1. Introduction
Selective serotonin reuptake inhibitors (SSRIs) are among the most widely prescribed classes of antidepressants worldwide and represent the first-line pharmacological treatment for major depressive disorder, generalized anxiety disorder, and several other psychiatric conditions [
1]. Their clinical appeal stems from a more favorable safety profile, particularly regarding cardiovascular and anticholinergic side effects, compared to older antidepressants such as tricyclics or MAO inhibitors [
2]. As a result, their use has expanded steadily over the past two decades, particularly among elderly populations and individuals with multimorbidity [
3].
However, emerging evidence suggests that SSRIs may increase the risk of bleeding, particularly in the gastrointestinal tract [
4]. This association is thought to be mediated by the inhibition of serotonin uptake into platelets, thereby impairing platelet aggregation and extending bleeding time [
5]. Platelets rely exclusively on serotonin uptake via the serotonin transporter (SERT), as they cannot synthesize it de novo. SSRIs inhibit this uptake, leading to the depletion of intraplatelet serotonin, reduced aggregation, and prolonged bleeding time [
5]. While gastrointestinal bleeding associated with SSRIs is well established, data regarding the potential link between SSRIs and intracranial hemorrhage (ICH) remain inconsistent and often rely on limited sample sizes or exploratory signal detection studies, such as the one by Renoux et al. [
6].
Several observational studies and meta-analyses have reported a modest but statistically significant association between SSRI use and ICH, particularly in patients concurrently taking antithrombotic agents, as demonstrated in a population-based cohort study by Renoux et al. [
6,
7] and supported by additional data from Parkin et al. [
8]. The combination of SSRIs with anticoagulants or antiplatelet therapy may act synergistically to impair hemostasis and increase the risk of serious bleeding events [
9]. Nevertheless, due to the rarity of ICH, absolute risk estimates remain low, and findings across studies have been inconsistent [
10].
Another key question pertains to whether all SSRIs carry the same risk or whether differences exist between individual agents. Marked pharmacokinetic and pharmacodynamic differences among SSRIs may lead to divergent safety profiles [
11]. These include differences in half-life (e.g., fluoxetine > 72 h vs. paroxetine ~21 h), CYP450 metabolism (e.g., CYP2D6 involvement), and blood–brain barrier penetration, which may impact both efficacy and safety [
11]. Furthermore, demographic factors such as patient age and gender, along with clinical parameters such as comorbidity burden and co-medication use, may further modulate bleeding risk. It has been hypothesized that pharmacogenomics and biological sex differences may further modulate these risks, although this was not directly tested in our study.
In this context, pharmacovigilance data from large spontaneous reporting systems, such as the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS), offer a valuable source for detecting rare but clinically significant adverse drug reactions, including ICH [
12]. Although such systems have inherent limitations, including a lack of exposure denominator data, underreporting, and inconsistent report quality, they allow for the early identification of safety signals that may not be captured in clinical trials or traditional epidemiologic studies [
13]. In addition, indication bias may influence the results, particularly in elderly patients who are more likely to receive both SSRIs and antithrombotic agents due to multimorbidity. Disproportionality signals may be affected by factors unrelated to pharmacological risk, such as differences in prescription volumes, stimulated reporting due to litigation or media attention, and regional reporting practices.
The objective of this study was to analyze all reports of ICH associated with SSRI use in the FAERS database, with particular focus on differences among individual SSRIs, patient subgroups (by age and gender), and potential interactions with anticoagulant or antiplatelet therapy. It is important to emphasize that disproportionality analyses based on spontaneous reporting systems like FAERS do not establish causality. These analyses are hypothesis-generating and do not confirm causality. By conducting disproportionality analyses, we aimed to generate real-world evidence that could inform clinical decision-making and guide future research.
4. Discussion
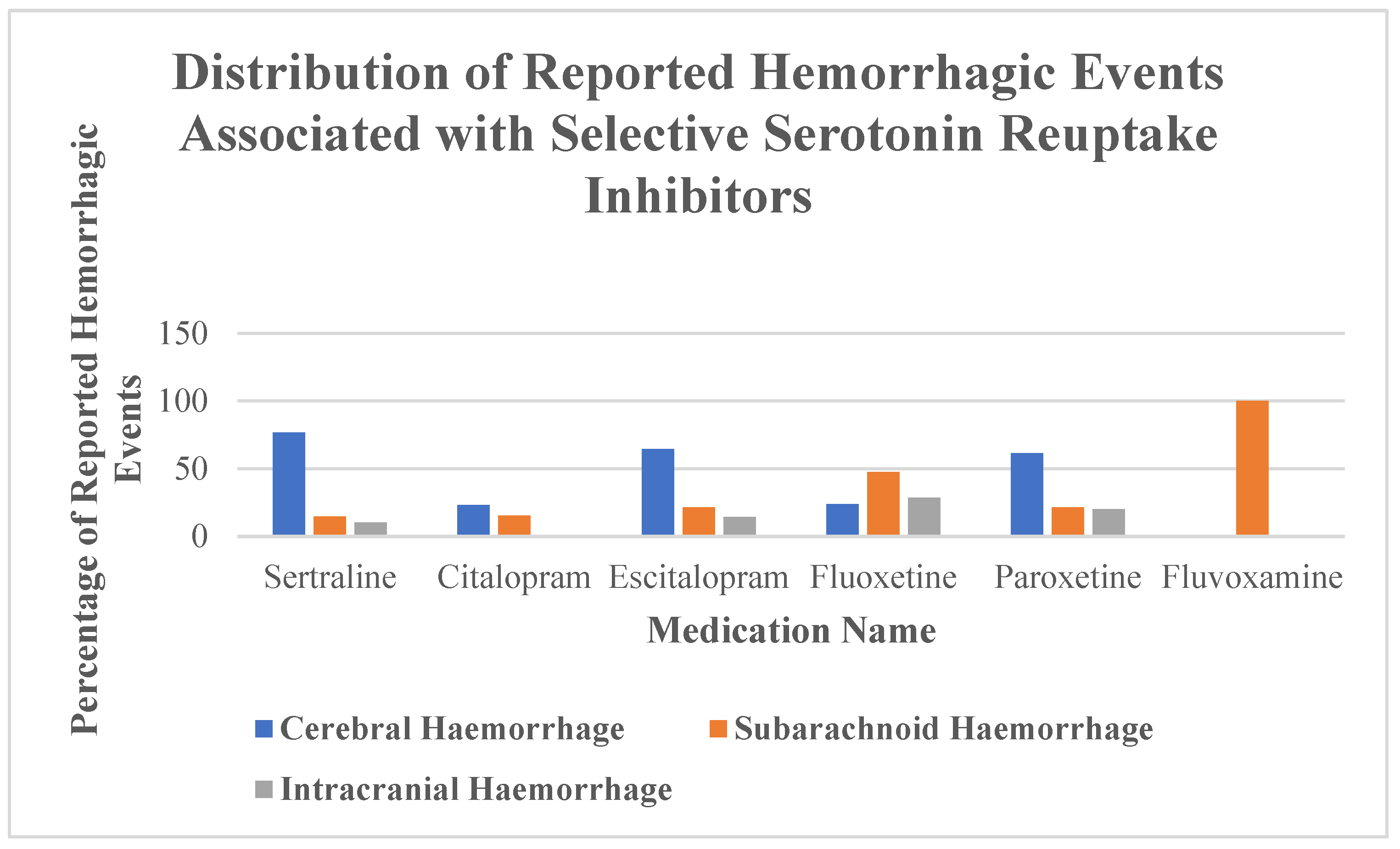
In this pharmacovigilance study using data from the FDA Adverse Event Reporting System (FAERS) database, we identified a total of 226 reports of intracranial hemorrhage (ICH) associated with the use of selective serotonin reuptake inhibitors (SSRIs). The results reveal considerable differences between individual SSRIs in terms of reported frequency, patient demographics, hemorrhage subtypes, and clinical outcomes.
Sertraline, paroxetine, and fluoxetine were the most frequently implicated agents.
Table 1 shows that ICH cases associated with sertraline were predominantly reported in older individuals and female patients, a distribution that may reflect prescribing trends or differential vulnerability. While their dominance likely reflects broader prescription patterns [
12], our disproportionality analysis suggests that these agents may also differ in their intrinsic bleeding risk. However, these findings may in part reflect prescription patterns. For instance, if sertraline is more commonly prescribed to elderly patients or those requiring anticoagulation, the observed disproportionality signals may be confounded by differential exposure rather than intrinsic pharmacological risk. Specifically, sertraline showed a strong signal for cerebral hemorrhage, whereas fluoxetine was disproportionately associated with subarachnoid and unspecified intracranial hemorrhage. In contrast, citalopram appeared to be inversely associated with cerebral hemorrhage, although the total number of cases was small. Due to the extremely limited number of reports involving fluvoxamine, no reliable inferences can be made about its association with ICH.
This is consistent with prior observational and pharmacovigilance studies reporting elevated ICH risk in elderly or anticoagulated SSRI users [
13,
14]. It has been hypothesized that the biological mechanism involves reduced serotonin uptake into platelets, leading to impaired aggregation and increased bleeding tendency, although this was not directly assessed in our study [
15]. Co-medication with anticoagulants or antiplatelet agents may amplify this risk [
16], as supported by our finding that sertraline combined with anticoagulants yielded a markedly elevated ROR of 9.56.
Older patients appear to be particularly susceptible to SSRI-associated ICH. Our age-stratified disproportionality analysis revealed a highly significant signal for sertraline in patients over 60 years of age (ROR = 7.92), consistent with prior evidence that age is an independent risk factor for spontaneous ICH [
17]. The lower representation of fluoxetine among elderly patients may reflect prescribing behavior or underlying pharmacokinetic considerations, such as its long half-life [
18]. In addition to its prolonged half-life, fluoxetine’s high permeability across the blood–brain barrier may increase its interaction with neurovascular structures, potentially contributing to the observed association with subarachnoid hemorrhage. Moreover, serotonin plays a role in modulating endothelial function and vascular tone. By interfering with serotonergic signaling in the cerebral vasculature, SSRIs may impair cerebrovascular autoregulation, further increasing susceptibility to hemorrhagic events, particularly in vulnerable populations [
18].
The gender-stratified analyses revealed that women were overrepresented in sertraline-related ICH reports (ROR = 2.34), whereas paroxetine was disproportionately reported in men (ROR = 1.81). These findings may relate to gender-specific prescription patterns, pharmacokinetics, and biological differences in platelet reactivity or vascular structure [
19,
20]. However, FAERS data do not allow for direct adjustment for exposure prevalence by sex, and these observations must be interpreted with caution.
Spontaneous reporting systems like FAERS have well-known limitations, including underreporting, differential reporting bias, and a lack of denominator data [
21,
22]. Nonetheless, such systems are useful for generating hypotheses and detecting rare but serious adverse events that may not be captured in randomized controlled trials. Notably, our study provides novel insights by systematically differentiating between types of ICH, exploring demographic modifiers, and highlighting potential drug–drug interactions.
Our results support prior studies demonstrating an increased bleeding risk when SSRIs are used in combination with antithrombotic agents [
23]. Given the widespread use of SSRIs in patients with cardiovascular or cerebrovascular conditions—many of whom are already on anticoagulation—these findings have clear clinical relevance. For example, patients with atrial fibrillation, mechanical heart valves, or prior thromboembolic events often require anticoagulation, and the addition of SSRIs may further increase bleeding risk [
24].
Although the absolute incidence of ICH in SSRI users remains low, the consequences of such events are often devastating. Careful risk–benefit assessment is warranted when prescribing SSRIs to high-risk individuals, especially elderly patients and those on oral anticoagulants or antiplatelet therapy. In such cases, alternative antidepressants with a more favorable bleeding profile should be considered, or at minimum, close monitoring should be implemented.
Further research is needed to confirm these associations and quantify absolute risk. Large-scale population-based studies using linked prescription, hospital, and mortality databases could help identify causality and clarify the temporal sequence between drug exposure and hemorrhage onset [
25]. Advanced data mining methods, including machine learning and Bayesian signal detection, may also enhance pharmacovigilance efforts [
26].
Despite its limitations, FAERS remains a valuable tool for early signal detection and pharmacovigilance surveillance. The heterogeneity in bleeding risk observed across individual SSRIs in this study challenges the notion that the SSRI class is pharmacologically homogeneous in terms of safety [
27]. Instead, our results support the emerging view that safety profiles must be assessed at the substance level rather than the drug class level [
28].
In conclusion, sertraline and fluoxetine were associated with elevated reporting signals for ICH in FAERS, particularly in elderly patients and those on anticoagulants. These findings underscore the importance of personalized prescribing, post-marketing surveillance, and further investigation into the comparative safety of antidepressants. As the use of SSRIs continues to rise globally, especially among high-risk populations, these safety concerns should not be overlooked [
29,
30].
Finally, our findings also highlight the need for regulatory bodies to consider drug-specific warnings rather than class-wide alerts when new safety data emerge. While SSRIs are widely considered a homogeneous pharmacological class, differences in molecular structure, metabolism, and off-target effects may result in divergent safety profiles. Post-marketing surveillance programs should therefore aim to stratify risk at the compound level. Furthermore, improved clinician education on pharmacovigilance principles could help bridge the gap between early signal detection and clinical practice. The integration of electronic health records with real-time pharmacovigilance alerts might enable more timely interventions and individualized patient monitoring, especially in high-risk groups. These findings raise important considerations for practical prescribing decisions in clinical care. Ongoing pharmacovigilance efforts and interprofessional collaboration remain vital in optimizing patient safety.
While our study focused exclusively on SSRIs, it would be valuable to compare the observed bleeding signals to those reported with other antidepressant classes, such as serotonin–norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs). Prior research has suggested that TCAs may carry a lower bleeding risk, whereas some SNRIs, such as venlafaxine, may also inhibit platelet function. Including these agents as comparators in future studies could provide a more comprehensive safety profile for clinicians.
4.1. Clinical Implications
The findings of this study have direct clinical relevance for physicians prescribing SSRIs, particularly in vulnerable populations. Given the observed association between sertraline and cerebral hemorrhage, and between fluoxetine and subarachnoid or unspecified intracranial bleeding, prescribers should consider individual bleeding risk when selecting an antidepressant. This is especially pertinent in elderly patients and those receiving concomitant anticoagulation or antiplatelet therapy, such as warfarin, direct oral anticoagulants (DOACs), aspirin, or clopidogrel.
For patients with multiple risk factors for bleeding—such as advanced age, hypertension, a history of stroke, or dual antithrombotic therapy—clinicians may consider the following:
Choosing SSRIs with lower bleeding signals (e.g., citalopram, based on this study);
Close monitoring during the initiation phase, especially within the first weeks when risk may be highest;
The regular review of anticoagulation indication and dosing;
Educating patients and caregivers for them to promptly recognize warning signs of neurological deterioration.
In psychiatric patients with cardiovascular comorbidity, multidisciplinary collaboration between psychiatrists, internists, and neurologists may help tailor safer and more effective treatment strategies. Pharmacogenetic testing and individualized risk modeling could also improve safety profiles in the future.
These implications highlight the importance of moving beyond a one-size-fits-all approach in antidepressant prescribing and emphasize the need for vigilance in pharmacotherapy of high-risk groups.
Beyond prescribing choices, these results emphasize the importance of shared decision-making. Physicians should engage patients in discussions about the potential bleeding risks of SSRIs, particularly when alternative treatment options exist. Patient-reported symptoms such as sudden headache, confusion, visual disturbance, or focal neurological signs should prompt immediate medical evaluation. Establishing clear follow-up plans, especially during the early treatment phase or during dose changes, can improve the early detection of adverse events. Furthermore, deprescribing strategies should be considered when the indication for SSRI use is no longer present or when bleeding risk outweighs potential benefit.
4.2. Limitations
This study has several important limitations inherent to the use of spontaneous reporting systems such as FAERS. First, underreporting is a well-documented phenomenon in pharmacovigilance databases, and the true number of intracranial hemorrhage (ICH) cases related to SSRI use may be substantially higher than captured. Conversely, reporting bias—driven by media attention, recent publications, or litigation—can lead to the overrepresentation of certain drugs or events, distorting relative signal strengths. Moreover, FAERS does not provide consistent information on the time interval between drug initiation and adverse events, limiting our ability to evaluate temporal associations. Furthermore, information on SSRI dosage and duration of treatment was not available. These factors are known to influence bleeding risk and may have contributed to the observed heterogeneity in adverse event reporting.
Second, the lack of denominator data (i.e., total number of patients exposed to each SSRI) precludes the calculation of incidence rates or absolute risk estimates. As a result, disproportionality analysis can only detect relative reporting signals, which are hypothesis-generating rather than confirmatory in nature.
Third, clinical details are frequently missing or incomplete in FAERS reports. Data on drug dosage, the duration of exposure, the temporal relationship between drug initiation and ICH onset, imaging confirmation, underlying comorbidities, and laboratory parameters are typically not available. This lack of granularity limits causal interpretation and makes it difficult to distinguish true adverse drug reactions from confounding by indication or comorbidity.
Fourth, co-medications may not be consistently or accurately reported, leading to the possible underestimation of interaction effects, particularly for anticoagulants, antiplatelet agents, or nonsteroidal anti-inflammatory drugs (NSAIDs), which are known to elevate bleeding risk.
Finally, spontaneous reporting systems are not designed for comparative effectiveness or safety analyses between drugs. Differences in reporting rates between individual SSRIs may reflect variations in market share, prescriber preference, patient populations, or regional reporting practices, rather than true pharmacological differences.
Despite these limitations, FAERS remains a valuable tool for detecting rare but serious adverse events and generating safety signals that can inform future research and regulatory decision-making.
5. Conclusions
This pharmacovigilance study identified notable safety signals for intracranial hemorrhage (ICH) associated with selective serotonin reuptake inhibitors (SSRIs), particularly sertraline and fluoxetine. Disproportionality analyses suggest that individual SSRIs may differ in their hemorrhagic risk profiles, with sertraline being significantly associated with cerebral hemorrhage and fluoxetine with subarachnoid and unspecified intracranial bleeding. These risks appear to be modified by patient age, sex, and the concomitant use of anticoagulants.
The markedly increased reporting odds for ICH in elderly patients and those co-treated with anticoagulants highlight clinically relevant safety concerns that warrant careful consideration during SSRI prescribing. Although these findings do not establish causality, they underscore the importance of personalized risk assessment, particularly in vulnerable populations.
Further studies using population-based data and controlled designs are needed to confirm these associations, quantify absolute risks, and guide clinical decision-making. Until then, clinicians should remain vigilant when prescribing SSRIs, especially in patients with elevated bleeding risk, and consider alternative treatment strategies when appropriate.
Until such data become available, the close monitoring of neurological warning signs, cautious risk stratification, and judicious prescribing are key. Clinicians must remain alert to signs of ICH, especially in patients presenting with atypical neurological symptoms during SSRI therapy. Enhanced pharmacovigilance remains essential to ensure the safe use of SSRIs globally. Early neuroimaging and interdisciplinary evaluation should be considered in high-risk patients reporting such symptoms, particularly during the initial phase of SSRI treatment or in combination with anticoagulants.