Cognitive Effects of Cannabis Use: A Comprehensive Review Across Domains
Abstract
1. Introduction
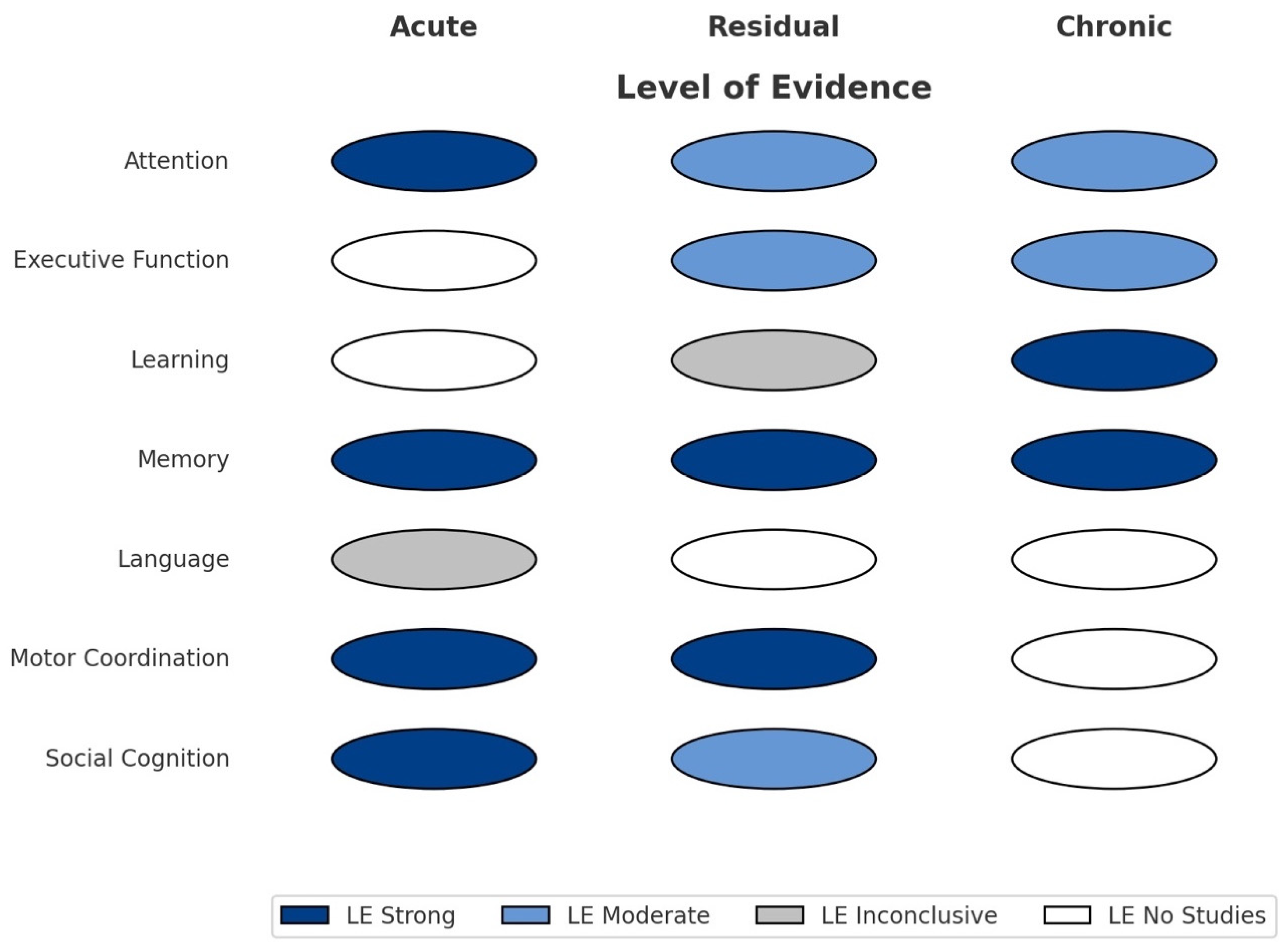
- Attention: The ability to selectively concentrate on relevant stimuli.
- Executive Function: Planning, inhibition, working memory, and cognitive flexibility.
- Learning: The capacity to acquire new information.
- Memory: The processes involved in the storage and retrieval of information.
- Language: Verbal comprehension and production.
- Motor Coordination: Fine and gross motor control.
- Social Cognition: Recognition and interpretation of others’ emotions, intentions, and social signals.
2. Attention
3. Executive Function
4. Learning
5. Memory
6. Language
7. Motor Coordination
8. Social Cognition
9. Discussion
10. Conclusions
Funding
Conflicts of Interest
References
- Ren, M.; Tang, Z.; Wu, X.; Spengler, R.; Jiang, H.; Yang, Y.; Boivin, N. The origins of cannabis smoking: Chemical residue evidence from the first millennium BCE in the Pamirs. Sci. Adv. 2019, 5, eaaw1391. [Google Scholar] [CrossRef]
- Cerdá, M.; Mauro, C.; Hamilton, A.; Levy, N.S.; Santaella-Tenorio, J.; Hasin, D.; Wall, M.M.; Keyes, K.M.; Martins, S.S. Association Between Recreational Marijuana Legalization in the United States and Changes in Marijuana Use and Cannabis Use Disorder From 2008 to 2016. JAMA Psychiatry 2020, 77, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mennis, J.; McKeon, T.P.; Stahler, G.J. Recreational cannabis legalization alters associations among cannabis use, perception of risk, and cannabis use disorder treatment for adolescents and young adults. Addict. Behav. 2023, 138, 107552. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.; Kuhns, L.; Cousijn, J. The short-term and long-term effects of cannabis on cognition: Recent advances in the field. Curr. Opin. Psychol. 2021, 38, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Englund, A.; Morrison, P.D.; Nottage, J.; Hague, D.; Kane, F.; Bonaccorso, S.; Stone, J.M.; Reichenberg, A.; Brenneisen, R.; Holt, D.; et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 2012, 27, 19–27. [Google Scholar] [CrossRef]
- Volkow, N.D.; Hampson, A.J.; Baler, R.D. Don’t worry, be happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 285–308. [Google Scholar] [CrossRef]
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, cannabinoids, and health. Dialogues Clin. Neurosci. 2017, 19, 309–316. [Google Scholar] [CrossRef]
- World Health Organization. The Health and Social Effects of Nonmedical Cannabis Use; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241510240 (accessed on 15 January 2025).
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. 1992. Available online: https://apps.who.int/iris/handle/10665/37958 (accessed on 13 January 2025).
- World Health Organization. Alcohol, Drugs and Addictive Behaviours Unit. World Health Organization. (n.d.) Available online: https://www.who.int/teams/mental-health-and-substance-use/alcohol-drugs-and-addictive-behaviours/drugs-psychoactive/cannabis (accessed on 2 April 2025).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Gerra, M.C.; Manfredini, M.; Cortese, E.; Antonioni, M.C.; Leonardi, C.; Magnelli, F.; Somaini, L.; Jayanthi, S.; Cadet, J.L.; Donnini, C. Genetic and Environmental Risk Factors for Cannabis Use: Preliminary Results for the Role of Parental Care Perception. Subst. Use Misuse 2019, 54, 670–680. [Google Scholar] [CrossRef]
- Badiani, A.; Boden, J.M.; De Pirro, S.; Fergusson, D.M.; Horwood, L.J.; Harold, G.T. Tobacco smoking and cannabis use in a longitudinal birth cohort: Evidence of reciprocal causal relationships. Drug Alcohol Depend. 2015, 150, 69–76. [Google Scholar] [CrossRef]
- Galimberti, M.; Levey, D.F.; Deak, J.D.; Zhou, H.; Stein, M.B.; Gelernter, J. Genetic influences and causal pathways shared between cannabis use disorder and other substance use traits. Mol. Psychiatry 2024, 29, 2905–2910. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.R.; Najman, J.M.; Bor, W.; O’CAllaghan, M.J.; Williams, G.M. Multiple risk factor model predicting cannabis use and use disorders: A longitudinal study. Am. J. Drug Alcohol Abus. 2009, 35, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Matheson, J.; Le Foll, B. Impacts of recreational cannabis legalization on use and harms: A narrative review of sex/gender differences. Front. Psychiatry 2023, 14, 1127660. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, J.G.; Kauert, G.; Theunissen, E.L.; Toennes, S.W.; Moeller, M.R. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology 2006, 34, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Crean, R.D.; Crane, N.A.B.; Mason, B.J. An evidence-based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yücel, M.; Solowij, N. Acute and chronic effects of cannabinoids on human cognition: A systematic review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef]
- Scott, J.C.; Slomiak, S.T.; Jones, J.D.; Rosen, A.F.G.; Moore, T.M.; Gur, R.C. Association of Cannabis with Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 585–595. [Google Scholar] [CrossRef]
- Figueiredo, P.R.; Tolomeo, S.; Steele, J.D.; Baldacchino, A. Neurocognitive consequences of chronic cannabis use: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 108, 358–369. [Google Scholar] [CrossRef]
- Pocuca, N.; Walter, T.J.; Minassian, A.; Young, J.W.; Geyer, M.A.; Perry, W. The Effects of Cannabis Use on Cognitive Function in Healthy Aging: A Systematic Scoping Review. Arch. Clin. Neuropsychol. 2021, 36, 673–685. [Google Scholar] [CrossRef]
- Adam, K.C.S.; Doss, M.K.; Pabon, E.; Vogel, E.K.; de Wit, H. Δ9-Tetrahydrocannabinol (THC) impairs visual working memory performance: A randomized crossover trial. Neuropsychopharmacology 2020, 45, 1807–1816. [Google Scholar] [CrossRef]
- Anderson, B.M.; Rizzo, M.; Block, R.I.; Pearlson, G.D.; O’Leary, D.S. Sex, drugs, and cognition: Effects of marijuana. J. Psychoact. Drugs 2010, 42, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.E.; Bedi, G.; de Wit, H. Effects of delta-9-tetrahydrocannabinol on evaluation of emotional images. J. Psychopharmacol. 2012, 26, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Böcker, K.B.E.; Gerritsen, J.; Hunault, C.C.; Kruidenier, M.; Mensinga, T.T.; Kenemans, J.L. Cannabis with high Δ9-THC contents affects perception and visual selective attention acutely: An event-related potential study. Pharmacol. Biochem. Behav. 2010, 96, 67–74. [Google Scholar] [CrossRef]
- Doss, M.K.; Weafer, J.; Gallo, D.A.; de Wit, H. Tetrahydrocannabinol at Retrieval Drives False Recollection of Neutral and Emotional Memories. Biol. Psychiatry. 2018, 84, 743–750. [Google Scholar] [CrossRef]
- Fried, P.; Watkinson, B.; James, D.; Gray, R. Current and former marijuana use: Preliminary findings of a longitudinal study of effects on IQ in young adults. Can. Med. Assoc. J. 2002, 166, 887–891. [Google Scholar]
- Gruber, S.A.; Sagar, K.A.; Dahlgren, M.K.; Racine, M.; Lukas, S.E. Age of onset of marijuana use and executive function. Psychol. Addict. Behav. 2012, 26, 496–506. [Google Scholar] [CrossRef]
- Hartley, S.; Simon, N.; Larabi, A.; Vaugier, I.; Barbot, F.; Quera-Salva, M.-A.; Alvarez, J.C. Effect of Smoked Cannabis on Vigilance and Accident Risk Using Simulated Driving in Occasional and Chronic Users and the Pharmacokinetic-Pharmacodynamic Relationship. Clin. Chem. 2019, 65, 684–693. [Google Scholar] [CrossRef]
- Kloft, L.; Otgaar, H.; Lynn, S.J.; Ramaekers, J.G.; Monds, L.A.; Merckelbach, H. Cannabis increases susceptibility to false memory. Proc. Natl. Acad. Sci. USA 2020, 117, 943–947. [Google Scholar] [CrossRef]
- Matheson, J.; Sproule, B.; Di Ciano, P.; Fares, A.; Le Foll, B. Acute and residual mood and cognitive performance of young adults following smoked cannabis. Pharmacol. Biochem. Behav. 2020, 194, 172937. [Google Scholar] [CrossRef]
- McClure, E.A.; Lydiard, J.B.; Goddard, S.D.; Gray, K.M. Objective and subjective memory ratings in cannabis-dependent adolescents. Am. J. Addict. 2015, 24, 47–52. [Google Scholar] [CrossRef]
- Messinis, L.; Kyprianidou, A.; Malefaki, S.; Papathanasopoulos, P. Neuropsychological deficits in long-term frequent cannabis users. Neurology 2006, 66, 737–739. [Google Scholar] [CrossRef]
- Ranganathan, M.; Radhakrishnan, R.; Addy, P.H.; Schnakenberg-Martin, A.M.; Williams, A.H.; Carbuto, M.; Elander, J.; Pittman, B.; Sewell, R.A.; Skosnik, P.D.; et al. Tetrahydrocannabinol (THC) impairs encoding but not retrieval of verbal information. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79 Pt B, 176–183. [Google Scholar] [CrossRef]
- Ross, J.M.; Ellingson, J.M.; Rhee, S.H.; Hewitt, J.K.; Corley, R.P.; Lessem, J.M.; Friedman, N.P. Investigating the causal effect of cannabis use on cognitive function with a quasi-experimental co-twin design. Drug Alcohol Depend. 2020, 206, 107712. [Google Scholar] [CrossRef]
- Schuster, R.M.; Gilman, J.; Schoenfeld, D.; Evenden, J.; Hareli, M.; Ulysse, C.; Nip, E.; Hanly, A.; Zhang, H.; Evins, A.E. One month of cannabis abstinence in adolescents and young adults is associated with improved memory. J. Clin. Psychiatry 2018, 79, 18m12138. [Google Scholar] [CrossRef]
- Valdiviezo, J.J.; Ramírez Flores, M.J.; Chainé, S.M. Cognición social y funcionamiento ejecutivo en hombres jóvenes con consumo de marihuana. Rev. Chil. De Neuropsicol. 2020, 15, 8–13. [Google Scholar] [CrossRef]
- Weckowicz, T.E.; Collier, G.; Spreng, L. Field dependence, cognitive functions, personality traits, and social values in heavy cannabis users and nonuser controls. Psychol. Rep. 1977, 41, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.; Brickner, O.; Lerman, H.; Greemland, M.; Bloch, M.; Lester, H.; Chisin, R.; Sarne, Y.; Mechoulam, R.; Bar-Hamburger, R.; et al. A study investigating the acute dose-response effects of 13 mg and 17 mg Delta 9- tetrahydrocannabinol on cognitive-motor skills, subjective and autonomic measures in regular users of marijuana. J. Psychopharmacol. 2008, 22, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, C.T.; Liguori, A.; Livengood, L.B.; Hart, S.L.; Mussat-Whitlow, B.J.; Lamborn, C.M.; Laurienti, P.J.; Porrino, L.J. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004, 76, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Solowij, N.; Pesa, N. Anormalidades cognitivas no uso da cannabis [Cognitive abnormalities and cannabis use]. Braz. J. Psychiatry 2010, 32 (Suppl. 1), S31–S40. [Google Scholar] [CrossRef]
- Gorey, C.; Kuhns, L.; Smaragdi, E.; Kroon, E.; Cousijn, J. Age-related differences in the impact of cannabis use on the brain and cognition: A systematic review. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, A.T.; Whitney, P.; Cuttler, C.; Spradlin, A.; Hinson, J.M.; McLaughlin, R.J. Altered attentional control strategies but spared executive functioning in chronic cannabis users. Drug Alcohol Depend. 2017, 181, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.E.; Bruno, R.; Johnston, J.; Matthews, A.; McGregor, I.; Allsop, D.J.; Lintzeris, N. Cognitive, physical, and mental health outcomes between long-term cannabis and tobacco users. Addict. Behav. 2018, 79, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Prashad, S.; Filbey, F.M. Cognitive motor deficits in cannabis users. Curr. Opin. Behav. Sci. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Ajmera, N.; Collins, P.F.; Weiss, H.; Luciana, M. Initiation of moderately frequent cannabis use in adolescence and young adulthood is associated with declines in verbal learning and memory: A longitudinal comparison of pre- versus post-initiation cognitive performance. J. Int. Neuropsychol. Soc. 2021, 27, 621–636. [Google Scholar] [CrossRef]
- Schreiner, A.M.; Dunn, M.E. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Exp. Clin. Psychopharmacol. 2012, 20, 420–429. [Google Scholar] [CrossRef]
- Riba, J.; Valle, M.; Sampedro, F.; Rodríguez-Pujadas, A.; Martínez-Horta, S.; Kulisevsky, J.; Rodríguez-Fornells, A. Telling true from false: Cannabis users show increased susceptibility to false memories. Mol. Psychiatry 2015, 20, 772–777. [Google Scholar] [CrossRef]
- Roten, A.; Baker, N.L.; Gray, K.M. Cognitive performance in a placebo-controlled pharmacotherapy trial for youth with marijuana dependence. Addict. Behav. 2015, 45, 119–123. [Google Scholar] [CrossRef]
- Becker, M.P.; Collins, P.F.; Luciana, M. Neurocognition in college-aged daily marijuana users. J. Clin. Exp. Neuropsychol. 2014, 36, 379–398. [Google Scholar] [CrossRef]
- Hunault, C.C.; Mensinga, T.T.; Böcker, K.B.E.; Schipper, C.M.A.; Kruidenier, M.; Leenders, M.E.C.; de Vries, I.; Meulenbelt, J. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology 2009, 204, 85–94. [Google Scholar] [CrossRef]
- Scott, J.C.; Wolf, D.H.; Calkins, M.E.; Bach, E.C.; Weidner, J.; Ruparel, K.; Moore, T.M.; Jones, J.D.; Jackson, C.T.; Gur, R.E.; et al. Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia Neurodevelopmental Cohort. Psychol. Addict. Behav. 2017, 31, 423–434. [Google Scholar] [CrossRef]
- Kroon, E.; Kuhns, L.; Hoch, E.; Cousijn, J. Heavy cannabis use, dependence and the brain: A clinical perspective. Addiction 2020, 115, 559–572. [Google Scholar] [CrossRef]
- Batalla, A.; Bhattacharyya, S.; Yücel, M.; Fusar-Poli, P.; Crippa, J.A.; Nogué, S.; Torrens, M.; Pujol, J.; Farré, M.; Martin-Santos, R.; et al. Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PLoS ONE 2013, 8, e55821. [Google Scholar] [CrossRef]
| First Author (Year) | Sample Characteristics | Study Design and Methods | Abstinence Period | Main Findings |
|---|---|---|---|---|
| Adam et al. (2020) [25] | N = 23 per experiment Age: Exp. 1 = 23 (SD = 3.6); Exp. 2 = 23.4 (SD = 4.3) Gender: M = F Country: USA | Design: Randomized Controlled Trial Conditions: THC vs. placebo (1-week interval) Administration: Inhaled THC Task: Visual working memory task (stimulus, delay, and recognition test) Measures: Task performance and metacognitive accuracy | ≥72 h, confirmed by urine, oral fluid, and breath testing Participant state during testing: Under the acute influence of 7.5 mg or 15 mg oral THC, randomized crossover | Memory—Participants performed significantly worse under THC (fewer correct items). Metacognitive accuracy was impaired (less awareness of own performance). |
| Anderson et al. (2010) [26] | N = 70 Age: Mean = 20.9 years (SD = 2.9) Gender: M = 35 and F = 35 Country: USA | Design: Randomized Controlled Trial Tasks: Stroop Test, Digit Span, Reaction Time, and UFOV Measures: Selective and divided attention, working memory, and cognitive flexibility | Participants were sober prior to testing; duration not clearly stated Participant state during testing: Under acute cannabis intoxication (2.9% THC) or placebo | Attention—Cannabis significantly increased the time needed to detect stimuli in both selective and divided attention tasks (UFOV). Users had difficulty identifying relevant information in complex visual environments. |
| Ballard et al. (2012) [27] | N = 25 Age: 18–35 years Gender: Not reported Country: USA | Design: Randomized Controlled Trial Conditions: 7.5 mg oral THC vs. placebo (cross-over) Tasks: Emotional image viewing (positive, neutral, and negative) Measures: Physiological response and subjective ratings | ≥12 h, verified by oral drug and breath alcohol testing Participant state during testing: Under acute oral THC (7.5 or 15 mg) or placebo | Social Cognition—THC reduced emotional reactivity to negative images, with participants reporting lower aversion and emotional intensity. No significant differences were observed for positive or neutral images. Participants felt less anxious and stressed under THC. |
| Böcker et al. (2010) [28] | N = 24 Age: Mean = 22.8 years Gender: M = 12 and F = 12 Country: The Netherlands | Design: Randomized Controlled Trial Conditions: High-THC cannabis (300 µg/kg) vs. Placebo Tasks: Visual selective attention (color and shape discrimination) Measures: Task performance and ERP (P1 and N2 components) | Required before each session (duration not specified), verified by urine drug screen Participant state during testing: Under the acute influence of high-THC cannabis (up to 69.4 mg THC) or placebo | Attention—THC impaired accuracy and reaction speed in visual attention tasks. Significant reductions in the P1 and N2 event-related potential (ERP) components were observed under THC. These early brain responses are associated with attentional resource allocation (P1) and conflict detection or cognitive control (N2), indicating impaired early-stage attentional processing. |
| Doss et al. (2018) [29] | N = 23 Age: 18–29 yrs Gender: F:M (12:11) Country: USA | Design: Randomized Controlled Trial (double-blind and placebo-controlled) Conditions: THC (15 mg oral capsule) vs. placebo Procedure: Two sessions spaced 48 h apart Phase 1: Participants viewed emotional (positive, negative, or neutral) images and semantically related word lists Phase 2: Memory retrieval tested for emotional memory and false recall Measures: Memory performance (hits and false alarms), emotional valence modulation, and subjective effect ratings | Participants were sober before THC administration, but no exact duration was specified Participant state during testing: Under the acute effect of 15 mg oral THC at the retrieval stage | Memory Cue Recall: THC did not impact correct or high-confidence hits but significantly increased false alarms and high-confidence false alarms. Emotional valence had no effect. DRM Recognition: THC increased false alarms for both critical lures and unrelated items, reducing adjusted hit rates. Correlation: False alarms positively correlated with subjective THC effect ratings, indicating that stronger perceived drug effects were linked to increased susceptibility to false memories. |
| Fried et al. (2002) [30] | N = 70 Age: 17–20 yrs Gender: Not available Country: USA | Design: Comparative Study Procedure: Longitudinal assessment of IQ and cognitive functions across multiple developmental stages Cannabis Use: Assessed via structured interviews and self-report questionnaires Cognitive Measures: IQ and domain-specific assessments with a focus on attention and working memory | Participants were categorized based on their current and past cannabis use, as verified by self-report and urinalysis: Current users were further divided into the following groups: - Heavy users: ≥5 joints/week - Light users: <5 joints/week Former users: Those who Those who had not used cannabis regularly for at least 3 months Non-users: Those who had never used more than once per week and no use in the past two weeks Importantly, although subjects reported no use on the day of testing, heavy current users were likely still under the residual influence of THC due to the drug’s long half-life | Attention and Memory: Chronic cannabis users showed a significant decline in cognitive performance over time, especially in attention and working memory domains. IQ Decline: Average IQ dropped by approximately 4 points from childhood to adulthood among chronic users, indicating a long-term cognitive impact associated with sustained cannabis use. |
| Gruber et al. (2012) [31] | N = 34 cannabis users + 28 non-users Age: Users = 22.8 yrs (SD = 6.57); Non-users = 24.3 yrs (SD = 6.64) Gender: Users = 5F:29M; Non-users = 9F:19M Country: USA | Design: Comparative Study Groups: Users divided into early-onset (<16 years) and late-onset (≥16 years) Assessments: Neuropsychological battery evaluating executive functions, including working memory, inhibitory control, cognitive flexibility, and processing speed | Users were required to abstain for at least 12 h before testing, verified by urine samples. | Working Memory: Early-onset users had significantly poorer performance in manipulating and storing information. Inhibitory Control: These users struggled more to suppress impulsive responses, reflecting reduced attentional regulation. Cognitive Flexibility: Lower capacity to adapt to changing rules or shift strategies, impairing decision-making and problem-solving. Processing Speed: Slower response times in early-onset users indicated diminished cognitive efficiency compared to late-onset users. |
| Hartley et al. (2019) [32] | N = 30 (15 chronic users [CC] and 15 occasional users [OC]) Age: 20–34 years Gender: Male Country: France | Design: Randomized Controlled Trial; Procedure: Double-blind; participants smoked cannabis in doses adjusted to their typical usage levels Tasks: High-precision simulated driving tasks assessing vigilance and accident risk. Measures: THC levels measured via blood and saliva samples, correlated with cognitive and motor performance | Chronic users were required to abstain for at least 12 h prior to the study. Occasional users were required to abstain for at least 48 h before the session. | Motor Coordination—Driving Vigilance: Cannabis significantly reduced vigilance in both groups. Chronic users exhibited a smaller decline, suggesting partial tolerance. Accident Risk: Both groups faced increased crash risk after cannabis use, with occasional users showing greater impairment. Pharmacodynamic Relationship: Higher THC concentrations were strongly associated with cognitive and motor impairments, though occasional users were more affected than chronic users at comparable THC levels. |
| Kloft et al. (2020) [33] | N = 64 Age: 22.7 yrs (SD = 2.6) Gender: F:M (32:32) Country: Australia and the Netherlands | Design: Randomized Controlled Trial (double-blind, placebo-controlled) Procedure: Participants (occasional cannabis users) attended two sessions, receiving THC in one and a placebo in the other. Memory was assessed immediately and after one week. Tasks: DRM Word Recognition: Test of false recognition of semantically related word lists. Virtual Reality (VR) Fight Scenario: Eyewitness memory after being exposed to misinformation. Virtual Reality Theft Scenario: Perpetrator’s memory distorted by misleading peer testimony | At least 7 days, confirmed by urine drug screening. Participant state during testing: Under the acute influence of THC, administered in a controlled laboratory setting. | Memory—DRM Task: THC increased false recognition of non-presented words, impairing participants’ ability to differentiate between seen and unseen information. VR Fight Scenario: The THC group was more likely to incorporate misleading information into their memory of the event, demonstrating increased susceptibility to misinformation-based false memories. VR Theft Scenario: Cannabis-intoxicated participants were more prone to accepting false testimony as part of their own recollection. Overall Implication: THC exposure significantly heightened the formation of false memories across all three paradigms. |
| Matheson et al. (2020) [34] | N = 91 Age: 19 to 25 yrs Gender: F = M Country: USA | Design: Randomized Controlled Trial Procedure: Participants were randomly assigned to receive either active cannabis (12.5% THC) or placebo. Assessments: Mood: Standardized mood scales were applied one hour after cannabis use. Cognitive Performance: Memory and other cognitive tests were administered at two timepoints: one hour and 48 h post-consumption | Yes, 12+ h, confirmed by oral and breath tests Participant state during testing: Under acute THC (12.5%) or in residual phase (24 h later) | Memory—Acute Effects: Participants in the THC group showed decreased word retention shortly after use. Residual Effects (48 h): No significant differences in cognitive performance between THC and placebo groups were observed after 48 h. Social Cognition/Mood: Mood: The THC group reported elevated excitement and positive mood shortly after consumption. Additional Effects: Increased sociability, euphoria, and confusion were reported more frequently in the THC group than in the placebo group. |
| McClure et al. (2015) [35] | N = 70 Age: 15 to 21 yrs Gender: Not available Country: USA | Design: Randomized Controlled Trial Procedure: Cannabis-dependent adolescents were compared to non-user controls. All participants completed both objective neuropsychological memory tests and subjective self-report memory questionnaires | 12 to 24 h, based on self-report. Those who appeared intoxicated at screening were excluded from testing. | Memory—Cannabis-using adolescents significantly underestimated their memory impairments when compared to objective test results. Subjective ratings of memory function did not accurately reflect the cognitive deficits observed in standardized assessments. |
| Messinis et al. (2006) [36] | N = 64 (20 long-term heavy users, 20 short-term heavy users, and 24 controls) Age: 32.7 yrs Gender: F:M = 15:25 Country: Greece | Design: Comparative Study Procedure: Participants were divided into three groups: long-term heavy cannabis users (≥10 years), short-term heavy users (5–9 years), and controls (used cannabis ≤20 times lifetime, none in the past 2 years). All abstained from cannabis for ≥24 h prior to testing. Cognitive domains were assessed using the following: Rey Auditory Verbal Learning Test (RAVLT); Trail Making Test—Part A (TMT-A); Trail Making Test—Part B (TMT-B); Boston Naming Test (BNT) Covariates controlled included age, IQ, education, and depressive symptoms | Participants were required to abstain from cannabis use for at least 24 h prior to testing. The actual abstinence range was from 36 to 240 h, as verified by urine samples collected before and during testing. | Attention/Psychomotor Speed—Both user groups showed slower TMT-A completion than controls. Long-term users were the slowest (p = 0.036), followed by short-term users (p < 0.001). Executive Function—Both groups had impaired TMT-B performance, indicating reduced mental flexibility. Long-term users were more impaired than controls (p = 0.011); short-term users also differed significantly (p < 0.001). Memory— Long-term users performed worse than controls on all RAVLT measures, including delayed recall (p < 0.001) and recognition (p = 0.015). Short-term users showed milder but significant memory deficits (p < 0.01). Language—Only long-term users showed reduced BNT performance (p = 0.008), suggesting lexical retrieval deficits not observed in the short-term group. |
| Ramaekers et al. (2006) [18] | N = 20 Age: 19 to 29 yrs Gender: F:M = 6:14 Country: Germany | Design: Randomized Controlled Trial Procedure: Participants were administered varying concentrations of THC, after which blood and saliva samples were collected to determine THC levels. | At least 72 h (3 days) verified through urine and oral fluid testing. | Motor Coordination and Composite Performance—Higher THC concentrations in serum and saliva were directly associated with greater impairment in cognitive and motor functions. Cognitive and motor deterioration increased with THC dose. Recovery varied based on individual THC clearance, with slower declines in THC linked to prolonged deficits. |
| Ranganathan et al. (2017) [37] | N = 79 (38 in Session 1; 57 in Session 2) Age: Not available Gender: Not available Country: USA | Design: Randomized Controlled Trial Procedure: Participants completed a series of verbal memory tasks under both THC and placebo conditions. Tasks were divided into the following: Encoding phase: Word lists were presented for memorization Retrieval phase: Participants recalled the memorized words after a delay Analysis: Performance was compared across conditions to assess THC effects on encoding vs. retrieval | Participants were sober during testing, but the duration is not specified, confirmed by urine testing Participant state during testing: Under the acute effects of oral THC (15 mg) in a double-blind, placebo-controlled, within-subject design | Memory-Encoding: THC significantly impaired the encoding of new verbal information. Participants remembered fewer words under THC than under the placebo. Retrieval: No significant differences were found in the recall of previously encoded material, indicating that THC primarily disrupts the learning process, not memory retrieval. Additional Findings: Statistical controls for THC metabolism and baseline cognitive function confirmed the robustness of the encoding-specific impairment. |
| Ross et al. (2021) [38] | N = 2410 twin pairs Age: Not available Gender: Male and female Country: USA | Design: Longitudinal Twin Study Procedure: Cognitive outcomes were compared within twin pairs discordant for cannabis use (one user and one non-user). This design allowed for control of genetic and shared environmental confounding Assessments: Standardized neuropsychological tests were used to evaluate attention, executive function, memory, and information processing speed | There is no experimental manipulation of cannabis use or controlled abstinence period | Attention—Cannabis users showed deficits in sustained and divided attention. They had increased difficulty maintaining focus during repetitive tasks and in switching attention between different stimuli. Executive Function—A slight but statistically significant impairment was observed in cannabis users. Performance was lower on tasks requiring planning, decision-making, and impulse control, indicating reduced capacity for managing complex behaviors. Memory—Cannabis users had significantly reduced short-term memory compared to their non-using co-twins. The deficit was especially evident in tasks that required holding information for short durations. |
| Schuster et al. (2018) [39] | N = 88 Age: 16 to 25 years Gender: Female–Male = 37:51 Country: USA | Design: Randomized Controlled Trial Groups: MJ-Abst (n = 62): Underwent four weeks of cannabis abstinence using contingency management (CM); MJ-Mon (n = 26): Received non-contingent monitoring with no abstinence requirement, matched for time and attention Assessments: Cognitive testing with a focus on learning and memory, administered across the intervention period | 30 days (MJ-Abst group), confirmed by urine toxicology MJ-Abst: abstinent; MJ-Mon: continued cannabis use | Learning—Abstaining from cannabis significantly enhanced the ability to learn new information. This finding supports the idea that cannabis may disrupt academic learning processes in youth. Memory—Participants in the abstinent group demonstrated marked improvements in learning and recall of new information. Memory performance improved most significantly during the first week of abstinence and continued to improve throughout the four-week period. |
| Valdiviezo et al. (2020) [40] | N = 36 (18 consumers vs. 18 non-consumers) Age: Consumers = 18.94 yrs (SD = 3.4); Non-consumers = 19.22 yrs (SD = 2.5) Gender: Male Country: Mexico | Design: Cross-sectional comparative observational study Assessments: Executive function tasks: card sorting, semantic classification, and metamemory; social cognition tasks: social judgment, absurdity detection, cause-effect reasoning Statistical analysis: Student’s t-test and Spearman correlation with cannabis use duration and dependence scores | 24 h before the assessment | Executive Function—Card Sorting: Lower accuracy (p = 0.01); more perseverative errors, indicating cognitive rigidity; Semantic Classification: Reduced abstract grouping performance (p = 0.03; p = 0.002 for abstract reasoning); Metamemory: More errors (p = 0.047); impaired self-monitoring and adaptive regulation of behavior. Social Cognition—Judgment: Lower performance (p = 0.007); Absurdities: Poorer identification of incongruities (p = 0.001); Causes: Weaker cause identification (p = 0.034); Consequences: Impaired consequence reasoning (p = 0.01). Interpretation: Cannabis users demonstrated significant deficits in executive functioning and social cognition. Difficulties were particularly evident in tasks requiring flexible thinking, behavioral adaptation, and understanding social cues and cause–effect relationships. |
| Weckowicz et al. (1977) [41] | N = 48 (24 cannabis users and 24 non-users) Age: 22.5 yrs Gender: Male only Country: Canada | Design: Comparative study Assessments: Field Dependence: Embedded Figures Test and Rod-and-Frame Test Selective Attention: Stroop Test and Selective Listening Task Convergent/Divergent Thinking: Wechsler Memory Scale, Guilford Number Facility Test, Letter Finding Test, and Word Association Test Personality and Values: Personality inventories, social values, and aesthetic judgment questionnaires Tests conducted in the laboratory and at home; total testing time ≈ 3 h | Not specified. Participants in the cannabis user group were active heavy users, with a history of daily cannabis use for no less than three years and an average of approximately 1200 uses. Eight participants smoked several times per day. No abstinence period was imposed prior to testing. | Attention—Cannabis users outperformed non-users in both selective attention tasks (Stroop and Selective Listening), indicating greater ability to filter relevant from irrelevant stimuli. The findings suggest enhanced selective attention among regular cannabis users, potentially due to long-term neuroadaptation or cognitive compensation. |
| Weinstein et al. (2008) [42] | N = 14 regular cannabis users Age: Mean = 27 yrs (SD = 7.45) Gender: 4 females and 10 males Country: Israel | Design: Comparative study (within-subject) Procedures: Two test sessions per participant; one day: smoked 17 mg THC cigarette and one placebo cigarette; other day: smoked 13 mg THC cigarette and one placebo cigarette Assessments: Cognitive/Motor Tests—Virtual maze (motor precision); Wisconsin Card Sorting Test (WCST) for mental flexibility; gambling task (risk-based decision-making); and time and distance estimation Physiological Measures: Heart rate and blood pressure before/after use. Subjective Measures: Questionnaire on intoxication and pleasure (“high”). | 24 h before each test session, verified by self-report, and urine drug screens were conducted to confirm recent use history | Motor and Cognition—Motor Skills: 17 mg THC increased collisions in the virtual maze more than 13 mg. Executive Function: Both doses impaired performance on WCST, with more errors under 17 mg, suggesting reduced cognitive flexibility. Decision-Making: 17 mg led to more high-risk choices in the gambling task, indicating riskier decision-making under higher THC. Physiological Effects: Elevated blood pressure and heart rate post-consumption. Subjective Effects: Increased intoxication and pleasure reported with both doses, more pronounced at 17 mg. |
| Whitlow et al. (2004) [43] | N = 20 (10 chronic cannabis users and 10 non-users) Age: Mean = 28 yrs (users) and 25 yrs (non-users) Gender: 2 females and 8 males in each group Country: USA | Design: Comparative study Procedures: Cannabis users: daily use for at least 5 years Control group: no cannabis use Assessments: Decision-Making: Iowa Gambling Task (IGT); Cognitive Battery: CANTAB tests for recognition memory and cognitive flexibility (set-shifting); and Mental Health: Anxiety and depression questionnaires | Participants in the cannabis user group were required to abstain from cannabis use for at least 12 h before testing. On average, they had abstained for 14.6 ± 3.1 h, with a range from 10 to 18 h. Urine cannabinoid levels were used to verify a decrease in concentration between day 1 and day 2 as confirmation of compliance | Executive Function and Memory Recognition Memory: No significant differences between groups Cognitive Flexibility (Set-Shifting): Cannabis users made more errors on set-shifting, though not statistically significant. This trend suggests potential subtle difficulties in adapting to changing rules or strategies. Limitations: The small sample size may have limited statistical power to detect significant differences. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, A.P.d.S.; Pozzolo Pedro, M.O.; Waisman Campos, M.; Torales, J.; Ventriglio, A.; Castaldelli-Maia, J.M. Cognitive Effects of Cannabis Use: A Comprehensive Review Across Domains. Neurol. Int. 2025, 17, 107. https://doi.org/10.3390/neurolint17070107
Queiroz APdS, Pozzolo Pedro MO, Waisman Campos M, Torales J, Ventriglio A, Castaldelli-Maia JM. Cognitive Effects of Cannabis Use: A Comprehensive Review Across Domains. Neurology International. 2025; 17(7):107. https://doi.org/10.3390/neurolint17070107
Chicago/Turabian StyleQueiroz, Andréia Pucinelli de Souza, Maria Olivia Pozzolo Pedro, Marcela Waisman Campos, Julio Torales, Antonio Ventriglio, and João Mauricio Castaldelli-Maia. 2025. "Cognitive Effects of Cannabis Use: A Comprehensive Review Across Domains" Neurology International 17, no. 7: 107. https://doi.org/10.3390/neurolint17070107
APA StyleQueiroz, A. P. d. S., Pozzolo Pedro, M. O., Waisman Campos, M., Torales, J., Ventriglio, A., & Castaldelli-Maia, J. M. (2025). Cognitive Effects of Cannabis Use: A Comprehensive Review Across Domains. Neurology International, 17(7), 107. https://doi.org/10.3390/neurolint17070107








