The Impact of Concurrent Chronic Heart Failure and Chronic Kidney Dysfunction on Post-Stroke Rehabilitation Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
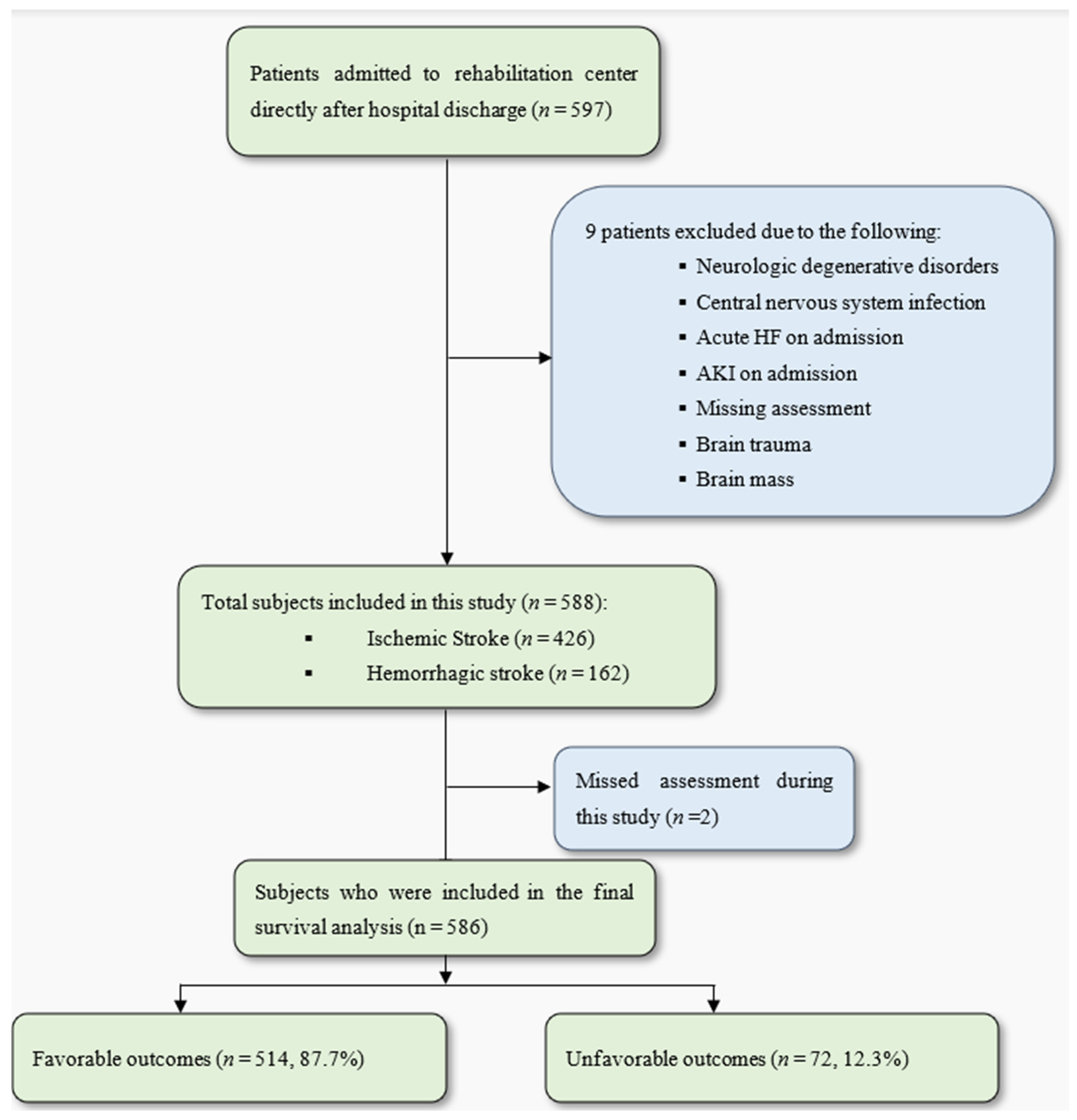
2.2. Patient Population
- Age < 18 years old.
- Brain mass.
- Cerebral injury due to trauma.
- Central nervous system infection.
- Neurological degenerative disorders.
- Acute kidney injury (AKI) on admission (an increase in serum Cr by ≥ 0.3 mg/dL within 48 h; or an increase in serum Cr to ≥1.5 times baseline within the prior 7 days; or urine volume ≤ 0.5 mL/kg/h for 6 h) [16].
- Acute decompensated heart failure (HF) on admission (new or worsening signs and symptoms of HF).
2.3. Study Endpoint
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DALYs | disability-adjusted life years lost |
| CHF | chronic heart failure |
| CKD | chronic renal dysfunction |
| AKI | acute kidney injury |
| ERBI | Early Rehabilitation Barthel Index |
| ECG | electrocardiogram |
| MDRD | Modification of Diet in Renal Disease |
| HR | hazard ratio |
| AF | atrial fibrillation |
References
- The GBD 2016 Lifetime Risk of Stroke Collaborators. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef]
- Tsagalis, G.; Bakirtzi, N.; Spengos, K.; Vemmou, A.; Manios, E.; Xinos, K.; Vemmos, K. Long-term prognosis of combined chronic heart failure and chronic renal dysfunction after acute stroke. Eur. J. Heart Fail. 2010, 12, 849–854. [Google Scholar] [CrossRef]
- Tsagalis, G.; Akrivos, T.; Alevizaki, M.; Manios, E.; Stamatellopoulos, K.; Laggouranis, A.; Vemmos, K.N. Renal dysfunction in acute stroke: An independent predictor of long-term all combined vascular events and overall mortality. Nephrol. Dial. Transplant. 2009, 24, 194–200. [Google Scholar] [CrossRef]
- Yahalom, G.; Schwartz, R.; Schwammenthal, Y.; Merzeliak, O.; Toashi, M.; Orion, D.; Sela, B.A.; Tanne, D. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke 2009, 40, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Böhm, M.; Boriani, G.; Christersson, C.; Coats, A.J.; Haeusler, K.G.; Jones, I.D.; Lip, G.Y.; Metra, M.; Ntaios, G. Interaction of heart failure and stroke: A clinical consensus statement of the ESC Council on Stroke, the Heart Failure Association (HFA) and the ESC Working Group on Thrombosis. Eur. J. Heart Fail. 2023, 25, 2107–2129. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, L.J.; Diprose, W.K.; Wang, M.T.; Barber, P.A. Chronic kidney disease and outcome following endovascular thrombectomy for acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104665. [Google Scholar] [CrossRef]
- Zamberg, I.; Assouline-Reinmann, M.; Carrera, E.; Sood, M.M.; Sozio, S.M.; Martin, P.-Y.; Mavrakanas, T.A. Epidemiology, thrombolytic management, and outcomes of acute stroke among patients with chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2022, 37, 1289–1301. [Google Scholar] [CrossRef]
- Toyoda, K. Cerebrorenal interaction and stroke. In Brain, Stroke and Kidney; Karger Publishers: Basel, Switzerland, 2013; Volume 179, pp. 1–6. [Google Scholar]
- Chelluboina, B.; Vemuganti, R. Chronic kidney disease in the pathogenesis of acute ischemic stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1893–1905. [Google Scholar] [CrossRef]
- Ghoshal, S.; Freedman, B.I. Mechanisms of Stroke in Patients with Chronic Kidney Disease. Am. J. Nephrol. 2019, 50, 229–239. [Google Scholar] [CrossRef]
- Bobot, M.; Suissa, L.; Hak, J.-F.; Burtey, S.; Guillet, B.; Hache, G. Kidney disease and stroke: Epidemiology and potential mechanisms of susceptibility. Nephrol. Dial. Transplant. 2023, 38, 1940–1951. [Google Scholar] [CrossRef]
- Cherng, Y.G.; Lin, C.S.; Shih, C.C.; Hsu, Y.H.; Yeh, C.C.; Hu, C.J.; Chen, T.L.; Liao, C.C. Stroke risk and outcomes in patients with chronic kidney disease or end-stage renal disease: Two nationwide studies. PLoS ONE 2018, 13, e0191155. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Rothwell, P.M. Impact of multimorbidity on risk and outcome of stroke: Lessons from chronic kidney disease. Int. J. Stroke 2021, 16, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Sato, W.; Ishikawa, K.; Shinjo, H.; Miyagawa, Y.; Noda, Y.; Imai, E.; Yamada, K. KDIGO (Kidney Disease: Improving Global Outcomes) criteria could be a useful outcome predictor of cisplatin-induced acute kidney injury. Oncology 2012, 82, 354–359. [Google Scholar] [CrossRef]
- Rollnik, J.D. The Early Rehabilitation Barthel Index (ERBI). Rehabilitation 2011, 50, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Ciculation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of Cardiology/American heart association task force on practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease: A Statement From the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Sundström, J.; Bodegard, J.; Bollmann, A.; Vervloet, M.G.; Mark, P.B.; Karasik, A.; Taveira-Gomes, T.; Botana, M.; Birkeland, K.I.; Thuresson, M.; et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: The CaReMe CKD study. Lancet Reg. Health-Eur. 2022, 20, 100438. [Google Scholar] [CrossRef] [PubMed]
- Munikrishnappa, D. Chapter 6: Limitations of Various Formulae and Other Ways of Assessing GFR in the Elderly: Is There a Role for Cystatin C? 2009. Available online: https://www.asn-online.org/education/distancelearning/curricula/geriatrics/Chapter6.pdf (accessed on 14 August 2024).
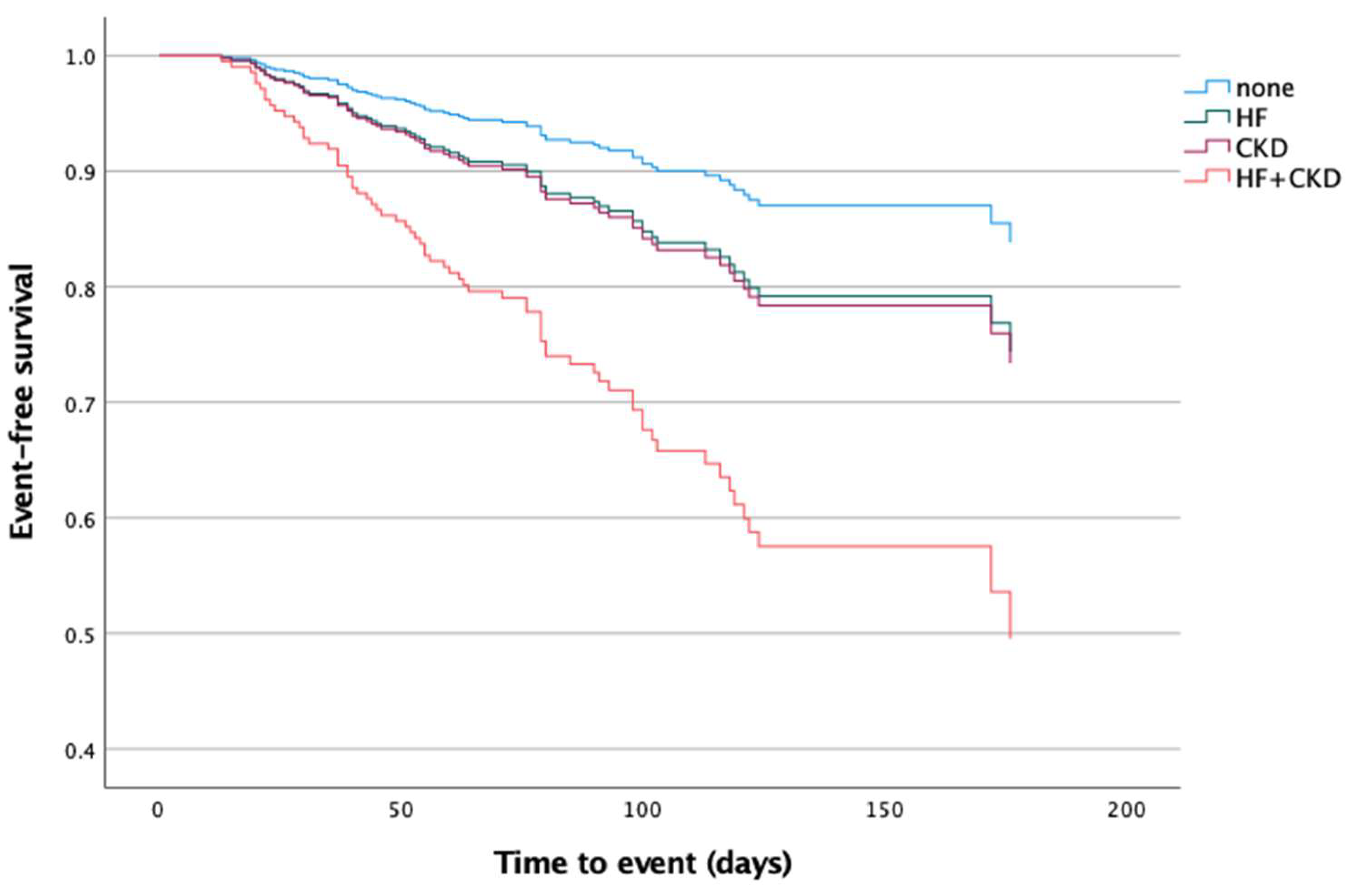
| Dependent Variable | Composite Unfavorable Outcome [HR (95% CI)] * | p-Value |
|---|---|---|
| CHF | 2.28 (1.21–4.29) | 0.01 |
| CKD | 2.19 (1.24–3.87) | 0.007 |
| Parameters | No CHF + No CKD (n = 449) | CHF + No CKD (n = 30) | No CHF + CKD (n = 86) | CHF + CKD (n = 21) | p-Value |
|---|---|---|---|---|---|
| Mean age, years (SD) | 68.78 (13) | 69.93 (14.69) | 72.79 (11.3) | 77.3 (10.02) | 0.003 |
| Gender [2] (%) | 233 (51.9%) | 13 (43.3%) | 50 (58.1%) | 11 (52.4%) | NS |
| Disability (ERBI) | −3.28 (60.40) | −23.33 (66.33) | −1.88 (58.28) | −20 (62.72) | NS |
| Stroke type | |||||
| Ischemic | 315 (70.2%) | 24 (80%) | 66 (76.7%) | 19 (90.5) | NS |
| Hemorrhagic | 134 (29.8%) | 6 (20%) | 20 (23.3%) | 2 (9.5%) | |
| Comorbidities | |||||
| HTN (%) | 335 (74.9%) | 22 (73.3%) | 77 (89.5%) | 17 (81%) | 0.02 |
| DM (%) | 121 (27.1%) | 13 (43.3%) | 40 (46.5%) | 9 (42.9%) | <0.001 |
| CVD (%) | 77 (17.2%) | 22 (73.3%) | 31 (36%) | 19 (90.5%) | <0.001 |
| HLP (%) | 105 (23.5%) | 4 (13.3%) | 20 (23.3%) | 8 (38.1%) | NS |
| AF (%) | 135 (30.2%) | 17 (56.7%) | 36 (41.9%) | 13 (61.9%) | <0.001 |
| Biochemical characteristics | |||||
| Cr (mg/L) | 74.99 (40.42) | 82.76 (41.92) | 173.47 (160.71) | 215.66 (217.67) | <0.001 |
| GFR (mL/min) | 99.74 (41.52) | 88.8 (40.32) | 52.79 (30.88) | 50.2 (32.47) | <0.001 |
| Total protein (g/L) | 66.92 (39.45) | 5.51 (7.66) | 60.94 (15.21) | 60.41 (6.6) | NS |
| CRP (mg/L) | 31.46 (58.61) | 31.98 (45.32) | 30.21 (44.15) | 22.1 (37.23) | NS |
| Hb (g/dL) | 8.57 (16.9) | 7.46 (1.14) | 7.35 (1.41) | 6.81 (1.28) | NS |
| WBC (×106/μL) | 8.62 (3.08) | 8.91 (3.53) | 9.12 (5.13) | 8.08 (2.98) | NS |
| Dependent Variable | Composite Unfavorable Outcome [HR (95% CI)] * | p-Value |
|---|---|---|
| CHF and CKD ¶ | ||
| No CHF + No CKD (reference point) | 1 | |
| CHF + No CKD | 1.88 (0.78–4.49) | 0.15 |
| No CHF + CKD | 1.96 (1.01–3.8) | 0.04 |
| CHF + CKD | 5.8 (2.5–13.44) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.; Jauert, N.; Schikora, M.; Joebges, M.; Doehner, W. The Impact of Concurrent Chronic Heart Failure and Chronic Kidney Dysfunction on Post-Stroke Rehabilitation Outcomes. Neurol. Int. 2025, 17, 70. https://doi.org/10.3390/neurolint17050070
Fischer A, Jauert N, Schikora M, Joebges M, Doehner W. The Impact of Concurrent Chronic Heart Failure and Chronic Kidney Dysfunction on Post-Stroke Rehabilitation Outcomes. Neurology International. 2025; 17(5):70. https://doi.org/10.3390/neurolint17050070
Chicago/Turabian StyleFischer, Azadeh, Nadja Jauert, Martin Schikora, Michael Joebges, and Wolfram Doehner. 2025. "The Impact of Concurrent Chronic Heart Failure and Chronic Kidney Dysfunction on Post-Stroke Rehabilitation Outcomes" Neurology International 17, no. 5: 70. https://doi.org/10.3390/neurolint17050070
APA StyleFischer, A., Jauert, N., Schikora, M., Joebges, M., & Doehner, W. (2025). The Impact of Concurrent Chronic Heart Failure and Chronic Kidney Dysfunction on Post-Stroke Rehabilitation Outcomes. Neurology International, 17(5), 70. https://doi.org/10.3390/neurolint17050070






