The Central Variant of Posterior Reversible Encephalopathy Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality and Risk of Bias Assessment
2.5. Statistical Analysis and Meta Analysis
2.6. Data Validation
3. Results
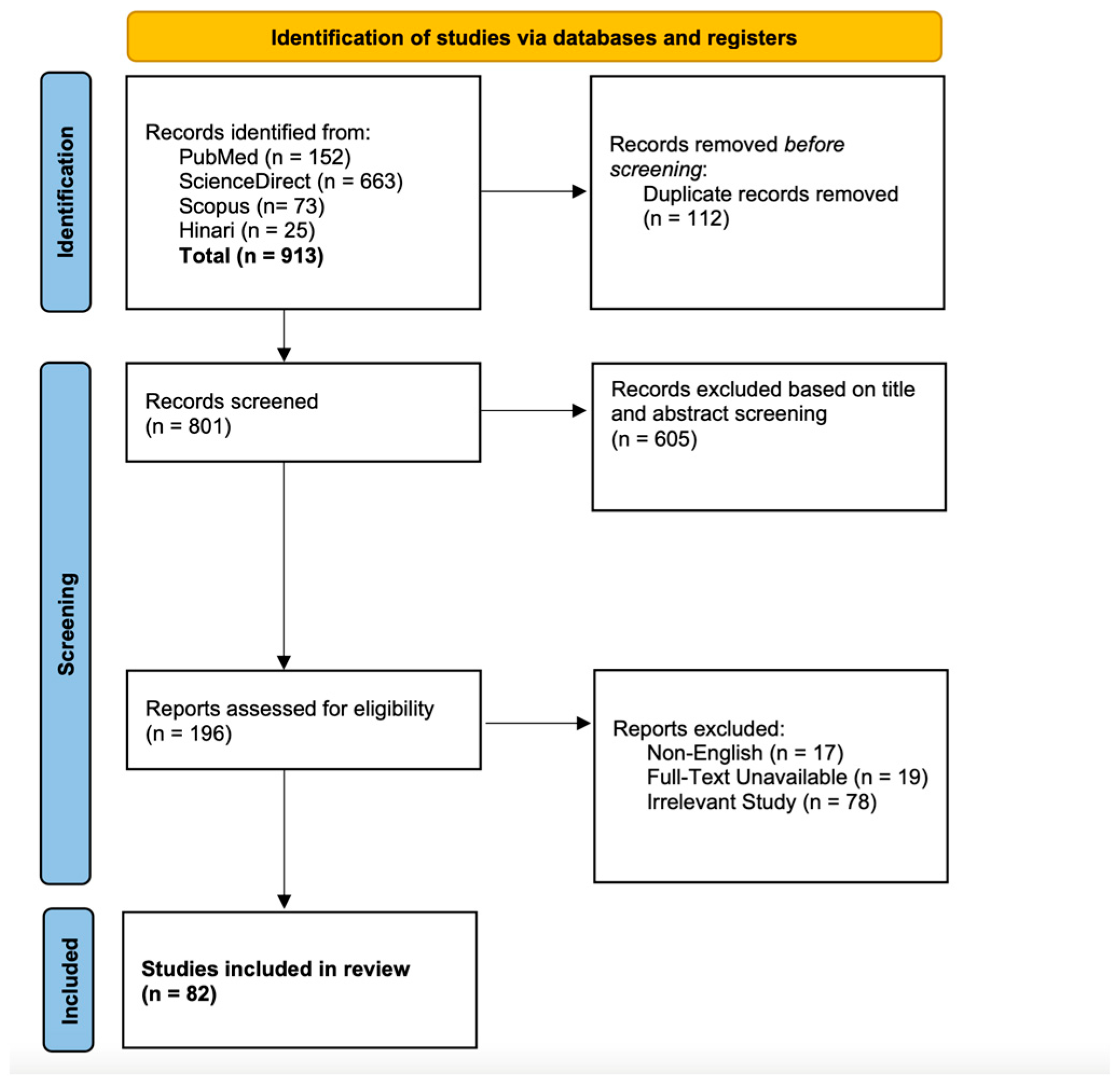
3.1. Search Results
3.2. Clinical and Radiographic Characteristics
3.3. Retrospective Studies
3.4. PRES-SCI
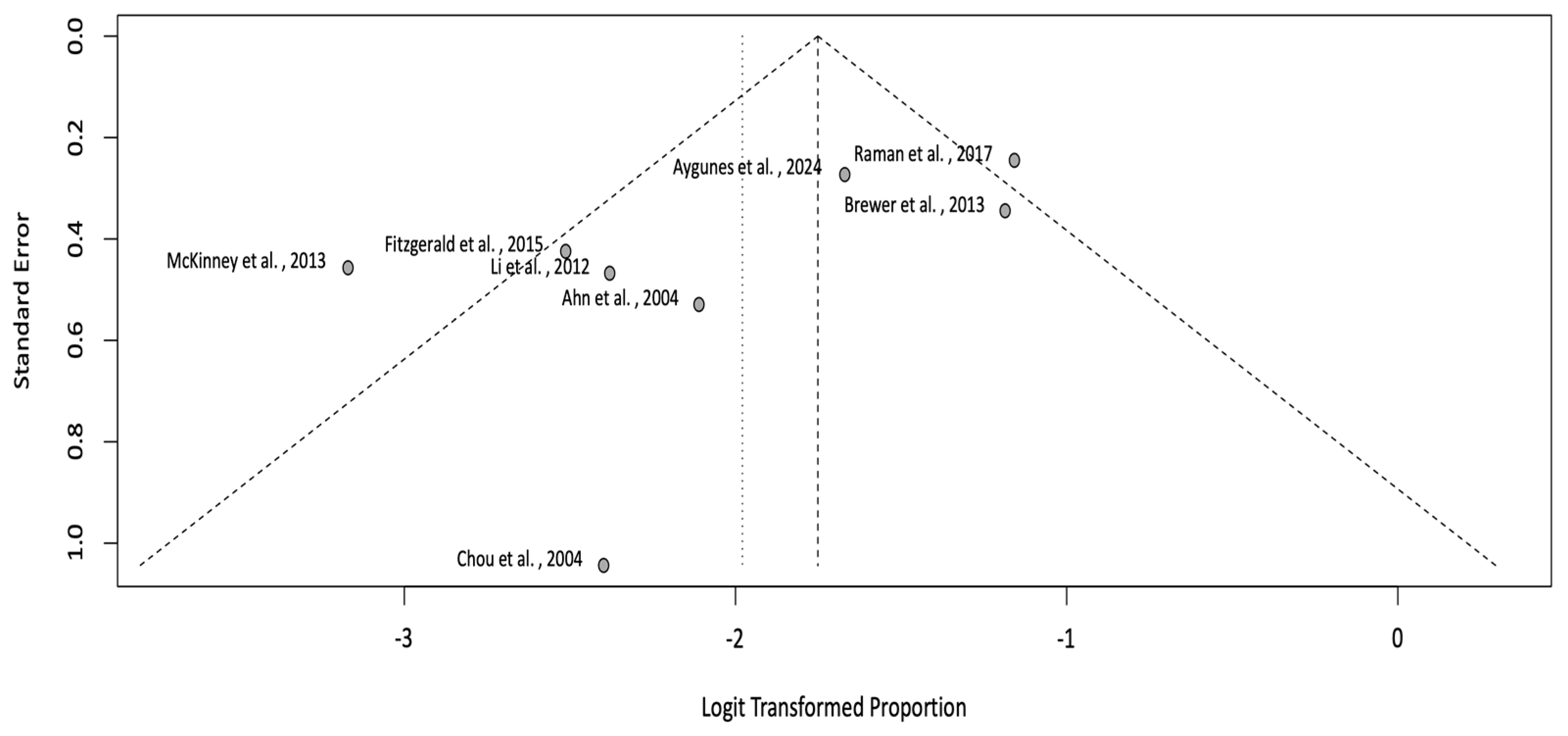
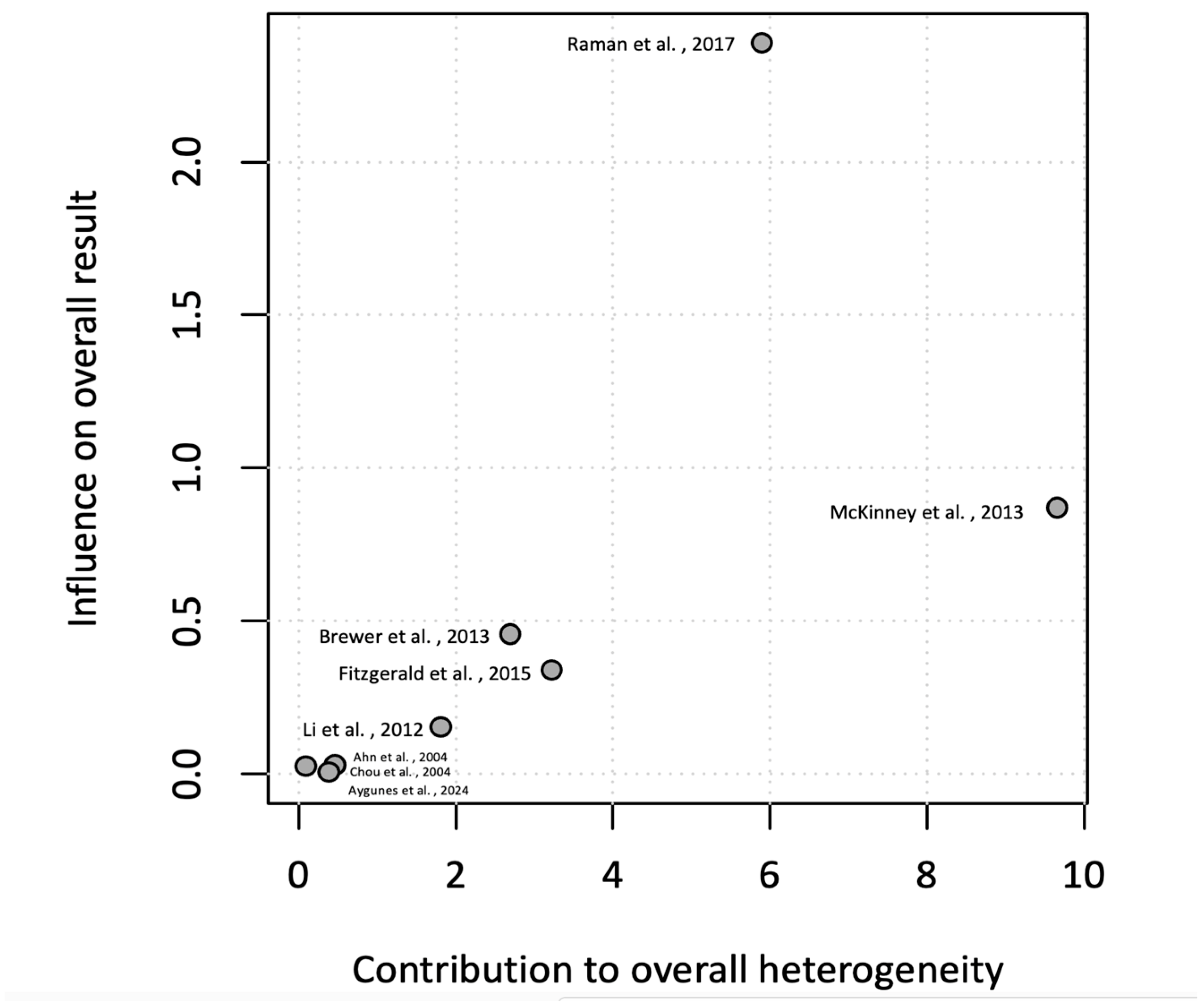
3.5. Meta-Analysis of Incidence Rate
3.6. Quality and Risk of Bias Assessment of Case Reports/Series
3.7. Quality and Risk of Bias Assessment of Cohort Studies
4. Discussion
| Case No. | Author | Publication Type | Year | Age | Gender | Risk Factors | Blood Pressure | Clinical Sequelae | CT Findings | MRI Findings | Vessel Imaging | CSF Examination | Treatment | Purported Cause | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abe et al. [4] | Case Report | 2014 | 59 | M | HTN, ESRD | 181/81 mmHg | Headache, nausea, dysarthria, tic, and weakness involving the bilateral arms and legs | Hypodense lesion involving pons and cerebellum | T2/FLAIR hyperintense lesion involving the pons and cerebellum | NA | Normal | Blood Pressure management and RRT (HD) | HTN | Radiographic and clinical improvement in 13 days |
| 2 | Abraham et al. [5] | Case Report | 2020 | 25 | F | SLE, Lupus nephritis (grade IV) | SBP 190 mmHg | GTC seizure, direction-changing torsional nystagmus, horizontal ophthalmoplegia, and symmetric weakness | T2/FLAIR hyperintense and ADC hypointense lesion involving bilateral paramedian thalamic and pontine hyperintensity. SWI punctate hypointensity within the areas of restricted diffusion in the bilateral thalami and pons, compatible with microhemorrhages | MRA: Subtle short segment stenoses involving bilateral external carotid arteries, the right V4 segment, and the right P2 segment. | Normal | Blood pressure management and RRT | HTN | Clinical improvement at 3 months. SWI imaging showed persistent punctate microhemorrhages in the bilateral thalami and pons. | |
| 3 | Abusabha et al. [6] | Case Report | 2017 | 52 | M | HTN | SBP 260 mmHg | Headaches, vertigo, blurred vision | NA | T2/FLAIR and DWI hyperintensities involving the bilateral cerebellum, pons, and occipital lobe | NA | NA | EVD insertion and suboccipital craniectomy and C1 | HTN | Radiographic and clinical improvement in 3 weeks |
| 4 | Ahn et al. [7] | Retrospective Study | 2004 | 29 | F | NA | 155/100 mmHg | Generalized tonic clonic seizures | NA | T2/FLAIR hyperintensities and ADC hypointensities involving bilateral basal ganglia | NA | NA | Blood pressure management | Postpartum eclampsia | Radiographic and clinical resolution |
| 5 | Ahn et al. [7] | Retrospective Study | 2004 | 28 | F | NA | 180/120 mmHg | Generalized tonic clonic seizures | NA | T2/FLAIR hyperintensities and ADC hypointensities involving bilateral basal ganglia and thalami | NA | NA | Blood pressure management | Eclampsia | Radiographic and clinical resolution |
| 6 | Ahn et al. [7] | Retrospective Study | 2004 | 41 | M | HTN, CKD (diabetic nephropathy) | 240/140 mmHg | Alteration in mental status | NA | T2/FLAIR hyperintensities and ADC hypointensities involving bilateral pons | NA | NA | Blood pressure management | HTN | Radiographic and clinical resolution |
| 7 | Ahn et al. [7] | Retrospective Study | 2004 | 32 | F | HTN, CKD | 190/100 mmHg | Alteration in mental status | NA | T2/FLAIR hyperintensities and ADC hypointensities involving bilateral pons | NA | NA | Blood pressure management | HTN | Radiographic and clinical resolution |
| 8 | Akhondian et al. [8] | Case Report | 2022 | 4 | F | Secondary hyperaldosteronism, HTN. | 180/110 mmHg | Alteration in mental status and status epilepticus | NA | T2/FLAIR and DWI hyperintensities in splenium of the corpus callosum, in cerebellum, brainstem, and cervical spinal cord | NA | NA | Seizure management (phenytoin, and phenobarbital). Blood pressure management (hydralazine, furosemide, and captopril). | HTN from secondary hyperaldosteronism | Radiographic and clinical resolution within one month |
| 9 | Andour et al. [9] | Case Series | 2023 | 59 | F | Diabetes, HTN | 170/100 mmHg | Alteration in mental status | Normal | T2/FLAIR, DWI hyperintense and ADC hypointense lesions involving temporal and occipital regions, in corpus callosum, in the brainstem and cerebellum | NA | NA | Blood pressure management | HTN | Clinical resolution of symptoms |
| 10 | Andour et al. [9] | Case Series | 2023 | 34 | M | Kidney failure | NA | GTC seizure | NA | T2/FLAIR hyperintensity left putamen with gadolinium enhancement | NA | NA | HD | Renal failure | Clinical and radiographic resolution of symptoms |
| 11 | Arai et al. [10] | Case Report | 1997 | 57 | M | CKD, polyarteritis nodosa | 200/140 mmHg | GTC seizures | Parietal lobe hypodensity | T2/FLAIR hyperintensities emporo-occipital white matter, the thalamus, the posterior limbs of the internal capsules, the external capsules, the midbrain, the pons, and the middle cerebellar peduncles | NA | NA | Blood pressure management | HTN | NA |
| 12 | Aridon et al. [11] | Case Report | 2011 | 53 | M | Thrombotic thrombocytopenic purpura | 260/180 mmHg | Disturbances of gait, dizziness, urinary incontinence and lethargy | Enlargement of the lateral and third ventricles | T2/FLAIR hyperintensities involving white matter of cerebral and cerebellar hemispheres with the involvement of midbrain and cerebellar peduncles | NA | Elevated protein (77 mg/dL) | Blood pressure management (nitroprusside, furosemide, and clonidine) and plasma exchange | HTN | Clinical and radiographic resolution of symptoms at 3 months |
| 13 | Bag et al. [14] | Case Report | 2010 | 23 | F | SLE | Normal | Alteration in mental status. Being treated for SLE with IV methylprednisolone 40 mg every 8 h. | T2/FLAIR and DWI hyperintensities involving bilateral thalamic and cerebral peduncle | NA | NA | Methylprednisolone 500 mg daily and cyclophosphamide. | SLE | Clinical and radiographic resolution of symptoms at 8 months | |
| 14 | Bălaşa et al. [15] | Case Report | 2015 | 42 | M | None | 190/110 mmHg | Occipital headache, nausea, vomiting and disequilibrium. Elevated creatinine 9.88 mg/dL on arrival. | Multiple white matter hypodensities in both cerebellar hemispheres and the brainstem | T2/FLAIR hyperintensities involving the cerebellar white matter as well as in the pons and midbrain | NA | NA | Hemodialysis and blood pressure management | HTN (due to kidney disease) | Clinical and radiographic resolution of symptoms at 2 weeks |
| 15 | Ball et al. [16] | Case Report | 2023 | 53 | M | Alcohol abuse | NA | 3 days of slurred speech, headache, and dizziness | T2/FLAIR hyperintensities involving the bilateral cerebellum | Normal | NA | Magnesium infusion | Hypomagnesemia (<0.5 mg/dL) | Clinical and radiographic resolution of symptoms (unspecified follow-up time) | |
| 16 | Bandeo et al. [17] | Case Report | 2018 | 26 | F | Ulcerative colitis Adalimumab (40 mg every other week) | NA | Thunderclap headache with photophobia, nausea, and vomiting. | NA | Left frontal cSAH and hyperintense lesions on T2-weighted and FLAIR sequences located in both occipital lobes, left cerebellar hemisphere, and brainstem | DSA: Normal | NA | Discontinuation of adalimumab | Adalimumab | Clinical and radiographic resolution of symptoms at 2 months |
| 17 | Bansal et al. [18] | Retrospective Study | 2020 | 16 | F | Viral hepatitis, and acute kidney injury | NA | Alteration in mental status, seizures, vomiting | NA | T2/FLAIR hyperintensities including basal ganglia, deep white mater and temporal lobe | NA | NA | Blood pressure management | HTN | NA |
| 18 | Barnaure et al. [19] | Case Report | 2014 | 57 | M | HTN | Elevated (unspecified) | Gait ataxia | NA | T2/FLAIR hyperintensities involving e pons, medulla, and cerebellum, and a small zone of contrast enhancement in the pons | NA | Normal | Blood pressure management | HTN | Clinical and radiographic resolution of symptoms at 1 week |
| 19 | Bing et al. [20] | Case Report | 2019 | 53 | M | None | 220/120 mmHg | Acute onset aphasia, fever | NA | T2/FLAIR hyperintensities involving the pons, medulla, and cervical spinal cord | NA | NA | Blood pressure management (nicardipine and urapidil) | HTN | Clinical and radiographic resolution of symptoms in one month |
| 20 | Braatz et al. [21] | Case Report | 2014 | 39 | M | NA | 230/140 mmHg | Headache, nausea, blurriness, and cortical blindness | NA | T2/FLAIR and DWI hyperintensities involving the pons and medulla | NA | NA | Blood pressure management | HTN | NA |
| 21 | Chakroun-Walha et al. [22] | Case Report | 2016 | 14 | M | Renal insufficiency | 200/150 mmHg | Headaches, ataxia, hemianopsia, bilateral strabismus, GTC seizure | Hypodensities in the brainstem | T2/FLAIR hyperintensities involving the brainstem and partially cerebellum | NA | NA | Blood pressure management and hemodialysis | HTN (Renal etiology) | Persistent strabismus and hemianopsia |
| 22 | Chaudhari et al. [23] | Case Report | 2018 | 28 | F | Caesarean section one week prior to presentation | NA | Seizure (abnormal movements of limbs and tongue-biting) | T2/FLAIR hyperintensities involving bilateral basal ganglia, and left cerebellum | NA | Normal | Seizure management (levetiracetam, and phenytoin) | Postpartum? | Clinical and radiographic improvement in 8 weeks | |
| 23 | Chen et al. [109] | Case Report | 2014 | 55 | M | HTN | 210/140 mmHg | Headache and dizziness | NA | T2/FLAIR hyperintensities in the pons, midbrain bilateral thalami, and cerebellar hemispheres with multiple microbleeds at bilateral basal ganglia | NA | NA | Blood pressure management | HTN | Clinical and radiographic improvement in 1 month |
| 24 | Chiang et al. [25] | Case Report | 2019 | 47 | M | ESRD, missed three dialysis sessions | 194/114 mmHg | Comatose | Hypodentisities in the brainstem | T2/FLAIR hyperintensities involving the pons and cerebellum | NA | Normal | RRT and blood pressure management | HTN (missed dialysis sessions) | Clinical and radiographic improvement in 2 weeks |
| 25 | Chou et al. [26] | Retrospective Study | 2004 | 52 | M | HTN | 194/120 | Seizure, aphasia | NA | T2/FLAIR hyperintensities involving deep white matter, thalamus, and pons | NA | NA | Blood pressure management, anticonvulsants, and RRT. | HTN | Clinical and radiographic improvement in 4 months |
| 26 | Decker et al. [27] | Case Report | 2009 | 74 | M | HTN | 224/144 mmHg | Slurred speech, right-sided facial droop, and incontinence of urine | NA | T2/FLAIR hyperintensities involving the brainstem (pons and midbrain) | NA | Normal (opening pressure of 16 cm of water, a protein of 129 mg/dL, WBC count of 1/mm3 , and glucose of 65 mg/dL (serum glucose 137 mg/dL). | Blood pressure management | HTN | Clinical and radiographic improvement in 3 months |
| 27 | Deguchi et al. [28] | Case Report | 2012 | 42 | F | HTN, CKD, thrombocytopenia | 270/150 mmHg | Headache, nausea | NA | T2/FLAIR hyperintensities involving the brainstem and cerebellum | NA | NA | Blood pressure management (amlodipine, nifedipine, arotinolol) | HTN | Clinical and radiographic improvement in 1 month |
| 28 | Dhawan et al. [29] | Case Report | 2010 | 10 | F | Pheochromocytoma | 260/190 mmHg | Facial palsy and bilateral papilledema | NA | T2/FLAIR hyperintensities in the bilateral caudate, putamen, thalamus, left-sided posterior limb of internal capsule, midbrain | NA | NA | Blood pressure management | HTN (pheochromocytoma) | Clinical and radiographic improvement in 2 years |
| 29 | Di Stefano et al. [30] | Case Report | 2019 | 46 | M | Mononucleosis, GERD | 200/140 mmHg | One-month history of headache and blurry vision in the right eye | NA | T2/FLAIR hyperintensities involving brainstem (midbrain, pons, medulla) and cervical spinal cord (C5–C6) | Normal | NA | Blood pressure management (ramipril, doxazosin) | HTN | Clinical and radiographic improvement in 3 weeks |
| 30 | Doi et al. [101] | Case Report | 2020 | 73 | M | Metastatic colorectal cancer | NA | Acute onset visual disturbance | NA | Increased T2-weighted signal within the pons, cerebellum, and bilateral optic nerves. Diffusion restriction within the pons and cerebellum | NA | NA | NA | NA | NA |
| 31 | Doi et al. [31] | Case Report | 2006 | 35 | M | None | 180/118 mmHg | Headache, nausea, blurred vision | NA | Increased T2-weighted signal in the pons, cerebellum, and basal ganglia | NA | NA | Blood pressure management | HTN | Clinical and radiographic improvement in 1 month |
| 32 | Doi et al. [31] | Case Report | 2006 | 52 | F | HTN | 200/130 mmHg | Altered mental status | NA | Increased T2-weighted signal in the midbrain, basal ganglia, and cerebellum | NA | NA | Blood pressure management | HTN | Radiographic improvement in 2 weeks |
| 33 | Fujii et al. [32] | Case Report | 2023 | 57 | M | None | 173/134 mmHg | Rigidity | NA | Increased signal on T2WI within the bilateral cerebellum, optic tract, cerebellar vermis, and cervical spinal cord. With DWI and ADC correlate in most lesions | NA | NA | RRT and blood pressure management | HTN | Radiographic improvement in five weeks |
| 34 | Gowan et al. [33] | Case Report | 2019 | 47 | F | HTN, T2DM, ESRD | 180/99 mmHg | 1-week history of altered mental status, difficulties walking, fatigue, and syncope | Normal | Increased signal on T2WI involving the pons and cerebellum | NA | NA | RRT and blood pressure management | HTN | Radiographic improvement on day 9 |
| 35 | Grossbach et al. [34] | Case Report | 2014 | 65 | F | Colon cancer | 217/113 mmHg | Comatose | Increased signal on T2WI involving the bilateral cerebellum | NA | CSF: Normal | Mechanical ventilation, sub-occipital craniectomy, EVD placement, | HTN | Modified Rankin score (mRS) 0 in six months | |
| 36 | Hama et al. [35] | Case Report | 2019 | 49 | F | CKD | 242/144 mmHg | 2-week history of vomiting and malaise | NA | Increased signal on T2WI involving the pons and medulla | NA | NA | RRT and blood pressure management | HTN and kidney disease | Clinical and radiographic improvement in 3 weeks |
| 37 | Han et al. [36] | Case Report | 2019 | 46 | M | HTN | 147/103 mmHg | Acute onset dysarthria and mild dysphagia | NA | Increased signal on T2WI brainstem, cerebellum and corticospinal Tracts. Diffusion restriction involving pons. Microhemorrhages seen on SWI within pons | NA | CSF: Elevated protein 55 mg/dL | Conservative management | Acute kidney injury | Clinical and radiographic improvement within 4 weeks |
| 38 | Hayashi et al. [37] | Case Report | 2022 | 71 | M | NA | 209/124 mmHg | Decreased level of consciousness | NA | Increased signal on T2WI within the pons | NA | NA | Blood pressure management | HTN | Clinical improvement and discharge on day 20 |
| 39 | Hebant et al. [38] | Case Report | 2019 | 80 | F | HTN, HLD | 190/110 mmHg | Acute alteration in mental status | NA | Increased signal on T2WI involving the medulla and right cerebellar peduncle | NA | CSF: Elevated protein. | Blood pressure management | HTN | Clinical and radiographic improvement within a few days |
| 40 | Ho et al. [12] | Case Report | 2016 | 49 | M | NA | 202/138 mmHg | Vertigo, cognitive decline, and difficulty ambulating | Hypodensity involving pons | Increased signal on T2WI involving the pons | NA | NA | Blood pressure management | HTN | Radiographic resolution in 10 days, clinical improvement in |
| 41 | Honda et al. [13] | Case Report | 2019 | 46 | F | NA | 208/140 mmHg | Visual impairment | NA | Increased signal on T2WI involving the pons and bilateral cerebellar hemispheres | NA | NA | Blood pressure management | HTN | Clinical and radiographic resolution |
| 42 | Jesrani et al. [39] | Case Report | 2021 | 39 | F | NA | 118/74 mmHg | GTC seizures | NA | Increased signal on T2WI involving the right caudate nucleus, bilateral thalami, and left globus pallidus | NA | NA | Anti-seizure medications | SLE | Death on day 4 |
| 43 | Jia et al. [40] | Case Report | 2017 | 14 | F | Neurogenic bladder | 120/81 mmHg | Decreased level of consciousness (comatose) | NA | Increased signal on T2WI involving the midbrain and pons | NA | NA | Erythropoietin, ferrous sulfate, bicarbonate | Renal failure (obstructive nephropathy) | Discharged on day 14 at baseline and radiographic resolution at 2 months |
| 44 | Kachi et al. [41] | Case Report | 2023 | 71 | F | HTN, Sjogren’s disease | 197/108 mmHg | Unsteadiness and weakness of the left lower extremity | NA | Increased signal on T2WI involving the basal ganglia, thalamus, brainstem, cerebellum | NA | CSF: Elevated protein, oligoclonal bands anti-SSA/SSB, IL-6 | IVMP 1000 mg for 3 days. Followed by oral prednisone 1 mg/kg. | Sjogren disease | Significant improvement on discharge |
| 45 | Katano et al. [42] | Case Report | 2010 | 54 | M | NA | 201/113 mmHg | Dysarthria and altered mental status | NA | Increased signal on T2WI involving the pons with ADC correlate | NA | NA | Antihypertensive therapy with hemodialysis | HTN | Continued renal dysfunction, unspecified neurological outcome |
| 46 | Kitaguchi et al. [43] | Case Series | 2005 | 73 | M | CKD, Wernicke encephalopathy | 220/116 mmHg | Appetite loss and failure to thrive | NA | Increased signal on T2WI involving the pons, thalamus, and cerebellum | NA | NA | Antihypertensive therapy | HTN | Mild lateral gaze palsy at 16-month follow-up |
| 47 | Kitaguchi et al. [43] | Case Series | 2005 | 49 | F | Aortic valve replacement | 88/40 mmHg | GTC seizure, flu-like symptoms | CT scan showed a lacunar infarction in the left internal capsule of the brain | Increased signal on T2WI involving the pons | NA | CSF: Normal | Supportive treatment | Undefined | Clinical and radiographic improvement in 2 months. Residual lacunar infarction within pons |
| 48 | Lamotte et al. [44] | Case Report | 2021 | 51 | M | Nasopharyngeal carcinoma | 185/125 mmHg | Dysarthria and gait instability | NA | Increased signal on T2WI involving the bilateral cerebellum, pons, and temporal lobes | NA | NA | Hemodialysis | Acute renal failure | Resolution of neurological symptoms in 3 days |
| 49 | Lee et al. [45] | Case Report | 2017 | 47 | F | NA | 270/220 mmHg | AMS | NA | Increased signal on T2WI involving the pons | NA | CSF: Opening pressure 21 cm H2O. Protein 102 mg/dL, albumin 64 mg/dL. | Blood pressure management | HTN | Resolution of neurological symptoms in 3 days and radiographic resolution on 9th day |
| 50 | Liu et al. [46] | Case Report | 2018 | 37 | F | HTN | 240/140 mmHg | Headache and blurry vision | NA | Increased signal on T2WI with diffusion restriction involving the midbrain | Normal | NA | Blood pressure management | HTN | Clinical and radiographic resolution in 2 weeks |
| 51 | Maciel et al. [47] | Case Report | 2015 | 44 | F | NA | 150/110 mmHg | Subacute onset headache and visual disturbance | NA | Increased signal on T2WI involving the midbrain, pons, medulla, and cerebellar hemispheres | NA | NA | Blood pressure management | HTN | Clinical resolution in 2 weeks |
| 52 | Maier et al. [49] | Case Report | 2018 | 22 | F | SLE | Normal | GTC seizures | Symmetrical hypodense lesions within the basal ganglia | Increased signal on T2WI involving the bilateral basal ganglia | NA | NA | Anti-seizure medications and osmolar therapy | SLE associated Immunosuppressive therapy (cyclophosphamide and azathioprine) | Death due to septic shock |
| 53 | Malhotra et al. [48] | Case Report | 2017 | 42 | F | SLE, HTN, pulmonary hypertension | 217/75 mmHg | Confusion, dysarthria, R hemiparesis, and hemianesthesia | Normal | Increased signal on T2WI involving the bilateral basal ganglia and thalamus | DSA: Normal | EEG: Normal | Blood pressure management | HTN | Radiographic improvement at 5 weeks and discharge to a long-term care facility |
| 54 | Maruyama et al. [50] | Case Report | 2023 | 53 | M | HTN | 214/145 mmHg | Headache and left-sided weakness | NA | Increased signal on T2WI involving the pons and bilateral cerebellar hemispheres | NA | NA | Blood pressure management | HTN | Radiographic improvement in 3 weeks and clinical resolution at 8 weeks |
| 55 | Matsumoto et al. [51] | Case Report | 2023 | 70s | F | HTN | 199/111 mmHg | Generalized weakness | NA | Increased signal on T2WI involving the pons, cerebellum, and medulla | NA | NA | Blood pressure management | HTN | Radiographic resolution in 10 months |
| 56 | McCarron et al. [52] | Case Report | 2008 | 42 | M | NA | 195/115 mmHg | GTC seizure, right-sided hemiparesis | NA | Increased signal on T2WI involving the pons | NA | NA | Blood pressure management | HTN | Almost complete radiographic resolution at 3-month follow-up |
| 57 | Moosa et al. [53] | Case Report | 2011 | 8 | F | Cloacal exstrophy, omphalocele, Chiari malformation with myelomeningocele and syringomyelia, and renal dysplasia with end stage renal disease. | 180/120 mmHg | Status epilepticus | NA | Increased signal on T2WI involving the midbrain and pons | NA | NA | Blood pressure management and anti-seizure medications | HTN | Radiographic resolution in 9 days |
| 58 | Nagato et al. [54] | Case Report | 2009 | 14 | F | NA | 185/145 mmHg | Nausea, vomiting, abdominal pain | NA | Increased signal on T2WI involving the pons, medulla, cerebellum, and cervical spinal cord | NA | NA | Blood pressure management | HTN | Significant clinical and radiographic improvement over 5 months |
| 59 | Nanba et al. [55] | Case Report | 2016 | 47 | F | NA | 197/106 mmHg | Headache | NA | Increased signal on T2WI involving the pons, bilateral thalamus, bilateral basal ganglia, and periventricular white matter | NA | NA | Blood pressure management | HTN | Radiographic improvement in 2 weeks |
| 60 | Navarro-Ballester et al. [56] | Case Report | 2021 | 62 | M | HTN, HLD | 190/95 mmHg | Nausea and vomiting | NA | Increased signal on T2WI involving the midbrain with SAH | DSA: mildly hypoplastic right vertebral artery | NA | Blood pressure management | HTN | Modified Rankin score of one at 6-month follow-up |
| 61 | Ocek et al. [57] | Case Report | 2015 | 55 | F | Psoriatic arthritis | 120/80 mmHg | Seizure, headache, hemiparesis | NA | Increased signal on T2WI involving the basal ganglia and thalamus | NA | CSF: Normal. EEG: Mild background slowing | Removal of toxic agent | sulfasalazine | Radiographic resolution of symptoms in 1 month |
| 62 | Ogaki et al. [66] | Case Report | 2009 | 49 | F | NA | 260/170 mmHg | Subacute onset worsening blurry vision | NA | Increased signal on T2WI involving the pons, bilateral cerebellum, and thalami | NA | NA | Blood pressure management | HTN | Discharge in one month with radiographic improvement |
| 63 | Ohashi et al. [58] | Case Report | 2022 | 4-month-old | F | NA | SBP 100–130 mmHg | Cardiac arrest following immunization | NA | Increased signal on T2WI involving the bilateral basal ganglia | NA | NA | VA-ECMO, peritoneal dialysis | HTN | Discharge in 6 months |
| 64 | Onomura et al. [59] | Case Report | 2022 | 40s | F | Hypertension | 230/150 mmHg | Headache, fatigue, and nausea | NA | Increased signal on T2WI involving the supratentorial white matter, cerebellum, pons, and cerebellar peduncles. Numerous white matter microhemorrhages involving the cerebral white matter | NA | NA | Blood pressure management | HTN | Discharge on day 20 with moderate improvement |
| 65 | Osman et al. [60] | Case Report | 2013 | 32 | M | T1DM, HTN | 220/140 mmHg | GTC seizure | NA | Increased signal on T2WI involving the bilateral pons and midbrain | NA | CSF: Normal | Blood pressure management | HTN | Repeat neuroimaging 12 days later showed significant improvement |
| 66 | Ou et al. [61] | Case Report | 2018 | 40 | M | NA | 200/140 mmHg | Headache | NA | Increased signal on T2WI involving the pons | NA | NA | Blood pressure management | HTN | Clinical resolution within three days |
| 67 | Raya et al. [62] | Case Report | 2019 | 29 | M | HIV, ESRD, HTN | 245/141 mmHg | Headache and burry vision | NA | Increased signal on T2WI involving the bilateral cerebellar hemisphere with more subtle involvement of the bilateral occipital lobes | NA | NA | Blood pressure management | HTN | Discharged on day 7 of hospitalization with no symptoms |
| 68 | Resorlu et al. [63] | Case Report | 2017 | 39 | M | HTN | 170/110 mmHg | Headache | NA | Increased signal on T2WI involving the pons | NA | NA | Blood pressure management | HTN | Radiographic resolution on hospital day 20 |
| 69 | Ribeiro et al. [65] | Case Report | 2013 | 59 | F | HIV | 210/110 mmHg | Headache, nausea, vomiting, blurry vision | NA | Increased signal on T2WI involving the basal ganglia, thalamus, internal and external capsules, and pons. SWI: microhemorrhages within the pons | NA | NA | Blood pressure management | HTN | Clinical improvement on hospital day 3 |
| 70 | Sallah et al. [64] | Case Report | 2021 | 64 | F | ESRD, stroke | 207/110 mmHg | Lethargy | NA | Increased signal on T2WI involving the pons and bilateral middle cerebellar peduncles | NA | EEG: Findings within the ictal-interictal continuum. | Blood pressure management and hemodialysis | HTN + ESRD | Clinical and radiographic resolution on day 11 of hospitalization |
| 71 | Sharma et al. [67] | Case Report | 2017 | 7 | M | None | 190/100 mmHg | GTC seizure | NA | Increased signal on T2WI involving the midbrain, pons, medulla, and cervical spinal cord | NA | NA | Blood pressure management and anti-seizure medications | HTN + Grade IV vesicoureteral reflex. | Radiographic improvement within 3 weeks |
| 72 | Shimizu et al. [68] | Case Report | 2013 | 10 | F | T-ALL | 141/105 mmHg | Headache | NA | Increased signal on T2WI involving the bilateral cerebellum. Gd+ enhancement within the same area. DWI showing patchy diffusion restriction within the bilateral cerebellum | NA | NA | Blood pressure management | HTN+ chemotherapy (vincristine, danorubicin, dexamethasone, cytarabine, methotrexate, prednisolone) | Radiographic improvement within 6 months |
| 73 | Srinivasan et al. [69] | Case Report | 2017 | 71 | M | HTN | 200/140 mmHg | Headache, dizziness, loss of consciousness | NA | Increased signal on T2WI involving the pons with restricted diffusion on DWI | NA | NA | Blood pressure management | HTN | Clinical improvement. Repeat imaging not completed. |
| 74 | Tan et al. [70] | Case Report | 2019 | 52 | M | HTN, CKD | 267/159 mmHg | Headache, dizziness | CTH: Hypoattenuation involving the pons | NA | NA | NA | Blood pressure management | HTN | Clinical improvement on day 2 of hospitalization |
| 75 | Tari Capone et al. [71] | Case Report | 2014 | 37 | M | HTN | 270/160 mmHg | Headache, and blurry vision | NA | Increased signal on T2WI involving the midbrain and pons | NA | NA | Blood pressure management | HTN | Clinical improvement in 2 weeks and radiographic resolution within 3 months |
| 76 | Thambisetty et al. [72] | Case Series | 2003 | 38 | M | HTN, anemia, thrombocytopenia, chronic hyponatremia, ETOH abuse | 210/130 mmHg | Headache, right-sided weakness | Obstructive hydrocephalus, non-enhancing hypoattenuation in pons, midbrain | Obstructive hydrocephalus, increased T2 signal in the pons and midbrain | NA | NA | Blood pressure management | HTN | Clinical resolution |
| 77 | Thambisetty et al. [72] | Case Series | 2003 | 61 | M | None | 219/138 mm.Hg | Left-sided weakness | Old lacunar infarction in anterior limb of right internal capsule, 5-mm hemorrhagic focus in right putamen | Increased T2 signal in the pons, cerebral peduncles and basal ganglia bilaterally | NA | NA | Blood pressure management | HTN | Clinical resolution |
| 78 | Thambisetty et al. [72] | Case Series | 2003 | 46 | M | T2DM, HTN, anemia, stroke | 203/139 mmHg | Blurred vision, confusion | Hypoattenuation in the pons, cerebral peduncles and internal capsule bilaterally | Increased T2 signal in midbrain, pons, medulla and cerebral peduncles. Focus of hemorrhage in left basal ganglia | NA | NA | Blood pressure management | HTN | Clinical resolution |
| 79 | Tortora et al. [73] | Case Report | 2015 | 32 | F | Abortion (1-month ago) | 120/70 mmHg | Headache and fever | Hypoattenuation within the right basal ganglia | Increased T2 signal and diffusion restriction in the pons | NA | NA | Enoxaparin and magnesium sulfate | Unknown | Residual coordination deficits of the extremities, and radiographic findings of pontine ischemia |
| 80 | Tsutsumi et al. [74] | Case Report | 2012 | 54 | F | None | 260/142 mmHg | Dysarthria, right-sided hemiparesis | Normal | Increased T2 signal involving the pons. MR spectroscopy showed an elevated choline level | NA | NA | Blood pressure management | HTN due to pseudochromocytoma | Radiographic resolution on hospital day 18 |
| 81 | Tsutsumi et al. [74] | Case Report | 2012 | 44 | F | None | 209/130 mmHg | Headache, blurry vision, gait abnormalities | NA | Increased T2 signal involving the pons | NA | NA | Blood pressure management | HTN | Radiographic resolution on hospital day 20 |
| 82 | Vaysman et al. [75] | Case Report | 2019 | 22 | F | SLE | 197/121 mmHg | Headache, and joint pain | Normal | Increased T2 signal involving the midbrain | NA | NA | Blood pressure management and plasmapheresis | HTN + SLE | Clinical and radiographic resolution of the patients’ symptoms on day 10 |
| 83 | Wakely et al. [76] | Case Report | 2005 | 33 | M | None | 210/150 mmHg | Headache, and visual disturbance | Normal | Increased T2 signal and diffusion restriction involving the pons | NA | NA | Blood pressure management | HTN | Radiographic resolution of symptoms within 2 months |
| 84 | Wittgrove et al. [77] | Case Report | 2018 | 57 | M | None | 220 mmHg | Aphasia, altered mental status, right-sided weakness | Normal | Increased T2 signal involving the pons and cerebellar peduncles | NA | Elevated protein 80 mg/dL | Blood pressure management | HTN secondary to renal artery stenosis | Clinical and radiographic improvement of the patient’s symptoms |
| 85 | Yamagami et al. [78] | Case Report | 2019 | 41 | F | HTN | 237/142 mmHg | Headache | Hemorrhage involving the left thalamus and basal ganglia | Increased T2 signal involving the left cerebellum, pons, temporal lobes, and bilateral basal ganglia | DSA: Normal | NA | Blood pressure management | HTN | Transferred to an inpatient rehabilitation facility on hospital day 40 with a modified Rankin score of 3. Repeat MRI showing resolution of vasogenic edema |
| 86 | Yis et al. [96] | Case Report | 2016 | 9 | F | None | 225/110 mmHg | Headache, vomiting, visual disturbance | NA | Increased T2 signal involving the medulla and cervical spinal cord | NA | CSF: 57 mg/dL, IgG Index 0.9. | Blood pressure management and methylprednisolone | HTN | Clinical improvement in 7 days and radiographic resolution in 10 days |
| 87 | Yokoyama et al. [79] | Case Report | 2019 | 43 | M | Guillain Barre syndrome (GBS) | 152/88 mmHg | Decreased level of consciousness | NA | Increase T2 signal involving the pons, midbrain, cerebellar peduncle, and basal ganglia. Diffusion restriction | NA | NA | IVIG and blood pressure management | HTN secondary to GBS | Significant improvement with residual limb weakness |
| 88 | Zhang et al. [80] | Case Report | 2016 | 35 | M | HTN | 200/140 mmHg | Dizziness | Hypodensity involving the pons with focal hyperdensity consistent with acute hemorrhagic conversion | Increased T2 signal involving the pons | NA | CSF: Elevated opening pressure of 245 mm H2O | Blood pressure management | HTN | Clinical and radiographic improvement in one month |
| 89 | de Havenon et al. [81] | Case Report | 2014 | 50 | M | HTN, CKD | 180/110 mmHg | Headache, vomiting, confusion | NA | Increased T2 signal involving the parieto-occipital lobes, bilateral cerebellum, medulla and confluent central lesion involving the entire spinal cord | NA | NA | Blood pressure management | HTN | Near complete radiographic resolution 5 months. Clinically has residual mild lower extremity weakness |
| 90 | de Havenon et al. [81] | Case Report | 2014 | 25 | M | HTN | 225/160 mmHg | Headache, vision loss | NA | Increased T2 signal involving the medulla and central lesions involving the entire spinal cord | NA | NA | Blood pressure management | HTN | Complete clinical and radiographic resolution in 3 months |
| 91 | Milia et al. [82] | Case Report | 2008 | 44 | F | HTN | 240/140 mmHg | Headache, lower extremity weakness, blurry vision | NA | Increased T2 signal involving the pons, medulla, and a central cord lesion from the cervicomedullary junction to C5 | NA | CSF: Normal | Blood pressure management | HTN | Clinical and radiographic resolution in 6 months |
| 92 | Samara et al. [83] | Case Report | 2019 | 42 | M | HTN | 250/130 mmHg | Headache and blurry vision | Periventricular edema, enlarged ventricles, and effacement of the basilar cistern | Increased T2 signal involving the medulla, cerebellum, and upper cervical spinal cord. | NA | CSF: Normal | Blood pressure management | HTN | Clinical and radiographic resolution in 2 months |
| 93 | Liu et al. [84] | Case Report | 2019 | 20 | M | HTN | 260/140 mmHg | Blurry vision and weakness | NA | Increased T2 signal involving the medulla, cervical and thoracic spinal cord | NA | NA | Blood pressure management | HTN | Clinical and radiographic resolution in 10 days |
| 94 | Chan et al. [87] | Case Report | 2018 | 4 | M | NF1 | 180/80 mmHg | Tachycardia | NA | Increased T2 signal involving the pons, medulla, cerebellum, and complete spinal cord (most prominent in cervical cord) | NA | CSF: Normal | Blood pressure management | HTN from NF 1-related renal artery stenosis | Clinical and radiographic improvement in 6 weeks with some residual vasogenic edema within the medulla |
| 95 | Gocmen et al. [85] | Case Report | 2016 | 10 | M | HTN, ESRD | 170/120 mmHg | Headaches | NA | Increased T2 signal involving the medulla and cervical spinal cord (C1-C5) | NA | Deferred | Blood pressure management | HTN from ESRD | Clinical and radiographic resolution in 10 days |
| 96 | Marrone et al. [86] | Case Report | 2016 | 19 | M | Testicular carcinoma (bleomycin, cisplatin and etoposide) | 240/140 mmHg | Headache, nausea, and central scotoma of the left eye | NA | Increased T2 signal involving the bilateral basal ganglia, pons, medulla, and anterior portion of the entire cervical spinal cord | NA | NA | Blood pressure management | HTN from renal artery stenosis from paraaortic lymph node dissection | Clinical and radiographic resolution in two weeks |
| 97 | Agarwal et al. [88] | Case Report | 2016 | 14 | M | Appendectomy | 170/110 mmHg | Seizure, headache, and blurry vision | NA | Increased T2 signal involving the pons, medulla, and entire spinal cord. Contrast sequence showing diffuse leptomeningeal enhancement | NA | CSF: Normal | Blood pressure management | HTN from renal artery stenosis | Clinical and radiographic improvement in 28 days |
| 98 | Choh et al. [89] | Case Report | 2011 | 17 | M | None | 240/130 mmHg | Headache, visual disturbance, vomiting | NA | Increased T2 signal involving the medulla and cervical spinal cord | NA | NA | Blood pressure management | HTN from IgA nephropathy | Clinical and radiographic improvement within one month |
| 99 | Khokhar et al. [90] | Case Report | 2016 | 22 | M | None | 200/140 mmHg | Headache, vomiting, blurry vision | NA | Increased T2 signal involving the parieto-occipital lobes with predmoninant involvement of the medulla, cerebellar hemisphere, and cervical spinal cord | NA | CSF: Normal. EEG: Normal | Blood pressure management | HTN | Clinical and radiographic improvement in 25 days |
| 100 | Srichawla et al. [95] | Case Report | 2025 | 59 | F | HTN, COPD | 195 mmHg SBP | Seizures, altered mental status | NA | Increased T2 signal involving the bilateral cerebellar hemisphere | CTA: Normal | CSF: Normal. EEG: Bilateral intermittent rhythmic discharges. Generalized periodic discharges, lateralized rhythmic delta activity in the left frontal lobe. | Blood pressure management | HTN due to adrenal insufficiency | Clinical and radiographic improvement on day 15 |
| Reference | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall | Risk |
|---|---|---|---|---|---|---|---|---|---|---|
| Abe et al. (2014) [4] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Abraham et al. (2020) [5] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Abusabha et al. (2017) [6] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Akhondian et al. (2002) [8] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Andour et al. (2023) [9] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Arai et al. (1997) [10] | Y | Y | Y | Y | Y | N | N | N | 5 | Moderate |
| Aridon et al. (2011) [11] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Bag et al. (2010) [14] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Bălaşa et al. (2015) [15] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ball et al. (2023) [16] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Bandeo et al. (2018) [17] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Barnaure et al. (2014) [19] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Bing et al. (2009) [20] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Braatz et al. (2014) [21] | Y | Y | Y | Y | Y | Y | N | N | 6 | Moderate |
| Chakroun-Walha et al. (2018) [22] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Chaudhari et al. (2014) [23] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Chiang et al. (2019) [25] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Chou et al. (2004) [26] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Decker et al. (2009) [27] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Deguchi et al. (2012) [28] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Dhawan et al. (2010) [29] | Y | Y | Y | Y | Y | N | N | N | 5 | Low |
| Di Stefano et al. (2019) [30] | Y | Y | Y | Y | Y | N | N | N | 5 | Moderate |
| Doi et al. [101] | Y | Y | Y | Y | N | N | N | N | 4 | High |
| Doi et al. [31] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Fujii et al. [32] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Gowan et al. [33] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Grossbach et al. [34] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Hama et al. [35] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Han et al. [36] | Y | Y | Y | Y | Y | N | Y | Y | 7 | Low |
| Hayashi et al. [37] | Y | Y | Y | N | Y | N | N | Y | 5 | Mod |
| Hebant et al. [38] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ho et al. [12] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Honda et al. [13] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Jesrani et al. [39] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Jia et al. [40] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Kachi et al. [41] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Kitaguchi et al. [43] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Lamotte et al. [44] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Lee et al. [45] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Liu et al. [46] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Maciel et al. [47] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Maier et al. [50] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Malhotra et al. [48] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Maruyama et al. [50] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Matsumoto et al. [51] | Y | Y | Y | Y | Y | Y | N | N | 6 | Moderate |
| McCarron et al. [52] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Moosa et al. [53] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Nagato et al. [54] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Nanba et al. [55] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Navarro-Ballester et al. [56] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ocek et al. [57] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ogaki et al. [66] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ohashi et al. [58] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Onomura et al. [59] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Osman et al. [60] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ou et al. [61] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Raya et al. [62] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Resorlu et al. [63] | Y | Y | Y | Y | N | N | N | N | 4 | High |
| Ribeiro et al. [65] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Sallah et al. [64] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Sharma et al. [67] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Shimizu et al. [68] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Srinivasan et al. [69] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tan et al. [70] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tari Capone et al. [71] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Thambisetty et al. [72] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tortora et al. [73] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Vaysman et al. [75] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Wakely et al. [76] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Wittgrove et al. [77] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Yamagami et al. [78] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Yokoyama et al. [79] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Zhang et al. [80] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Havenon et al. [81] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Milia et al. [82] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Samara et al. [83] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Liu et al. [84] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Chan et al. [87] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Gocmen et al. [85] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Marrone et al. [86] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Agarwal et al. [88] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Choh et al. [89] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Khokhar et al. [90] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Srichawla et al. [95] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Study | Selection (4 Score) | Comparability (2 Score) | Outcome (3 Score) | Total |
|---|---|---|---|---|
| Ahn et al. (2004) [7] | *** | ** | *** | 8 |
| Bansal et al. (2020) [18] | *** | * | 4 | |
| Brewer et al. [97] | *** | ** | *** | 8 |
| Chen et al. (2017) [91] | **** | ** | *** | 9 |
| Chou et al. (2004) [26] | *** | ** | *** | 8 |
| Fitzgerald et al. (2015) [92] | *** | ** | *** | 8 |
| Li et al. [98] | *** | ** | *** | 8 |
| McKinney et al. (2013) [2] | *** | ** | *** | 8 |
| McKinney et al. (2007) [93] | *** | ** | *** | 8 |
| Raman et al. (2017) [99] | *** | ** | *** | 8 |
| Yoon et al. (2013) [100] | *** | ** | *** | 8 |
| Aygunes et al. (2024) [94] | *** | ** | *** | 8 |
Limitations and Future Direction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hinchey, J.; Chaves, C.; Appignani, B.; Breen, J.; Pao, L.; Wang, A.; Pessin, M.S.; Lamy, C.; Mas, J.L.; Caplan, L.R. A reversible posterior leukoencephalopathy syndrome. N. Engl. J. Med. 1996, 334, 494–500. [Google Scholar] [CrossRef] [PubMed]
- McKinney, A.M.; Jagadeesan, B.D.; Truwit, C.L. Central-variant posterior reversible encephalopathy syndrome: Brainstem or basal ganglia involvement lacking cortical or subcortical cerebral edema. AJR Am. J. Roentgenol. 2013, 201, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Tokuda, Y. Recurrent posterior reversible encephalopathy syndrome of the brainstem in a hypertensive patient with end-stage renal disease. J. Emergencies Trauma Shock 2014, 7, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Longardner, K.; Chen, P.; Huisa, B.; Handwerker, J. Case 279: Central-Variant Posterior Reversible Encephalopathy Syndrome. Radiology 2020, 296, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Abusabha, Y.; Petridis, A.K.; Kraus, B.; Kamp, M.A.; Steiger, H.J.; Beseoglu, K. Life-threatening posterior reversible encephalopathy syndrome in the cerebellum treated by posterior fossa decompression. Acta Neurochir. (Wien.) 2017, 159, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.J.; You, W.J.; Jeong, S.L.; Lee, J.W.; Kim, B.S.; Lee, J.H.; Yang, D.W.; Son, Y.M.; Hahn, S.T. Atypical manifestations of reversible posterior leukoencephalopathy syndrome: Findings on diffusion imaging and ADC mapping. Neuroradiology 2004, 46, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Akhondian, J.; Ashrafzadeh, F.; Seilanian Toosi, F.; Behnam, M.; Beiraghi Toosi, M.; Imannezhad, S.; Akhoundian, M.R.; Hashemi, N. A case report of Posterior reversible encephalopathy syndrome with spinal cord involvement (PRES-SCI) as an atypical presentation of PRES in children. Iran. J. Child Neurol. 2022, 16, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Andour, H.; Cheraqui, A.; Lahfidi, A.; Fikri, M.; Ech-Cherif El Kettani, N.; Jiddane, M.; Touarsa, F. Posterior reversible encephalopathy syndrome (PRES): Should more attention be paid to the atypical forms? Radiol. Case Rep. 2023, 18, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Shigeno, K.; Wada, M. A reversible posterior leukoencephalopathy syndrome in a patient with classical polyarteritis nodosa. Clin. Neurol. 1997, 37, 64–66. [Google Scholar]
- Aridon, P.; Ragonese, P.; Mazzola, M.A.; Quintini, G.; Lo Re, M.; Talamanca, S.; Terruso, V.; D'Amelio, M.; Savettieri, G. Reversible posterior leukoencephalopathy syndrome in a patient with thrombotic thrombocytopenic purpura. Neurol. Sci. 2011, 32, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Tsai, C.L.; Hsu, Y.D.; Lee, J.T.; Yang, F.C.; Hsu, C.C.; Lin, C.C. Posterior Reversible Encephalopathy Syndrome Mimicking Brainstem Infarction: A Dilemma. Acta Neurol. Taiwan. 2016, 25, 56–59. [Google Scholar] [PubMed]
- Honda, K.; Hashimoto, S. Brainstem Cerebellum-type Posterior Reversible Encephalopathy Syndrome. Intern. Med. 2020, 59, 1337–1338. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.K.; Curé, J.K.; Sullivan, J.C.; Roberson, G.H. Central variant of posterior reversible encephalopathy syndrome in systemic lupus erythematosus: New associations? Lupus 2010, 19, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Bălaşa, R.; Maier, S.; Baubec, E.G.; Bajkó, Z.; Bălaşa, A. Cerebellar and brainstem variant of posterior reversible encephalopathy syndrome. Acta Neurol. Belg. 2015, 115, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.M.; Rayi, A.; Gustafson, M. A Rare Association of Hypomagnesemia and Posterior Reversible Encephalopathy Syndrome (PRES). Cureus 2023, 15, e41572. [Google Scholar] [CrossRef] [PubMed]
- Bandeo, L.; Rausch, A.; Saucedo, M.; Chertcoff, A.; Cejas, L.L.; Roca, C.U.; Pacha, S.; Pardal, M.F.; Reisin, R.; Bonardo, P. Convexity Subarachnoid Hemorrhage Secondary to Adalidumab in a Patient with Ulcerative Colitis. J. Vasc. Interv. Neurol. 2018, 10, 62–64. [Google Scholar] [PubMed]
- Bansal, S.; Bansal, R.; Goyal, M.K.; Takkar, A.; Singh, R.; Singh, P.; Lal, V. Clinical, Etiological and Imaging Profile of Posterior Reversible Encephalopathy Syndrome: A Prospective and Follow-Up Study. Ann. Indian. Acad. Neurol. 2020, 23, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Barnaure, I.; Horvath, J.; Lovblad, K.O.; Vargas, M.I. Atypical brainstem presentation of posterior reversible encephalopathy syndrome (PRES). J. Neuroradiol. 2014, 41, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Bing, F.; M'Biene, S.; Gay, S. Brainstem Posterior Reversible Encephalopathy Syndrome With Spinal Cord Involvement (PRES-SCI). Rev. Neurol. (Paris) 2020, 176, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Braatz, V.L.; Lorenzoni, P.J.; Kay, C.S.; Chula, D.C.; Scola, R.H.; Werneck, L.C. Brainstem reversible leukoencephalopathy syndrome. Arq. Neuropsiquiatr. 2014, 72, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Chakroun-Walha, O.; Bacha, I.; Frikha, M.; Ben Mahfoudh, K.; Rekik, N. A rare case of acute posterior reversible encephalopathy syndrome involving brainstem in a child. J. Acute Dis. 2016, 5, 521–523. [Google Scholar] [CrossRef]
- Chaudhari, D.M.; Renjen, P.N.; Goyal, N.; Mishra, A. Central variant reversible encephalopathy syndrome. BMJ Case Rep. 2022, 15, 245636. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Tseng, Y.C.; Hsu, H.L.; Huang, Y.L.; Chen, C.J. Teaching neuroimages: Central variant of posterior reversible encephalopathy syndrome. Neurology 2014, 82, e164. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.F.; Chen, P.T.; Chen, Y.L.; Chen, M.H. Atypical posterior reversible encephalopathy syndrome in a noncompliant hemodialysis patient: Case report and literature review. Hemodial. Int. 2019, 23, e100–e103. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-C.; Lai, P.-H.; Yeh, L.-R.; Yuan, M.-K.; Liang, H.-L.; Chen, C.; Pan, H.-B.; Yang, C.-F.; Li, J.-Y.; Lo, Y.-K. Posterior Reversible Encephalopathy Syndrome: Magnetic Resonance Imaging and Diffusion-Weighted Imaging in 12 Cases. Kaohsiung J. Med. Sci. 2004, 20, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Decker, D.A.; Falchook, A.D.; Yachnis, A.T.; Waters, M.F. Radiographic and pathologic findings in an atypical brainstem variant of reversible posterior leukoencephalopathy syndrome. Neurologist 2009, 15, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, I.M.D.; Uchino, A.M.D.; Suzuki, H.M.D.; Tanahashi, N.M.D. Malignant Hypertension with Reversible Brainstem Hypertensive Encephalopathy and Thrombotic Microangiopathy. J. Stroke Cerebrovasc. Dis. 2012, 21, 915.e7–915.e20. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.R.; Goswami, J.N.; Suthar, R.; Dayal, D.; Vyas, S.; Singhi, P.D. A Child with Central Variant Posterior Reversible Encephalopathy Syndrome. Neuropediatrics 2019, 50, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Rispoli, M.G.; Onofrj, M.; De Angelis, M.V. Tumour-like presentation of atypical posterior reversible encephalopathy syndrome with prominent brainstem involvement. BMJ Case Rep. 2020, 13, 231687. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Kimura, F.; Fujiyama, T.; Fujimura, C.; Nishina, T.; Sato, T.; Hosokawa, T.; Uehara, H.; Ishida, S.; Hanafusa, T. Hypertensive brainstem encephalopathy without parieto-occipital lesion—Two case reports. Neurol. Med.-Chir. 2006, 46, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Fujii, H.; Matsuki, M.; Doi, S.; Isozaki, T.; Watanabe, Y.; Nakamata, A.; Fujita, A.; Mori, H. Optic pathway involvement in the posterior reversible encephalopathy syndrome: A case report and review of the literature. Radiol. Case Rep. 2023, 18, 3769–3772. [Google Scholar] [CrossRef] [PubMed]
- Gowan, J.M.; Liu, A. Isolated pan-pontine posterior reversible encephalopathy syndrome in a patient with uncontrolled hypertension. Clin. Case Rep. 2019, 7, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Grossbach, A.J.; Abel, T.J.; Hodis, B.; Wassef, S.N.; Greenlee, J.D.W. Hypertensive posterior reversible encephalopathy syndrome causing posterior fossa edema and hydrocephalus. J. Clin. Neurosci. 2014, 21, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Hama, R.; Oshikawa, H. Brainstem Posterior Reversible Encephalopathy Syndrome. Intern. Med. 2019, 58, 2901–2902. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Sun, Z.; Zhou, C.; Shen, Y.; Li, G.; Wang, J. Central-variant posterior reversible encephalopathy syndrome with bilateral corticospinal tract involvement: A case report. Acta Neurol. Belg. 2021, 121, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Hashida, T.; Yazaki, M.; Uchida, T.; Watanabe, E. Posterior reversible encephalopathy syndrome (PRES) of the isolated brainstem. Clin. Case Rep. 2022, 10, e05712. [Google Scholar] [CrossRef] [PubMed]
- Hebant, B.; Guegan-Massardier, E.; Triquenot-Bagan, A.; Ozkul-Wermester, O. Atypical MRI presentation of posterior reversible encephalopathy syndrome with predominant brainstem involvement. Acta Neurol. Belg. 2019, 119, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Jesrani, G.; Gupta, S.; Arya, Y.; Syal, A.; Gupta, M. Systemic Lupus Erythematosus Induced Central Variant of Posterior Reversible Encephalopathy Syndrome: A Rare Association. Cureus 2021, 13, e13431. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.J.; Qu, Z.Z.; Zhang, X.Q.; Tian, Y.J.; Wang, Y. Uremic encephalopathy with isolated brainstem involvement revealed by magnetic resonance image: A case report. BMC Neurol. 2017, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Kachi, S.; Nomura, T.; Yamada, K.; Oshima, Y.; Ura, S. Atypical posterior reversible encephalopathy syndrome associated with Sjögren's syndrome: A case report. Rinsho Shinkeigaku 2023, 63, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Katano, K.; Kakuchi, Y.; Nakashima, A.; Nakahama, K.; Kawano, M. Apparent diffusion coefficient map based on diffusion-weighted magnetic resonance imaging is useful in diagnosing the brainstem variant of reversible posterior leukoencephalopathy syndrome with uremia. Clin. Exp. Nephrol. 2010, 14, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, H.; Tomimoto, H.; Miki, Y.; Yamamoto, A.; Terada, K.; Satoi, H.; Kanda, M.; Fukuyama, H. A brainstem variant of reversible posterior leukoencephalopathy syndrome. Neuroradiology 2005, 47, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, G.; Lenka, A. Brainstem Predominant Posterior Reversible Encephalopathy Syndrome. Neurol. India 2021, 69, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, S.J. Central-Variant Posterior Reversible Encephalopathy Syndrome with Albuminocytologic Dissociation. Case Rep. Neurol. 2018, 10, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cao, J.; Su, Z.; Xu, S. Isolated brainstem involvement in posterior reversible encephalopathy syndrome: A case report and review of the literature. Int. J. Neurosci. 2019, 129, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Maciel, R.; Nzwalo, H.; Palma, R.; Martins, A.; Shamassa, M.; Pizhin, D. The Resolution of Central Variant of Posterior Reversible Encephalopathy Syndrome. Neurohospitalist 2015, 5, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Mazumder, R.; Blanco, M.B.; Liebeskind, D.S. Atypical case of central-variant posterior reversible encephalopathy syndrome. Acta Neurol. Belg. 2017, 117, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Monica, C.; Romaniuc, A.; Andone, S.; Bălaşa, R. Central-variant posterior reversible encephalopathy syndrome in a young patient with systemic lupus erythematosus. Acta Neurol. Belg. 2019, 119, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Fujikawa, H.; Takimiya, R.; Sato, H. Simultaneous Presentation of Brainstem and Cerebellar Posterior Reversible Encephalopathy Syndrome With Acute Cerebral Infarction. Cureus 2023, 15, e34843. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Ogawa, T.; Hishikawa, N.; Takao, Y.; Fujii, S. Dramatic Amelioration in Serial Magnetic Resonance Imaging in an "Isolated Brainstem" Reversible Encephalopathy Syndrome Case. Yonago Acta Med. 2023, 66, 297–299. [Google Scholar] [CrossRef] [PubMed]
- McCarron, M.O.; McKinstry, C.S. Vanishing brainstem edema. J. Stroke Cerebrovasc. Dis. 2008, 17, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.N.; Eagam, M.; Moodley, M. Reversible brainstem edema due to hypertensive encephalopathy in an 8-year-old girl. J. Child. Neurol. 2011, 26, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Nagato, M.; Takahashi, Y.; Yoshioka, M.; Nambu, M. A case of hypertensive encephalopathy with extensive spinal lesions on MRI. Brain Dev. 2010, 32, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Nanba, T.; Kashimura, H.; Saura, H.; Takeda, M. Subarachnoid hemorrhage due to ruptured intracranial aneurysm following posterior reversible encephalopathy syndrome. J. Neurosci. Rural. Pract. 2016, 7, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Ballester, A.; Revert-Espí, R. Unusual Presentation of a Posterior Reversible Encephalopathy Syndrome With Brainstem Involvement and Subarachnoid Haemorrhage. Cureus 2021, 13, e16295. [Google Scholar] [CrossRef] [PubMed]
- Ocek, L.; Sener, U.; Demirtas, B.S.; Ozcelik, M.M.; Oztekin, O.; Zorlu, Y. Central-Variant Posterior Reversible Encephalopathy due to Sulfasalazine: A Case Report. Med. Princ. Pract. 2015, 24, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, E.; Hayakawa, I.; Tsutsumi, Y.; Kamei, K.; Ide, K.; Abe, Y. Central-variant posterior reversible encephalopathy syndrome in an infant with mid-aortic syndrome: A rare case of symmetric basal ganglia lesions. Radiol. Case Rep. 2022, 17, 3475–3480. [Google Scholar] [CrossRef] [PubMed]
- Onomura, H.; Shimizu, T.; Suzuki, J.; Nakai, N.; Teramachi, Y.; Tomonori, K.; Akiguchi, I.; Ito, Y. Posterior reversible encephalopathy syndrome presenting with thrombotic microangiopathy triggered by malignant hypertension: A case report and literature review. BMJ Neurol. Open 2022, 4, e000296. [Google Scholar] [CrossRef] [PubMed]
- Osman, Y.; Imam, Y.Z.; Salem, K.; Al-Hail, H.; Uthman, B.; Deleu, D. Isolated Brainstem Involvement in a Patient with Hypertensive Encephalopathy. Case Rep. Neurol. Med. 2013, 2013, 540947. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Xia, L.; Wang, L.; Xia, L.; Zhou, Q.; Pan, S. Posterior Reversible Encephalopathy Syndrome With Isolated Involving Infratentorial Structures. Front. Neurol. 2018, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Raya, M.; Nasir, I.; Liu, A. Atypical presentation of cerebellar posterior reversible encephalopathy syndrome in a patient with HIV. Clin. Case Rep. 2020, 8, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Resorlu, M.; Karatag, O.; Aylanc, N.; Ozturk, M.O.; Toprak, C.A. Posterior reversible encephalopathy with brainstem involvement. Intern. Emerg. Med. 2018, 13, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Sallah, Y.H.; Zubair, A.S.; Dewey, J.J. Extensive Brainstem Posterior Reversible Encephalopathy Syndrome in a Hemodialysis Non-Adherent Patient. Cureus 2021, 13, e14523. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Monteiro, M.; Moreira, B.; França, M. Rare posterior reversible encephalopathy syndrome in a patient with HIV. BMJ Case Rep. 2013, 2013, 201495. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, K.; Fukae, J.; Noda, K.; Fujishima, K.; Hattori, N.; Okuma, Y. Blurred Vision With Acute Hypertension Indicating Hypertensive Brainstem Encephalopathy: Case Report. Neurol. Med. -Chir. 2009, 49, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, R.; Sehgal, R.; Aggarwal, K.C. Atypical presentation of posterior reversible encephalopathy: In a child with bilateral grade IV vesicoureteric reflux. J. Trop. Pediatr. 2014, 60, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Tha, K.K.; Iguchi, A.; Cho, Y.; Yoshida, A.; Fujima, N.; Tsukahara, A.; Shirato, H.; Terae, S. Isolated posterior fossa involvement in posterior reversible encephalopathy syndrome. Neuroradiol. J. 2013, 26, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.G.; Balasubramanian, P.; Mayilvaganan, K.R.; Kannan, U.N.; Bilal, M. Central variant of posterior reversible encephalopathy syndrome—A rare case report. J. Clin. Diagn. Res. 2017, 11, TD01–TD02. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Y.; Tan, K. Hypertensive brainstem encephalopathy: A diagnosis often overlooked. Clin. Med. (Lond.) 2019, 19, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Tari Capone, F.; Candela, S.; Bozzao, A.; Orzi, F. A new case of brainstem variant of posterior reversible encephalopathy syndrome: Clinical and radiological features. Neurol. Sci. 2015, 36, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Thambisetty, M.; Biousse, V.; Newman, N.J. Hypertensive brainstem encephalopathy: Clinical and radiographic features. J. Neurol. Sci. 2003, 208, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Caranci, F.; Belfiore, M.P.; Manzi, F.; Pagliano, P.; Cirillo, S. Brainstem variant of posterior reversible encephalopathy syndrome: A case report. Neuroradiol. J. 2015, 28, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, M.; Akimoto, J.; Nakajima, N.; Hashimoto, R.; Haraoka, J. Two cases of posterior reversible encephalopathy syndrome resembling brainstem glioma. Clin. Neurol. Neurosurg. 2012, 114, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Vaysman, T.; Xu, P.; Vartanian, T.; Michalak, P.; Pike, K.; Liu, A. "Highlighting" red nuclei by atypical posterior reversible encephalopathy syndrome in a patient with systemic lupus erythematosus. Clin. Case Rep. 2019, 7, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Wakely, S.L.; Ditchfield, A. Hypertensive encephalopathy: A rare case of isolated pons involvement. Clin. Radiol. Extra 2005, 60, E53–E56. [Google Scholar] [CrossRef]
- Wittgrove, C.; Kaur, H.; Siddiqui, J.H. Atypical Variant of Posterior Reversible Encephalopathy Syndrome in the Setting of Renovascular Hypertension: Case Report and Review of Literature. Cureus 2018, 10, e3573. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, K.; Maeda, Y.; Iihara, K. Variant Type of Posterior Reversible Encephalopathy Syndrome Associated with Deep Brain Hemorrhage: Case Report and Review of the Literature. World Neurosurg. 2020, 134, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, J.; Yamaguchi, H.; Koge, J.; Matsushita, T.; Isobe, N.; Yamasaki, R.; Kira, J.I. Brainstem posterior reversible encephalopathy syndrome in a case with Guillain–Barré syndrome. Clin. Exp. Neuroimmunol. 2019, 10, 267–271. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zheng, Y.; Zhang, B.J.; Zhang, Y.; Ding, M.P.; Zhang, B.R. Variant Type of Posterior Reversible Encephalopathy Syndrome with Diffuse Cerebral White Matter and Brainstem Involvement Associated with Intracranial Hemorrhage. J. Stroke Cerebrovasc. Dis. 2016, 25, e233–e235. [Google Scholar] [CrossRef] [PubMed]
- de Havenon, A.; Joos, Z.; Longenecker, L.; Shah, L.; Ansari, S.; Digre, K. Posterior reversible encephalopathy syndrome with spinal cord involvement. Neurology 2014, 83, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Milia, A.; Moller, J.; Pilia, G.; Mascia, M.G.; Marchi, P.; Mura, M.; Marrosu, M.G. Spinal cord involvement during hypertensive encephalopathy: Clinical and radiological findings. J. Neurol. 2008, 255, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Samara, A.; Berry, B.; Ghannam, M. Posterior reversible encephalopathy syndrome with isolated infratentorial involvement: A case report. Radiol. Case Rep. 2019, 14, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dai, D.; Cao, F.; Zhang, L.; Wang, X. Posterior reversible encephalopathy syndrome with spinal cord involvement but without hemisphere lesions: A case report. Medicine 2019, 98, e13649. [Google Scholar] [CrossRef] [PubMed]
- Gocmen, R.; Ardicli, D.; Erarslan, Y.; Duzova, A.; Anlar, B. Reversible Hypertensive Myelopathy-The Spinal Cord Variant of Posterior Reversible Encephalopathy Syndrome. Neuropediatrics 2017, 48, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Marrone, L.C.; Martins, W.A.; Brunelli, J.P.; Fussiger, H.; Carvalhal, G.F.; Filho, J.R.; Soder, R.B.; Schuck, M.; Viola, F.S.; Marrone, A.C.; et al. PRES with asymptomatic spinal cord involvement. Is this scenario more common than we know? Spinal Cord. Ser. Cases 2016, 2, 15001. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.J.; Tse, K.S.; Fok, W.S.E.; Poon, W.L. First case of neurofibromatosis with posterior reversible encephalopathy syndrome showing spinal cord involvement. Indian. J. Radiol. Imaging 2018, 28, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Sebastian, L.J.D.; Gaikwad, S.B.; Garg, A.; Mishra, N.K. Spinal cord involvement and contrast enhancement in posterior reversible encephalopathy syndrome. BJR Case Rep. 2016, 2, 20150326. [Google Scholar] [CrossRef] [PubMed]
- Choh, N.A.; Jehangir, M.; Rasheed, M.; Mira, T.; Ahmad, I.; Choh, S. Involvement of the cervical cord and medulla in posterior reversible encephalopathy syndrome. Ann. Saudi Med. 2011, 31, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, H.V.; Choudhary, P.; Saxena, S.; Arif, M. Posterior reversible encephalopathy syndrome with spinal cord involvement (PRES-SCI): A case report. Ann. Indian. Acad. Neurol. 2016, 19, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; Wu, T.-C.; Ko, C.-C.; Feng, I.J.; Tsui, Y.-K.; Lin, C.-J.; Chen, J.-H.; Lin, C.-P. Quantitative Magnetic Resonance Diffusion-Weighted Imaging Evaluation of the Supratentorial Brain Regions in Patients Diagnosed with Brainstem Variant of Posterior Reversible Encephalopathy Syndrome: A Preliminary Study. J. Stroke Cerebrovasc. Dis. 2017, 26, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.T.; Samant, R.S.; Kumar, M.; Van Hemert, R.; Angtuaco, E.J. Features of infratentorial-predominant posterior reversible encephalopathy syndrome. Acta Neurol. Belg. 2015, 115, 629–634. [Google Scholar] [CrossRef] [PubMed]
- McKinney, A.M.; Short, J.; Truwit, C.L.; McKinney, Z.J.; Kozak, O.S.; SantaCruz, K.S.; Teksam, M. Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am. J. Roentgenol. 2007, 189, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Aygunes, U.; Sasmaz, H.I.; Arpaci, T.; Akbas, T.; Ozcan, N.; Antmen, A.B. Clinical and Radiological Characteristics of Classical and Variant Type of Posterior Reversible Encephalopathy Syndrome on Prognosis Following Hematopoietic Stem Cell Transplantation in Pediatric Patients: A Single-Center Experience. Exp. Clin. Transplant. 2024, 22, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Srichawla, B.S.; Kipkorir, V.; Lalla, R. Central-variant posterior reversible encephalopathy syndrome in association with adrenal insufficiency: A case report. Med. (Baltim.) 2025, 104, e41625. [Google Scholar] [CrossRef] [PubMed]
- Yiş, U.; Karaoğlu, P.; Kurul, S.H.; Soylu, A.; Çakmakçi, H.; Kavukçu, S. Posterior reversible leukoencephalopathy syndrome with spinal cord involvement in a 9-year-old girl. Brain Dev. 2016, 38, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.; Owens, M.Y.; Wallace, K.; Reeves, A.A.; Morris, R.; Khan, M.; Lamarca, B.; Martin Jr, J.N. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am. J. Obstet. Gynecol. 2013, 208, 468.e1–468.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.D.P.; Gor, D.M.D.; Walicki, D.M.T.; Jenny, D.R.N.; Jones, D.M.D.; Barbour, P.M.D.; Castaldo, J.M.D. Spectrum and Potential Pathogenesis of Reversible Posterior Leukoencephalopathy Syndrome. J. Stroke Cerebrovasc. Dis. 2012, 21, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Devaramane, R.; Jagadish, G.M.; Chowdaiah, S. Various Imaging Manifestations of Posterior Reversible Encephalopathy Syndrome (PRES) on Magnetic Resonance Imaging (MRI). Pol. J. Radiol. 2017, 82, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.D.; Cho, B.M.; Oh, S.M.; Park, S.H.; Jang, I.B.; Lee, J.Y. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J. Cerebrovasc. Endovasc. Neurosurg. 2013, 15, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Yokote, H.; Uchihara, T.; Toru, S. Bilateral Optic Nerve Edema in Central-variant Posterior Reversible Encephalopathy Syndrome. Intern. Med. 2020, 59, 2333–2334. [Google Scholar] [CrossRef] [PubMed]
- Fugate, J.E.; Claassen, D.O.; Cloft, H.J.; Kallmes, D.F.; Kozak, O.S.; Rabinstein, A.A. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin. Proc. 2010, 85, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A.; Mandrekar, J.; Merrell, R.; Kozak, O.S.; Durosaro, O.; Fugate, J.E. Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J. Stroke Cerebrovasc. Dis. 2012, 21, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Srichawla, B.S.; Garcia-Dominguez, M.A. Regional dynamic cerebral autoregulation across anterior and posterior circulatory territories: A detailed exploration and its clinical implications. World J. Crit. Care Med. 2024, 13, 97149. [Google Scholar] [CrossRef] [PubMed]
- Srichawla, B.S.; Catton, R.M.; Lichtenberg, A.A.; Henninger, N. Clinical characteristics and risk factors for bilateral lateral geniculate body pathology: A systematic review of the literature. Neurol. Sci. 2023, 44, 3481–3493. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.B. Hyperperfusion encephalopathies: Hypertensive encephalopathy and related conditions. Neurologist 2002, 8, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Beausang-Linder, M.; Bill, A. Cerebral circulation in acute arterial hypertension--protective effects of sympathetic nervous activity. Acta Physiol. Scand. 1981, 111, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; Fugate, J.E.; Rabinstein, A.A. Central pontine and extrapontine myelinolysis: A systematic review. Eur. J. Neurol. 2014, 21, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-R.; Chen, S.-P. Posterior reversible encephalopathy as the first manifestation of Bickerstaff's brainstem encephalitis. BMC Neurol. 2016, 16, 215. [Google Scholar] [CrossRef] [PubMed]

| Database | Search String |
|---|---|
| PubMed/PubMedCentral/MEDLINE | (“Posterior Leukoencephalopathy Syndrome” OR “Posterior Reversible Encephalopathy Syndrome” OR “PRES”) AND (“central variant” OR “central variant” OR “brainstem” OR “spinal cord”) |
| ScienceDirect | (“Posterior Reversible Encephalopathy Syndrome” OR “PRES”) AND (“central variant” OR “brainstem” OR “spinal cord”) |
| Scopus | (“Posterior Leukoencephalopathy Syndrome” OR “Posterior Reversible Encephalopathy Syndrome” OR “PRES”) AND (“central-variant” OR “central variant” OR “brainstem” OR “spinal cord”) |
| Hinari | (“Posterior Leukoencephalopathy Syndrome” OR “Posterior Reversible Encephalopathy Syndrome” OR “PRES”) AND (“central variant” OR “central variant” OR “brainstem” OR “spinal cord”) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srichawla, B.S.; Garcia-Dominguez, M.A.; Silver, B. The Central Variant of Posterior Reversible Encephalopathy Syndrome: A Systematic Review and Meta-Analysis. Neurol. Int. 2025, 17, 113. https://doi.org/10.3390/neurolint17070113
Srichawla BS, Garcia-Dominguez MA, Silver B. The Central Variant of Posterior Reversible Encephalopathy Syndrome: A Systematic Review and Meta-Analysis. Neurology International. 2025; 17(7):113. https://doi.org/10.3390/neurolint17070113
Chicago/Turabian StyleSrichawla, Bahadar S., Maria A. Garcia-Dominguez, and Brian Silver. 2025. "The Central Variant of Posterior Reversible Encephalopathy Syndrome: A Systematic Review and Meta-Analysis" Neurology International 17, no. 7: 113. https://doi.org/10.3390/neurolint17070113
APA StyleSrichawla, B. S., Garcia-Dominguez, M. A., & Silver, B. (2025). The Central Variant of Posterior Reversible Encephalopathy Syndrome: A Systematic Review and Meta-Analysis. Neurology International, 17(7), 113. https://doi.org/10.3390/neurolint17070113






