The Association of Axonal Damage Biomarkers and Osteopontin at Diagnosis Could Be Useful in Newly Diagnosed MS Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Informed Consent
2.2. Clinical Characteristics
2.3. Laboratory Sampling
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Axonal and Inflammatory Biomarkers Positively Correlate at Diagnosis
3.3. Neurofilament Light Chains Are Higher in Patients with Acute Inflammation and High-Efficacy Treatments
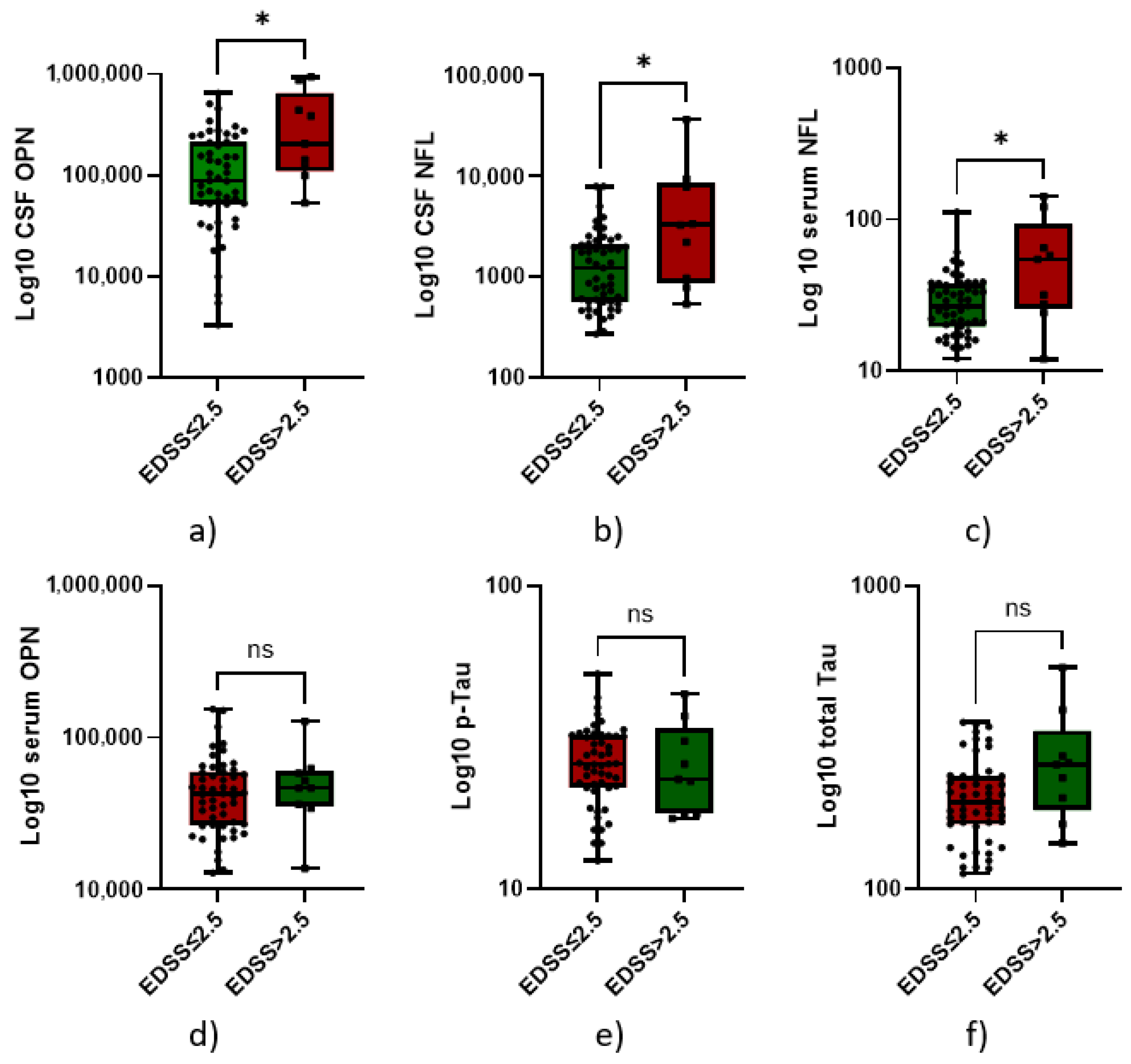
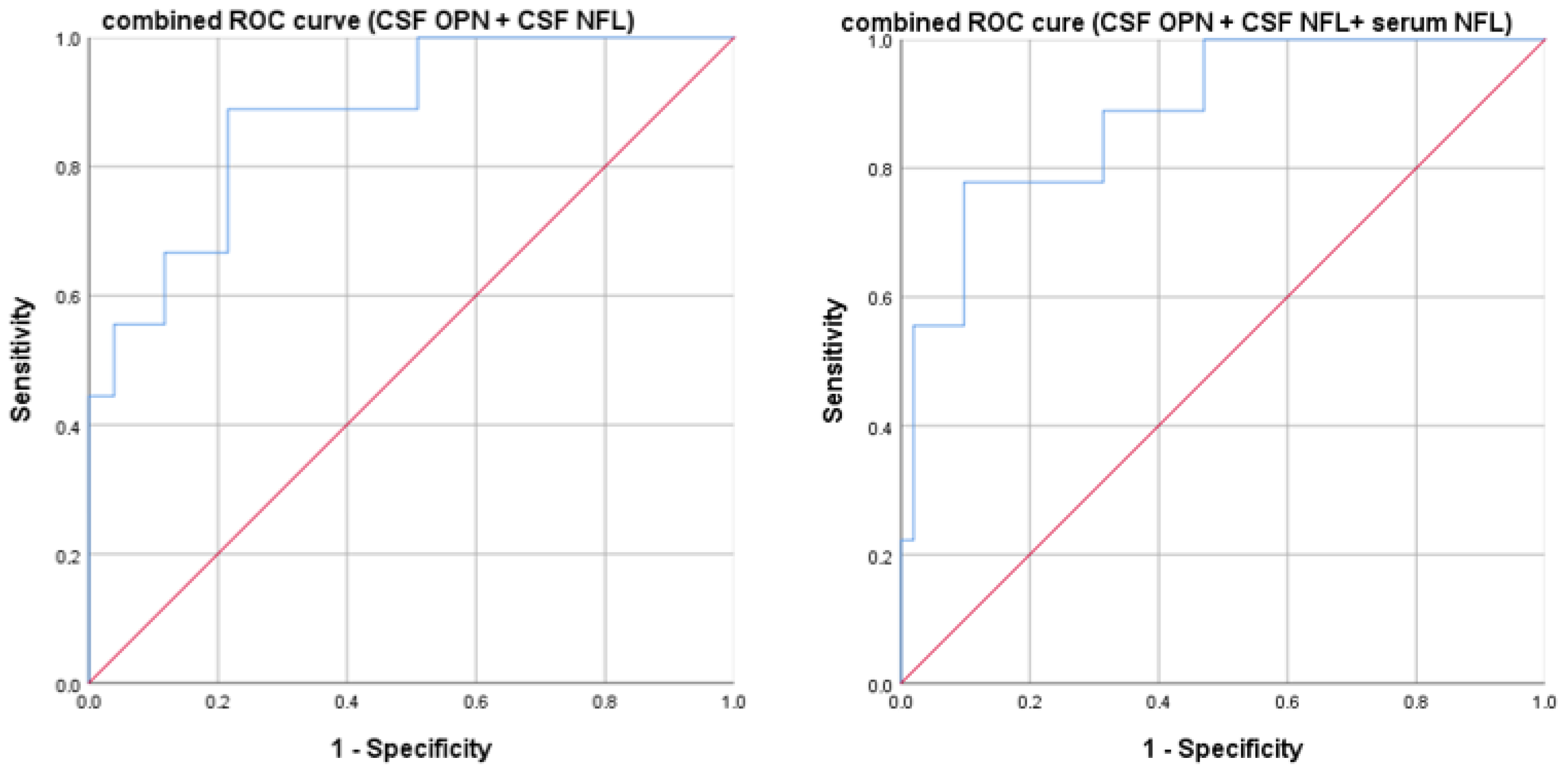
3.4. High Neurofilaments and Osteopontin Predict Higher Disability at Diagnosis
3.5. Cerebrospinal Fluid Neurofilament and First DMT Choices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CIS | clinically isolated syndrome |
| CSF | cerebrospinal fluid |
| Dd | disease duration |
| DMTs | disease-modifying therapies |
| EDSS | expanded disability status scale |
| Gd | gadolinium-enhancing |
| HE | highly effective |
| IQR | interquartile range |
| ME | moderate-efficacy |
| MS | multiple sclerosis |
| NFL | neurofilament light chain |
| OPN | osteopontin |
| PMS | progressive MS |
| p-Tau | phosporilated tau |
| RIS | radiologically isolated syndrome |
| ROC | receiver operating characteristic |
| RR | relapsing–remitting |
| SD | standard deviation |
| SIMOA | single-molecule assay |
| t-Tau | total tau |
References
- Di Sabatino, E.; Ferraro, D.; Gaetani, L.; Emiliano, E.; Parnetti, L.; Di Filippo, M. CSF biomarkers of B-cell activation in multiple sclerosis: A clinical perspective. J. Neurol. 2025, 272, 211. [Google Scholar] [CrossRef]
- Montobbio, N.; Cordioli, C.; Signori, A.; Bovis, F.; Capra, R.; Sormani, M.P. Relapse-Associated and Relapse-Independent Contribution to Overall Expanded Disability Status Scale Progression in Multiple Sclerosis Patients Diagnosed in Different Eras. Ann. Neurol. 2024, 97, 95–103. [Google Scholar] [CrossRef]
- Guo, J.; Wu, J.; Wang, L.; Liu, H.; Wu, X.; Yang, H.; Li, W.; Wang, H.; Bu, B.; Yang, C.; et al. Treatment algorithms of relapsing multiple sclerosis: An exploration based on the available disease-modifying therapies in China. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241239117. [Google Scholar] [CrossRef]
- Rashid, W.; Ciccarelli, O.; Leary, S.M.; Arun, T.; Doshi, A.; Evangelou, N.; Ford, H.L.; Hobart, J.; Jacob, S.; Muraro, P.A.; et al. Using disease-modifying treatments in multiple sclerosis: Association of British Neurologists (ABN) 2024 guidance. Pract. Neurol. 2024, 25, 18–24. [Google Scholar] [CrossRef]
- Bergamaschi, R. Prognosis of multiple sclerosis: Clinical factors predicting the late evolution for an early treatment decision. Expert. Rev. Neurother. 2006, 6, 357–364. [Google Scholar] [CrossRef]
- Bergamaschi, R. Prognostic factors in multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Lazibat, I.; Nevajda, B.; Grahovac, G.; Brinar, V.V. Should MS be treated by escalation or induction therapy? Coll. Antropol. 2014, 38, 385–393. [Google Scholar] [PubMed]
- Absinta, M. Multiple sclerosis: A smoldering disease. Trends Mol. Med. 2025, 31, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Comi, C.; Virgilio, E.; Vecchio, D.; Tesser, F.; Cappellano, G. Chapter 3—Biomarkers in multiple sclerosis. In Translational Neuroimmunology; Rezaei, N., Yazdanpanah, N., Eds.; Academic Press: New York, NY, USA, 2023; Volume 8, pp. 27–53. [Google Scholar] [CrossRef]
- Di Filippo, M.; Gaetani, L.; Centonze, D.; Hegen, H.; Kuhle, J.; Teunissen, C.E.; Tintoré, M.; Villar, L.M.; Willemse, E.A.J.; Zetterberg, H.; et al. Fluid biomarkers in multiple sclerosis: From current to future applications. Lancet. Reg. Health. Eur. 2024, 44, 101009. [Google Scholar] [CrossRef]
- Harris, V.K.; Sadiq, S.A. Disease biomarkers in multiple sclerosis: Potential for use in therapeutic decision making. Mol. Diagn. Ther. 2009, 13, 225–244. [Google Scholar] [CrossRef]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch. Neurol. 2005, 62, 865–870. [Google Scholar] [CrossRef]
- Thebault, S.; Booth, R.A.; Freedman, M.S. Blood Neurofilament Light Chain: The Neurologist’s Troponin? Biomedicines 2020, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Thebault, S.; Booth, R.A.; Rush, C.A.; MacLean, H.; Freedman, M.S. Serum Neurofilament Light Chain Measurement in MS: Hurdles to Clinical Translation. Front. Neurosci. 2021, 15, 654942. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Puricelli, C.; Malucchi, S.; Virgilio, E.; Martire, S.; Perga, S.; Passarelli, F.; Valentino, P.; Di Sapio, A.; Cantello, R.; et al. Serum and cerebrospinal fluid neurofilament light chains measured by SIMOA™, Ella™, and Lumipulse™ in multiple sclerosis naïve patients. Mult. Scler. Relat. Disord. 2024, 82, 105412. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Atuesta, C.; Reyes, S.; Giovanonni, G.; Gnanapavan, S. The Evolution of Neurofilament Light Chain in Multiple Sclerosis. Front. Neurosci. 2021, 15, 642384. [Google Scholar] [CrossRef]
- Nötzel, M.; Werder, L.I.; Ziemssen, T.; Akgün, K. Ella versus Simoa Serum Neurofilament Assessment to Monitor Treatment Response in Highly Active Multiple Sclerosis Patients. Int. J. Mol. Sci. 2022, 23, 12361. [Google Scholar] [CrossRef]
- Gauthier, A.; Viel, S.; Perret, M.; Brocard, G.; Casey, R.; Lombard, C.; Laurent-Chabalier, S.; Debouverie, M.; Edan, G.; Vukusic, S.; et al. Comparison of SimoaTM and EllaTM to assess serum neurofilament-light chain in multiple sclerosis. Ann. Clin. Transl. Neurol. 2021, 8, 1141–1150. [Google Scholar] [CrossRef]
- Virgilio, E.; De Marchi, F.; Contaldi, E.; Dianzani, U.; Cantello, R.; Mazzini, L.; Comi, C. The Role of Tau beyond Alzheimer’s Disease: A Narrative Review. Biomedicines 2022, 10, 760. [Google Scholar] [CrossRef]
- Hoehne, C.; Stuve, O.; Stopschinski, B.E. Tau in Multiple Sclerosis: A Review of Therapeutic Potential. Curr. Treat. Options Neurol. 2025, 27, 14. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Serino, R.; Cantello, R.; Dianzani, U.; Comi, C. Cerebrospinal Tau levels as a predictor of early disability in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103231. [Google Scholar] [CrossRef]
- Agnello, L.; Colletti, T.; Lo Sasso, B.; Vidali, M.; Spataro, R.; Gambino, C.M.; Giglio, R.V.; Piccoli, T.; Bivona, G.; La Bella, V.; et al. Tau protein as a diagnostic and prognostic biomarker in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021, 28, 1868–1875. [Google Scholar] [CrossRef]
- Leitão, M.J.; Silva-Spínola, A.; Santana, I.; Olmedo, V.; Nadal, A.; Le Bastard, N.; Baldeiras, I. Clinical validation of the Lumipulse G cerebrospinal fluid assays for routine diagnosis of Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 91. [Google Scholar] [CrossRef]
- Jaworski, J.; Psujek, M.; Janczarek, M.; Szczerbo-Trojanowska, M.; Bartosik-Psujek, H. Total-tau in cerebrospinal fluid of patients with multiple sclerosis decreases in secondary progressive stage of disease and reflects degree of brain atrophy. Ups. J. Med. Sci. 2012, 117, 284–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez, M.A.; Olsson, B.; Bau, L.; Matas, E.; Cobo Calvo, A.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martinez-Yelamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult. Scler. 2015, 21, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Agah, E.; Zardoui, A.; Saghazadeh, A.; Ahmadi, M.; Tafakhori, A.; Rezaei, N. Osteopontin (OPN) as a CSF and blood biomarker for multiple sclerosis: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0190252. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tao, Z.; Zhao, X. The Role of Osteopontin (OPN) in Regulating Microglia Phagocytosis in Nervous System Diseases. J. Integr. Neurosci. 2024, 23, 169. [Google Scholar] [CrossRef]
- Rosmus, D.D.; Lange, C.; Ludwig, F.; Ajami, B.; Wieghofer, P. The Role of Osteopontin in Microglia Biology: Current Concepts and Future Perspectives. Biomedicines 2022, 10, 840. [Google Scholar] [CrossRef]
- Cappellano, G.; Vecchio, D.; Magistrelli, L.; Clemente, N.; Raineri, D.; Barbero Mazzucca, C.; Virgilio, E.; Dianzani, U.; Chiocchetti, A.; Comi, C. The Yin-Yang of osteopontin in nervous system diseases: Damage versus repair. Neural Regen. Res. 2021, 16, 1131–1137. [Google Scholar] [CrossRef]
- Orsi, G.; Hayden, Z.; Cseh, T.; Berki, T.; Illes, Z. Osteopontin levels are associated with late-time lower regional brain volumes in multiple sclerosis. Sci. Rep. 2021, 11, 23604. [Google Scholar] [CrossRef]
- Marastoni, D.; Turano, E.; Tamanti, A.; Colato, E.; Pisani, A.I.; Scartezzini, A.; Carotenuto, S.; Mazziotti, V.; Camera, V.; Anni, D.; et al. Association of Levels of CSF Osteopontin With Cortical Atrophy and Disability in Early Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200265. [Google Scholar] [CrossRef]
- Karasalih, B.; Duman, H.; Bechelany, M.; Karav, S. Osteopontin: Its Properties, Recent Studies, and Potential Applications. Int. J. Mol. Sci. 2025, 26, 5868. [Google Scholar] [CrossRef]
- Kabdesh, I.; Tutova, O.; Akhmetzyanova, E.; Timofeeva, A.; Bilalova, A.; Mukhamedshina, Y.; Chelyshev, Y. Thoracic Spinal Cord Contusion Impacts on Lumbar Enlargement: Molecular Insights. Mol. Neurobiol. 2025, 62, 8551–8567. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Tintore, M.; Rovira, À.; Río, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arévalo, M.J.; Negrotto, L.; et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015, 138, 1863–1874. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Bowen, J.D.; Hartung, H.P.; Vermersch, P.; Hughes, B.; Damian, D.; Hyvert, Y.; Dangond, F.; Galazka, A.; Grosso, M.; et al. Subgroup analysis of clinical and MRI outcomes in participants with a first clinical demyelinating event at risk of multiple sclerosis in the ORACLE-MS study. Mult. Scler. Relat. Disord. 2021, 49, 102695. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A.; Bastianello, S.; Comi, G.; Gallo, P.; Gallucci, M.; Ghezzi, A.; Marrosu, M.G.; Minonzio, G.; Pantano, P.; et al. Guidelines from The Italian Neurological and Neuroradiological Societies for the use of magnetic resonance imaging in daily life clinical practice of multiple sclerosis patients. Neurol. Sci. 2013, 34, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Fraser, C.L.; Abegg, M.; Alroughani, R.; Alshowaeir, D.; Alvarenga, R.; Andris, C.; Asgari, N.; Barnett, Y.; Battistella, R.; et al. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022, 21, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Franciotta, D.; Avolio, C.; Capello, E.; Lolli, F. Consensus recommendations of the Italian Association for Neuroimmunology for immunochemical cerebrospinal fluid examination. J. Neurol. Sci. 2005, 237, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.M.; Fagan, A.M.; Grant, E.A.; Marcus, D.S.; Benzinger, T.L.; Mintun, M.A.; Holtzman, D.M.; Morris, J.C. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Arch. Neurol. 2011, 68, 1145–1151. [Google Scholar] [CrossRef]
- Fujirebio. Lumipulse G pTau 181. Available online: https://www.fujirebio.com/en/products-solutions/lumipulse-g-ptau-181 (accessed on 8 July 2025).
- Fujirebio. Kits for Fully Automated Testing. Available online: https://www.fujirebio.com/en/products-solutions/kits-for-fully-automated-testing (accessed on 8 July 2025).
- Kurtzke, J.F. Disability rating scales in multiple sclerosis. Ann. N. Y. Acad. Sci. 1984, 436, 347–360. [Google Scholar] [CrossRef]
- Zarghami, A.; Hussain, M.A.; van der Mei, I.; Simpson-Yap, S.; Ponsonby, A.L.; Lechner-Scott, J.; Broadley, S.A.; Lucas, R.M.; Zhou, Y.; Lin, X.; et al. Long-term disability trajectories in multiple sclerosis: A group-based trajectory analysis of the AusLong cohort. J. Neurol. Neurosurg. Psychiatry 2025, 96, 424–434. [Google Scholar] [CrossRef]
- Bsteh, G.; Dal-Bianco, A.; Krajnc, N.; Berger, T. Biomarkers of Progression Independent of Relapse Activity-Can We Actually Measure It Yet? Int. J. Mol. Sci. 2025, 26, 4704. [Google Scholar] [CrossRef]
- Toscano, S.; Spelman, T.; Ozakbas, S.; Alroughani, R.; Chisari, C.G.; Lo Fermo, S.; Prat, A.; Girard, M.; Duquette, P.; Izquierdo, G.; et al. First-year treatment response predicts the following 5-year disease course in patients with relapsing-remitting multiple sclerosis. Neurotherapeutics 2025, 22, e00552. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Arnold, D.L.; Alvarez, E.; Cross, A.H.; Willi, R.; Li, B.; Kukkaro, P.; Kropshofer, H.; Ramanathan, K.; Merschhemke, M.; et al. Prognostic Value of Serum Neurofilament Light Chain for Disease Activity and Worsening in Patients with Relapsing Multiple Sclerosis: Results from the Phase 3 ASCLEPIOS I and II Trials. Front. Immunol. 2022, 13, 852563. [Google Scholar] [CrossRef] [PubMed]
- Modvig, S.; Degn, M.; Horwitz, H.; Cramer, S.P.; Larsson, H.B.; Wanscher, B.; Sellebjerg, F.; Frederiksen, J.L. Relationship between cerebrospinal fluid biomarkers for inflammation, demyelination and neurodegeneration in acute optic neuritis. PLoS ONE 2013, 8, e77163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orsi, G.; Cseh, T.; Hayden, Z.; Perlaki, G.; Nagy, S.A.; Giyab, O.; Olsen, D.A.; Madsen, J.S.; Berki, T.; Illes, Z. Microstructural and functional brain abnormalities in multiple sclerosis predicted by osteopontin and neurofilament light. Mult. Scler. Relat. Disord. 2021, 51, 102923. [Google Scholar] [CrossRef]
- Tortorella, C.; Direnzo, V.; Ruggieri, M.; Zoccolella, S.; Mastrapasqua, M.; D’Onghia, M.; Paolicelli, D.; Cuonzo, F.D.; Gasperini, C.; Trojano, M. Cerebrospinal fluid neurofilament light levels mark grey matter volume in clinically isolated syndrome suggestive of multiple sclerosis. Mult. Scler. 2018, 24, 1039–1045. [Google Scholar] [CrossRef]
- Mondésert, E.; Schraen-Maschke, S.; Quadrio, I.; Bousiges, O.; Bouvier, D.; Delaby, C.; Bedel, A.; Lehmann, S.; Fourier, A. A French multicenter analytical evaluation of the automated Lumipulse G sNfL blood assay (Fujirebio®) and its comparison to four other immunoassays for serum neurofilament light chain assessment in clinical settings. Clin. Chim. Acta 2025, 565, 120007. [Google Scholar] [CrossRef]

| Characteristics | n/60, (%) | |
| Gender | Female | 38 (63%) |
| MS type | Relapsing–remitting | 53 (89%) |
| Clinically isolated syndrome | 2 (3%) | |
| Radiologically isolated syndrome | 2 (3%) | |
| Secondary progressive | 3 (5%) | |
| Mean ± SD (years) | ||
| Age | Onset | 33.0 ± 11.0 |
| Diagnosis | 36.5 ± 10.7 | |
| Mean, Median, IQR | ||
| EDSS | At diagnosis | 1.7, 1.5, 1–2.5 |
| Dd from onset to baseline | Mean ± SD 5.0 ± 7.2 | |
| Brain white matter lesion load | High lesion load (≥10) | 33 (55%) |
| Low lesion load (<10) | 27 (45%) | |
| Contrast enhancement (gd+) | Absent | 25 (42%) |
| Present | 38 (58%) | |
| Spinal lesions | Absent | 19 (32%) |
| Present | 41 (68%) | |
| Onset | Optic neuritis | 17 (29%) |
| Sensory/motor | 16 (26%) | |
| Brainstem/cerebellar | 10 (17%) | |
| Spinal | 14 (23%) | |
| Multifocal | 1 (2%) | |
| Radiologically isolated syndrome | 2 (3%) | |
| DMTs | H-E | 16 (27%) |
| M-E * | 44 (73%) | |
| Mean ± SD (pg/mL) | ||
| Biomarkers | CSF T-tau | 215 ± 79.4 |
| CSF p-Tau | 26.5 ± 7.9 | |
| CSF NFL | 2487 ± 4804 | |
| Serum NFL | 34.6 ± 24.6 | |
| CSF OPN | 174,013 ± 192,495 | |
| Serum OPN | 49,329 ± 30,418 |
| CSF NFL | Serum NFL | CSF T-tau | CSF p-Tau | CSF OPN | Serum OPN | |
|---|---|---|---|---|---|---|
| CSF NFL | - | 0.8 | 0.45 | 0.13 | 0.08 | 0.25 |
| p < 0.0001 | p = 0.0004 | p = 0.3 | p = 0.5 | p = 0.05 | ||
| Serum NFL | - | 0.29 | −0.03 | −0.08 | 0.43 | |
| p = 0.02 | p = 0.7 | p = 0.5 | p = 0.0005 | |||
| CSF T-tau | - | 0.76 | 0.26 | 0.06 | ||
| p < 0.0001 | p = 0.04 | p = 0.6 | ||||
| CSF p-Tau | - | 0.30 | −0.18 | |||
| p = 0.01 | p = 0.1 | |||||
| CSF OPN | - | −0.15 | ||||
| p = 0.2 | ||||||
| Serum OPN | - |
| Total Tau | p-Tau | ||||
| T0 | Mean ± SD | p-Value | Mean ± SD | p-Value | |
| Brain white matter lesion load | High lesion load (≥10) | 216.7 ± 83.9 | 0.9 | 26.7 ± 7.6 | 0.8 |
| Low lesion load (<10) | 212.9 ± 74.9 | 26.3 ± 8.5 | |||
| Spinal lesions | Present | 214.0 ± 79.19 | 0.8 | 26.9 ± 8.6 | 0.6 |
| Absent | 219.7 ± 82.9 | 25.5 ± 6.6 | |||
| Gd+ | Present | 218.9 ± 86.7 | 0.9 | 26.4 ± 7.9 | 0.8 |
| Absent | 208.6 ± 67.2 | 26.7 ± 8.1 | |||
| MS phenotype | RR | 213.5 ± 79.18 | 0.6 | 26.2 ± 7.6 | 0.8 |
| CIS | 174 ± 63.6 | 23.2 ± 6.9 | |||
| RIS | 265.5 ± 119.5 | 28 ± 5.9 | |||
| SP | 235 ± 94 | 33.4 ± 15.6 | |||
| Sex | Male | 236.1 ± 97.5 | 0.2 | 29.5 ± 9.9 | 0.08 |
| Female | 202.7 ± 65 | 24.8 ± 6.1 | |||
| Onset | Optic neuritis | 211 ± 72.7 | 0.6 | 26 ± 7.6 | 0.4 |
| Sensory/motor | 199.9 ± 79.9 | 23.4 ± 7.8 | |||
| Brainstem/cerebellar | 244.3 ± 115.9 | 30.9 ± 8.9 | |||
| Spinal | 211.3 ± 53.3 | 27.3 ± 7.8 | |||
| Multifocal | 179 ± 0 | 26 ± 0 | |||
| RIS | 265.5 ± 119.5 | 28 ± 5.9 | |||
| DMTs | H-E | 229 ± 104.9 | 0.7 | 24.4 ± 5.5 | 0.2 |
| M-E * | 209.8 ± 68.6 | 27.3 ± 8.6 | |||
| CSF NFL | Serum NFL | ||||
| Mean ± SD | p-Value | Mean ± SD | p-Value | ||
| Brain white matter lesion load | High lesion load (≥10) | 2742 ± 6092 | 0.3 | 36.2 ± 26.2 | 0.3 |
| Low lesion load (<10) | 2176 ± 2551 | 32.5 ± 22.7 | |||
| Spinal Lesions | Present | 2573 ± 5640 | 0.8 | 31.8 ± 21.7 | 0.2 |
| Absent | 2403 ± 2549 | 41.1 ± 29.6 | |||
| Gd+ | Present | 3270 ± 5964 | 0.01 | 39.4 ± 29.2 | 0.04 |
| Absent | 1228 ± 994.7 | 26.8 ± 11.3 | |||
| MS phenotype | RR | 2701 ± 5075 | 0.4 | 35.77 ± 25.86 | 0.7 |
| CIS | 541.5 ± 86.9 | 22.1 ± 6.8 | |||
| RIS | 1207 ± 907.9 | 29.3 ± 12.3 | |||
| SP | 861.7 ± 91.5 | 25.1 ± 2.3 | |||
| Sex | Male | 3372 ± 7447 | 0.7 | 35.23 ± 28.06 | 0.8 |
| Female | 1974 ± 2142 | 34.19 ± 22.7 | |||
| Onset | Optic neuritis | 1361 ± 911 | 0.1 | 30.2 ± 10.1 | 0.3 |
| Sensory/motor | 1771 ± 2408 | 31.59 ± 27.09 | |||
| Brainstem/cerebellar | 5108 ± 10,850 | 42.42 ± 37.59 | |||
| Spinal | 2603 ± 2332 | 32.83 ± 15.17 | |||
| Multifocal | 7812 ± 0 | 112 ± 0 | |||
| RIS | 1207 ± 907.9 | 29.3 ± 12.3 | |||
| DMTs | H-E | 4383 ± 8633 | 0.049 | 45.2 ± 36.7 | 0.1 |
| L-E * | 1797 ± 1960 | 30.7 ± 17.3 | |||
| CSF OPN | Serum OPN | ||||
| T0 | Mean ± SD | p-Value | Mean ± SD | p-Value | |
| Brain white matter lesion load | High lesion load (≥10) | 186,836 ± 203,762 | 0.6 | 46,239 ± 264,141 | 0.5 |
| Low lesion load (<10) | 158,340 ± 180,335 | 53,106 ± 34,842 | |||
| Spinal lesions | Present | 188,053 ± 210,440 | 0.6 | 47,358 ± 28,398 | 0.4 |
| Absent | 143,714 ± 147,058 | 53,583 ± 34,824 | |||
| Gd+ | Present | 199,157 ± 222,831 | 0.2 | 46,226 ± 23,619 | 0.8 |
| Absent | 133,563 ± 123,884 | 54,322 ± 39,068 | |||
| MS phenotype | RR | 175,163 ± 195,633 | 0.0508 | 50,249 ± 32,030 | 0.8 |
| CIS | 27,988 ± 34,892 | 34,401 ± 9568 | |||
| RIS | 58,773 ± 9643 | 51,225 ± 11,522 | |||
| SP | 327,868 ± 164,976 | 41,769 ± 13,186 | |||
| Sex | Male | 230,248 ± 210,723 | 0.01 | 53,520 ± 32,932 | 0.3 |
| Female | 141,455 ± 175,827 | 46,903 ± 29,041 | |||
| Onset | Optic neuritis | 148,648 ± 153,565 | 0.05 | 54,437 ± 31,892 | 0.8 |
| Sensory/motor | 223,811 ± 229,099 | 44,000 ± 18,341 | |||
| Brainstem/cerebellar | 126,245 ± 154,789 | 49,996 ± 42,790 | |||
| Spinal | 191,176 ± 230,598 | 43,593 ± 28,590 | |||
| Multifocal | 276,307 ± 0 | 117,614 ± 0 | |||
| RIS | 58,773 ± 9643 | 51,225 ± 11,522 | |||
| DMTs | H-E | 206,384 ± 249,459 | 0.7 | 44,912 ± 27,888 | 0.5 |
| M-E * | 162,241 ± 169,121 | 50,935 ± 31,438 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgilio, E.; Puricelli, C.; Clemente, N.; Ciampana, V.; Imperatore, Y.; Perga, S.; Stangalini, S.; Boggio, E.; Appiani, A.; Gigliotti, C.L.; et al. The Association of Axonal Damage Biomarkers and Osteopontin at Diagnosis Could Be Useful in Newly Diagnosed MS Patients. Neurol. Int. 2025, 17, 110. https://doi.org/10.3390/neurolint17070110
Virgilio E, Puricelli C, Clemente N, Ciampana V, Imperatore Y, Perga S, Stangalini S, Boggio E, Appiani A, Gigliotti CL, et al. The Association of Axonal Damage Biomarkers and Osteopontin at Diagnosis Could Be Useful in Newly Diagnosed MS Patients. Neurology International. 2025; 17(7):110. https://doi.org/10.3390/neurolint17070110
Chicago/Turabian StyleVirgilio, Eleonora, Chiara Puricelli, Nausicaa Clemente, Valentina Ciampana, Ylenia Imperatore, Simona Perga, Sveva Stangalini, Elena Boggio, Alice Appiani, Casimiro Luca Gigliotti, and et al. 2025. "The Association of Axonal Damage Biomarkers and Osteopontin at Diagnosis Could Be Useful in Newly Diagnosed MS Patients" Neurology International 17, no. 7: 110. https://doi.org/10.3390/neurolint17070110
APA StyleVirgilio, E., Puricelli, C., Clemente, N., Ciampana, V., Imperatore, Y., Perga, S., Stangalini, S., Boggio, E., Appiani, A., Gigliotti, C. L., Dianzani, U., Comi, C., & Vecchio, D. (2025). The Association of Axonal Damage Biomarkers and Osteopontin at Diagnosis Could Be Useful in Newly Diagnosed MS Patients. Neurology International, 17(7), 110. https://doi.org/10.3390/neurolint17070110









