Exploratory Evaluation for Functional Changes of Six-Month Systematic Non-Invasive Electrical Stimulation in a Whole-Body Suit on Children with Cerebral Palsy GMFCS III–V
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Rationale for the Study
2.2. Population
2.3. The Whole-Body Suit with TENS
2.4. Assessments
2.4.1. Spasticity and Passive Range of Motion
2.4.2. Goal Attainment Scale Using SMART Principles
2.4.3. Gross Motor Functions Measure-66 (GMFM-66) and Posture and Postural Ability Scale (PPAS)
2.5. Statistical Analysis
2.6. Declarations
3. Results
3.1. Subjects
3.1.1. Spasticity and Passive Range of Motion
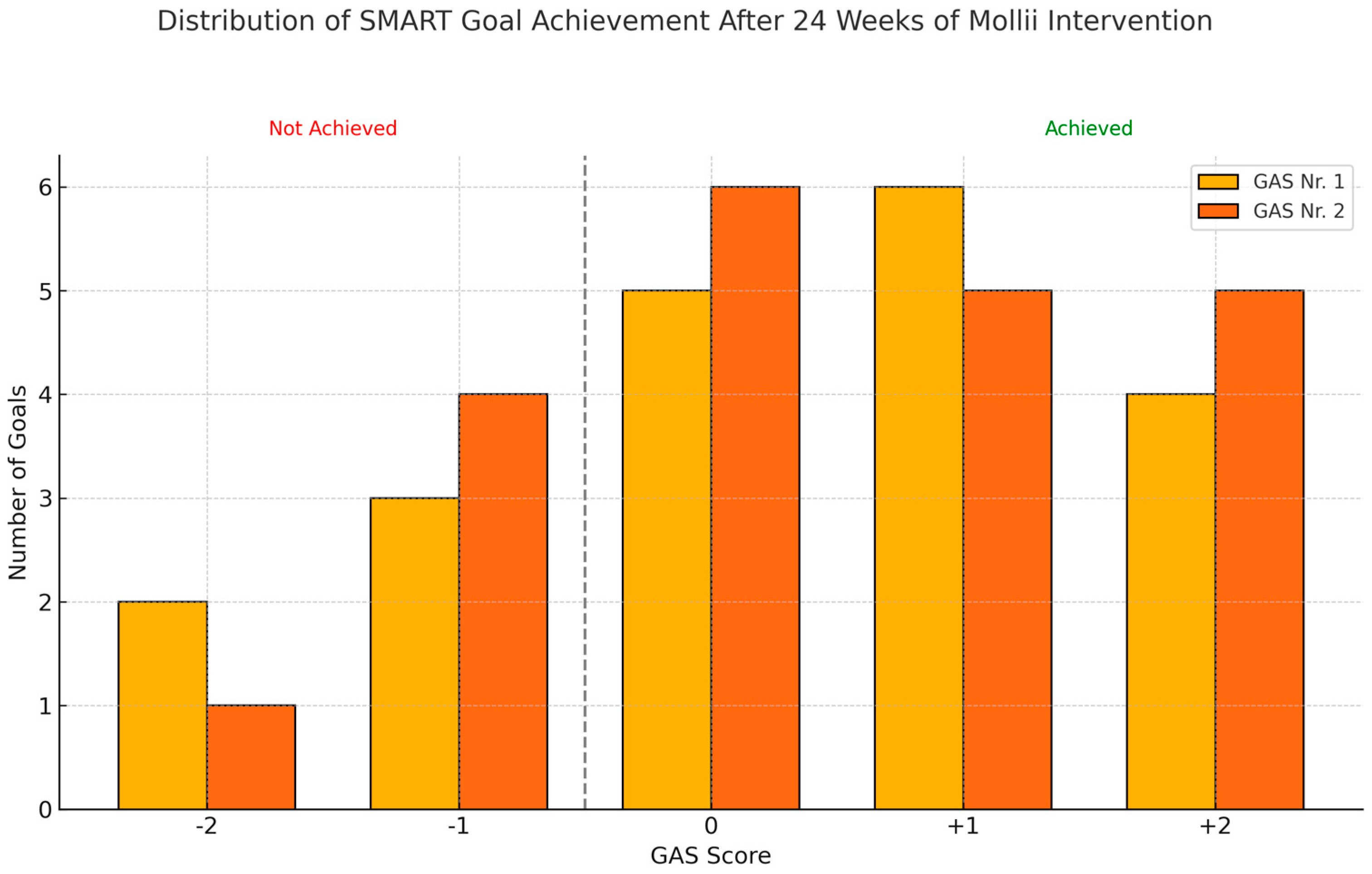
3.1.2. Goal Attainment Scale Using SMART Principles
3.1.3. Gross Motor Functions Measure-66 (GMFM-66) and Posture and Postural Ability Scale (PPAS)
4. Discussion
5. Clinical Implications/Limitations/Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TENS | Transcutaneous electrical nerve stimulation |
| Whole-body suit | Full-body garment suit with incorporated surface electrodes |
| CP | Cerebral palsy |
| GMFCS III–V | Gross Motor Function Classification System III–V |
| SD | Standard deviation |
| V1 | Angle of the catch of the very slow movement of the Modified Tardieu Scale |
| V3 | Angle of the catch of the quick stretch of the Modified Tardieu Scale |
| pROM | Passive Range of Motion |
| MAS | Modified Ashworth Scale |
| MTS | Modified Tardieu Scale |
| LE | Lower extremity |
| UE | Upper extremity |
| GAS | Goal Attainment Scale |
| SMART | Specific, Measurable, Achievable, Relevant, and Timed (SMART) principles |
| GMFM-66 | Gross Motor Functions Measure-66 |
| PPAS | Posture and Postural Ability Scale |
References
- Eunson, P. Aetiology and epidemiology of cerebral palsy. Paediatr. Child. Health 2016, 26, 367–372. Available online: https://www.physio-pedia.com/images/4/47/Aetiology_and_Epidemiology_of_Cerebral_Palsy.pdf (accessed on 28 April 2025). [CrossRef]
- Michael-Asalu, A.; Taylor, G.; Campbell, H.; Lelea, L.L.; Kirby, R.S. Cerebral Palsy: Diagnosis, Epidemiology, Genetics, and Clinical Update. Adv. Pediatr. 2019, 66, 189–208. [Google Scholar] [CrossRef]
- Colver, A.; Fairhurst, C.; Pharoah, P.O. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef]
- Graham, K.H.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar] [CrossRef]
- Multani, I.; Manji, J.; Hastings-Ison, T.; Khot, A.; Graham, K. Botulinum Toxin in the Management of Children with Cerebral Palsy. Pediatr. Drugs 2019, 21, 261–281. [Google Scholar] [CrossRef]
- Howard, J.J.; Graham, K.; Shortland, A.P. Understanding skeletal muscle in cerebral palsy: A path to personalized medicine? Dev. Med. Child. Neurol. 2021, 64, 289–295. [Google Scholar] [CrossRef]
- Howard, J.J.; Herzog, W. Skeletal Muscle in Cerebral Palsy: From Belly to Myofibril. Front. Neurol. 2021, 12, 620852. [Google Scholar] [CrossRef]
- Katusic, A.; Alimovic, S. The relationship between spasticity and gross motor capability in nonambulatory children with spastic cerebral palsy. Int. J. Rehabil. Res. 2013, 36, 205–210. [Google Scholar] [CrossRef]
- Mills, P.B.; Dossa, F. Transcutaneous electrical nerve stimulation for management of limb spasticity: A systematic review. Am. J. Phys. Med. Rehabil. 2016, 95, 309–318. [Google Scholar] [CrossRef]
- Kerr, C.; McDowell, B.; McDonough, S. Electrical stimulation in cerebral palsy: A review of effects on strength and motor function. Dev. Med. Child. Neurol. 2004, 46, 205–213. [Google Scholar] [CrossRef]
- Pennati, G.V. Theoretical Framework for Clinical Applications of Mollii—An Introductory Review; Molliiaustralia: Stockholm, Sweden, 2017; Available online: https://molliiaustralia.com.au/wp-content/uploads/2023/07/mollii-theoretical-framework.pdf (accessed on 28 April 2025).
- Schuhfried, O.; Crevenna, R.; Fialka-Moser, V.; Paternostro-Sluga, T. Non-invasive neuromuscular electrical stimulation in patients with central nervous system lesions: An educational review. J. Rehabil. Med. 2012, 44, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3375668/ (accessed on 28 April 2025).
- Doucet, B.M.; Griffin, L. High-versus low-frequency stimulation effects on fine motor control in chronic hemiplegia: A pilot study. Top Stroke Rehabil. 2013, 20, 299–307. [Google Scholar] [CrossRef]
- Fagerstedt, P.; Zelenin, P.V.; Deliagina, T.G.; Orlovsky, G.N.; Grillner, S. Crossed reciprocal inhibition evoked by electrical stimulation of the lamprey spinal cord. Exp. Brain Res. 2000, 134, 147–154. [Google Scholar] [CrossRef]
- Ertzgaard, P.; Alwin, J.; Sörbo, A.; Lindgren, M.; Sandsjö, L. Evaluation of a self-administered transcutaneous electrical stimulation concept for the treatment of spasticity: A randomized placebo-controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Schut, H.A.; Stam, H.J. Goals in rehabilitation teamwork. Disabil. Rehabil. 1994, 16, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Bovend’Eerdt, T.J.; Botell, R.E.; Wade, D.T. Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef]
- Zaza, C.; Stolee, P.; Prkachin, K. The application of goal attainment scaling in chronic pain settings. J. Pain. Symptom. Manag. 1999, 17, 55–64. [Google Scholar] [CrossRef]
- WHO Group. International Classification of Functioning, Disability and Health (ICF); WHO: Geneva, Switzerland, 2001; Available online: https://www.who.int/classifications/icf/en/ (accessed on 28 April 2025).
- Haugh, A.B.; Pandyan, A.D. A systematic review of the Tardieu Scale for the measurement of spasticity. Disabil. Rehabil. 2006, 28, 899–907. [Google Scholar] [CrossRef]
- Mutlu A, Livanelioglu A, Gunel MK Reliability of Ashworth and Modified Ashworth scales in children with spastic cerebral palsy. BMC Musculoskelet. Disord. 2008, 9, 44. [CrossRef]
- CPOP Group. Recommendations and Standardization for Leg and Foot Examination of the Danish Cerebral Palsy Observation Register; CPOP: Copenhagen, Denmark, 2024; Available online: https://cpop.dk/wp-content/uploads/Fysioterapeut-manual_revideret-09.01.24.pdf (accessed on 28 April 2025).
- CPOP Group. Recommendations and Standardization for Arm and Hand Examination of the Danish Cerebral Palsy Observation Register; CPOP: Copenhagen, Denmark, 2024; Available online: https://cpop.dk/wp-content/uploads/Ergoterapeutisk-manual_opdateret-09-01-2024-1.pdf (accessed on 28 April 2025).
- Norkin, C.C.; White, D.J.; Norkin, W. Measurement of Joint Motion: A Guide to Goniometry, 5th ed.; F.A. Davis Company: Philadelphia, PA, USA, 2016; ISBN 13: 978-0-8036-4566-0. Available online: https://www.fadavis.com/product/physical-therapy-measurement-joint-motion-goniometry-norkin-white-5 (accessed on 28 April 2025).
- Canchild Group. GMFM 66; Canchild Group: Hamilton, ON, Canada, 2021; Available online: https://canchild.ca/en/resources/44-gross-motor-function-measure-gmfm (accessed on 28 April 2025).
- CPOP Group. Recommendations and Standardization for PPAS of the Danish Cerebral Palsy Observation Register; CPOP: Copenhagen, Denmark, 2018; Available online: https://cpop.dk/wp-content/uploads/Dansk-Manual-PPAS_f%C3%A6rdig.pdf (accessed on 28 April 2025).
- Chen, C.L.; Chen, C.Y.; Chen, H.C.; Wu, C.Y.; Lin, K.C.; Hsieh, Y.W.; Shen, I.H. Responsiveness and minimal clinically important difference of Modified Ashworth Scale in patients with stroke. Eur. J. Phys. Rehabil. Med. 2019, 55, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Bakaniene, I.; Urbonaviciene, G.; Janaviciute, K.; Prasauskiene, A. Effects of the Inerventions method on gross motor function in children with spastic cerebral palsy. Neurol. Neurochir. Pol. 2018, 52, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Cusick, A.; McIntyre, S.; Novak, I.; Lannin, N.; Lowe, K. A comparison of goal attainment scaling and the Canadian Occupational Performance Measure for paediatric rehabilitation research. Pediatr. Rehabil. 2006, 9, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Steenbeek, D.; Ketelaar, M.; Galama, K.; Gorter, J.W. Goal attainment scaling in paediatric rehabilitation: A critical review of the literature. Dev. Med. Child. Neurol. 2007, 49, 550–556. [Google Scholar] [CrossRef]
- Palisano, R.J. Validity of goal attainment scaling in infants with motor delays. Phys. Ther. 1993, 73, 651–658. [Google Scholar] [CrossRef]
- Westerlund, M.; Sjöberg, E.; Sandell, J.; Sandström, C.; Lauritsen, H.K.; Lundqvist, F. The Inerventions Method—Follow up and long term use of a new possible therapy for patients with spasticity. Ortop. Magasin. 2014, 3, 45–46. Available online: https://molliiaustralia.com.au/wp-content/uploads/2023/07/inerventions-method.pdf (accessed on 28 April 2025).
- Gäverth, J.; Sandgren, M.; Lindberg, P.G.; Forssberg, H.; Eliasson, A.C. Test-retest and inter-rater reliability of a method to measure wrist and finger spasticity. Rehabil. Med. 2013, 45, 630–636. [Google Scholar] [CrossRef]

| Component | ICF Code | Description | Frequency |
|---|---|---|---|
| Body Functions | b455 | Exercise tolerance function | 2 |
| b735 | Muscle tone function | 3 | |
| b770 | Gait pattern function | 1 | |
| Activities | d410 | Changing basic body position | 4 |
| d4104 | Maintain body control | 2 | |
| d415 | Weight-bearing | 3 | |
| d440 | Fine hand use | 1 | |
| d445 | Hand and arm use | 6 | |
| d450 | Walking | 4 | |
| d455 | Moving around | 2 | |
| d465 | Moving around using equipment | 2 | |
| Body Structures | s510 | Improving drooling | 1 |
| Patient | Age | Sex | Wgt. | Hgt. | GFMCS | Classification |
|---|---|---|---|---|---|---|
| 1 | 16 | M | 50 | 165 | III | spastic tetraplegic |
| 2 | 14 | M | 30 | * | V | mixed ataxic/spastic tetraplegic |
| 3 | 17 | M | 50 | 168 | V | mixed dystonic/spastic tetraplegic |
| 4 | 16 | F | 47 | 158 | III | spastic tetraplegic |
| 5 | 9 | M | 25 | 129 | V | metachromatic leukodystrophy ** |
| 6 | 7 | F | 18.5 | 113 | V | spastic tetraplegic |
| 7 | 11 | M | 23.5 | 128 | V | spastic tetraplegic |
| 8 | 10 | F | 30 | 133 | V | spastic tetraplegic |
| 9 | 8 | M | 19.5 | 126 | V | spastic tetraplegic |
| 10 | 16 | F | 38 | 150 | V | mixed dystonic/spastic tetraplegic |
| 11 | 12 | F | 36.5 | 142 | III | mixed dystonic/spastic tetraplegic |
| 12 | 17 | M | 48.5 | 171 | V | spastic tetraplegic |
| 13 | 10 | F | 20 | 118 | V | mixed dystonic/spastic tetraplegic |
| 14 | 9 | M | 24 | 112 | III | spastic hemiplegic |
| 15 | 17 | M | 31 | 129 | V | mixed dystonic/spastic tetraplegic |
| 16 | 9 | M | 19 | 118 | IV | spastic tetraplegic |
| 17 | 10 | F | 24 | 110 | V | spastic tetraplegic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torabi, T.P.; Mortensen, K.; Michelsen, J.S.; Wong, C. Exploratory Evaluation for Functional Changes of Six-Month Systematic Non-Invasive Electrical Stimulation in a Whole-Body Suit on Children with Cerebral Palsy GMFCS III–V. Neurol. Int. 2025, 17, 102. https://doi.org/10.3390/neurolint17070102
Torabi TP, Mortensen K, Michelsen JS, Wong C. Exploratory Evaluation for Functional Changes of Six-Month Systematic Non-Invasive Electrical Stimulation in a Whole-Body Suit on Children with Cerebral Palsy GMFCS III–V. Neurology International. 2025; 17(7):102. https://doi.org/10.3390/neurolint17070102
Chicago/Turabian StyleTorabi, Tina P., Kristian Mortensen, Josephine S. Michelsen, and Christian Wong. 2025. "Exploratory Evaluation for Functional Changes of Six-Month Systematic Non-Invasive Electrical Stimulation in a Whole-Body Suit on Children with Cerebral Palsy GMFCS III–V" Neurology International 17, no. 7: 102. https://doi.org/10.3390/neurolint17070102
APA StyleTorabi, T. P., Mortensen, K., Michelsen, J. S., & Wong, C. (2025). Exploratory Evaluation for Functional Changes of Six-Month Systematic Non-Invasive Electrical Stimulation in a Whole-Body Suit on Children with Cerebral Palsy GMFCS III–V. Neurology International, 17(7), 102. https://doi.org/10.3390/neurolint17070102






