam. Med. 2021, 42, 2–28. [Google Scholar] [CrossRef] [PubMed]
- Monges, H.; Payan, H.; Legre, M.; Vignoli, R. Considerations on a case of benign multinodular adenoma of the liver. Arch. Mal. Appar. Dig. Mal. Nutr. 1963, 52, 725–737. [Google Scholar] [PubMed]
- Monaco, A.P.; Hallgrimsson, J.; Mcdermott, W.V.J. Multiple adenoma (hamartoma) of the liver treated by subtotal (90 percent) resection: Morphological and functional studies of regeneration. Ann. Surg. 1964, 159, 513–519. [Google Scholar] [CrossRef]
- Flejou, J.F.; Barge, J.; Menu, Y.; Degott, C.; Bismuth, H.; Potet, F.; Benhamou, J.P. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology 1985, 89, 1132–1138. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Rahnemai-Azar, A.A.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Moris, D.; Spartalis, E.; Cloyd, J.M.; Weber, S.M.; Pawlik, T.M. Current Approaches in the Management of Hepatic Adenomas. J. Gastrointest. Surg. 2019, 23, 199–209. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Laumonier, H.; Couchy, G.; Le Bail, B.; Sa Cunha, A.; Rullier, A.; Laurent, C.; Blanc, J.F.; Cubel, G.; Trillaud, H.; et al. Hepatocellular adenoma management and phenotypic classification: The Bordeaux experience. Hepatology 2009, 50, 481–489. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Rebouissou, S.; Thomas, C.; Blanc, J.-F.; Saric, J.; Sa Cunha, A.; Rullier, A.; Cubel, G.; Couchy, G.; Imbeaud, S.; et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007, 46, 740–748. [Google Scholar] [CrossRef]
- Rebouissou, S.; Bioulac-Sage, P.; Zucman-Rossi, J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J. Hepatol. 2008, 48, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Nault, J.C.; Couchy, G.; Balabaud, C.; Morcrette, G.; Caruso, S.; Blanc, J.F.; Bacq, Y.; Calderaro, J.; Paradis, V.; Ramos, J.; et al. Molecular Classification of Hepatocellular Adenoma Associates with Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology 2017, 152, 880–894.e6. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.W.; Marsh, J.W.; Steel, J.; Holloway, S.E.; Heckman, J.T.; Ochoa, E.R.; Geller, D.A.; Gamblin, T.C. Surgical management of hepatocellular adenoma: Take it or leave it? Ann. Surg. Oncol. 2008, 15, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Junior, M.A.F.; Chaib, E.; Saad, W.A.; D’Albuquerque, L.A.C.; Cecconello, I. Surgical management of spontaneous ruptured hepatocellular adenoma. Clinics 2009, 64, 775–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Aalten, S.M.; de Man, R.A.; IJzermans, J.N.M.; Terkivatan, T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br. J. Surg. 2012, 99, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Farges, O.; Ferreira, N.; Dokmak, S.; Belghiti, J.; Bedossa, P.; Paradis, V. Changing trends in malignant transformation of hepatocellular adenoma. Gut 2011, 60, 85–89. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Jeannot, E.; Nhieu, J.T.; Van Scoazec, J.-Y.; Guettier, C.; Rebouissou, S.; Bacq, Y.; Leteurtre, E.; Paradis, V.; Michalak, S.; et al. Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology 2006, 43, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.M.; van den Bos, I.C.; Dwarkasing, R.S.; Kuiper, J.W.; den Hollander, J. Hepatocellular adenoma: Findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur. Radiol. 2006, 16, 1873–1886. [Google Scholar] [CrossRef]
- Thapar, M.; Grapp, O.; Fisher, C. Management of Hepatic Adenomatosis. Curr. Gastroenterol. Rep. 2015, 17, 12. [Google Scholar] [CrossRef]
- Krause, K.; Tanabe, K.K. A Shifting Paradigm in Diagnosis and Management of Hepatic Adenoma. Ann. Surg. Oncol. 2020, 27, 3330–3338. [Google Scholar] [CrossRef]
- Nault, J.C.; Paradis, V.; Cherqui, D.; Vilgrain, V.; Zucman-Rossi, J. Molecular classification of hepatocellular adenoma in clinical practice. J. Hepatol. 2017, 67, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Pilati, C.; Letouzé, E.; Nault, J.-C.; Imbeaud, S.; Boulai, A.; Calderaro, J.; Poussin, K.; Franconi, A.; Couchy, G.; Morcrette, G.; et al. Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell 2014, 25, 428–441. [Google Scholar] [CrossRef] [Green Version]
- Torbenson, M. Hepatic Adenomas: Classification, Controversies, and Consensus. Surg. Pathol. Clin. 2018, 11, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.C.; Campbell, C.M.; Manoharan, B.; Bryant, R.; Cavallucci, D.; O’Rourke, N.; Clouston, A.D. Subclassification of hepatocellular adenomas: Practical considerations in the implementation of the Bordeaux criteria. Pathology 2018, 50, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Thomeer, M.G.; Broker, M.; Verheij, J.; Doukas, M.; Terkivatan, T.; Bijdevaate, D.; de Man, R.A.; Moelker, A.; IJzermans, J.N. Hepatocellular adenoma: When and how to treat? Update of current evidence. Ther. Adv. Gastroenterol. 2016, 9, 898–912. [Google Scholar] [CrossRef]
- Liau, S.-S.; Qureshi, M.S.; Praseedom, R.; Huguet, E. Molecular pathogenesis of hepatic adenomas and its implications for surgical management. J. Gastrointest. Surg. Off. J. Soc.Surg. Aliment. Tract. 2013, 17, 1869–1882. [Google Scholar] [CrossRef] [Green Version]
- Klompenhouwer, A.J.; de Man, R.A.; Dioguardi Burgio, M.; Vilgrain, V.; Zucman-Rossi, J.; Ijzermans, J.N.M. New insights in the management of Hepatocellular Adenoma. Liver Int. 2020, 40, 1529–1537. Available online: https://pubmed.ncbi.nlm.nih.gov/32464711 (accessed on 8 January 2022). [CrossRef] [PubMed]
- Agrawal, S.; Agarwal, S.; Arnason, T.; Saini, S.; Belghiti, J. Management of Hepatocellular Adenoma: Recent Advances. Clin. Gastroenterol. Hepatol. 2015, 13, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- van Rosmalen, B.V.; Coelen, R.J.S.; Bieze, M.; van Delden, O.M.; Verheij, J.; Dejong, C.H.C.; van Gulik, T.M. Systematic review of transarterial embolization for hepatocellular adenomas. Br. J. Surg. 2017, 104, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Mironov, O.; Jaberi, A.; Beecroft, R.; Kachura, J.R. Retrospective Single-Arm Cohort Study of Patients with Hepatocellular Adenomas Treated with Percutaneous Thermal Ablation. Cardiovasc. Interv. Radiol. 2018, 41, 935–941. [Google Scholar] [CrossRef]
- Smolock, A.R.; Cristescu, M.M.; Potretzke, T.A.; Ziemlewicz, T.J.; Lubner, M.G.; Hinshaw, J.L.; Brace, C.L.; Lee, F.T., Jr. Microwave Ablation for the Treatment of Hepatic Adenomas. J. Vasc. Interv. Radiol. 2016, 27, 244–249. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chálim Rebelo, C.; Moura, D.B.; Flor de Lima, M.; Dutra, E.; Amaral, R.; Pereira, J.R.; Duarte, M.A. An Extreme Case of Liver Adenomatosis: Are They All the Same? Gastroenterol. Insights 2022, 13, 68-76. https://doi.org/10.3390/gastroent13010008
Chálim Rebelo C, Moura DB, Flor de Lima M, Dutra E, Amaral R, Pereira JR, Duarte MA. An Extreme Case of Liver Adenomatosis: Are They All the Same? Gastroenterology Insights. 2022; 13(1):68-76. https://doi.org/10.3390/gastroent13010008
Chicago/Turabian StyleChálim Rebelo, Carolina, Diogo Bernardo Moura, Margarida Flor de Lima, Eduardo Dutra, Rui Amaral, José Renato Pereira, and Maria Antónia Duarte. 2022. "An Extreme Case of Liver Adenomatosis: Are They All the Same?" Gastroenterology Insights 13, no. 1: 68-76. https://doi.org/10.3390/gastroent13010008
APA StyleChálim Rebelo, C., Moura, D. B., Flor de Lima, M., Dutra, E., Amaral, R., Pereira, J. R., & Duarte, M. A. (2022). An Extreme Case of Liver Adenomatosis: Are They All the Same? Gastroenterology Insights, 13(1), 68-76. https://doi.org/10.3390/gastroent13010008





