Current Management of Aneurysmal Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Clinical Aspects
3. SAH Classifications
4. Clinical Presentation
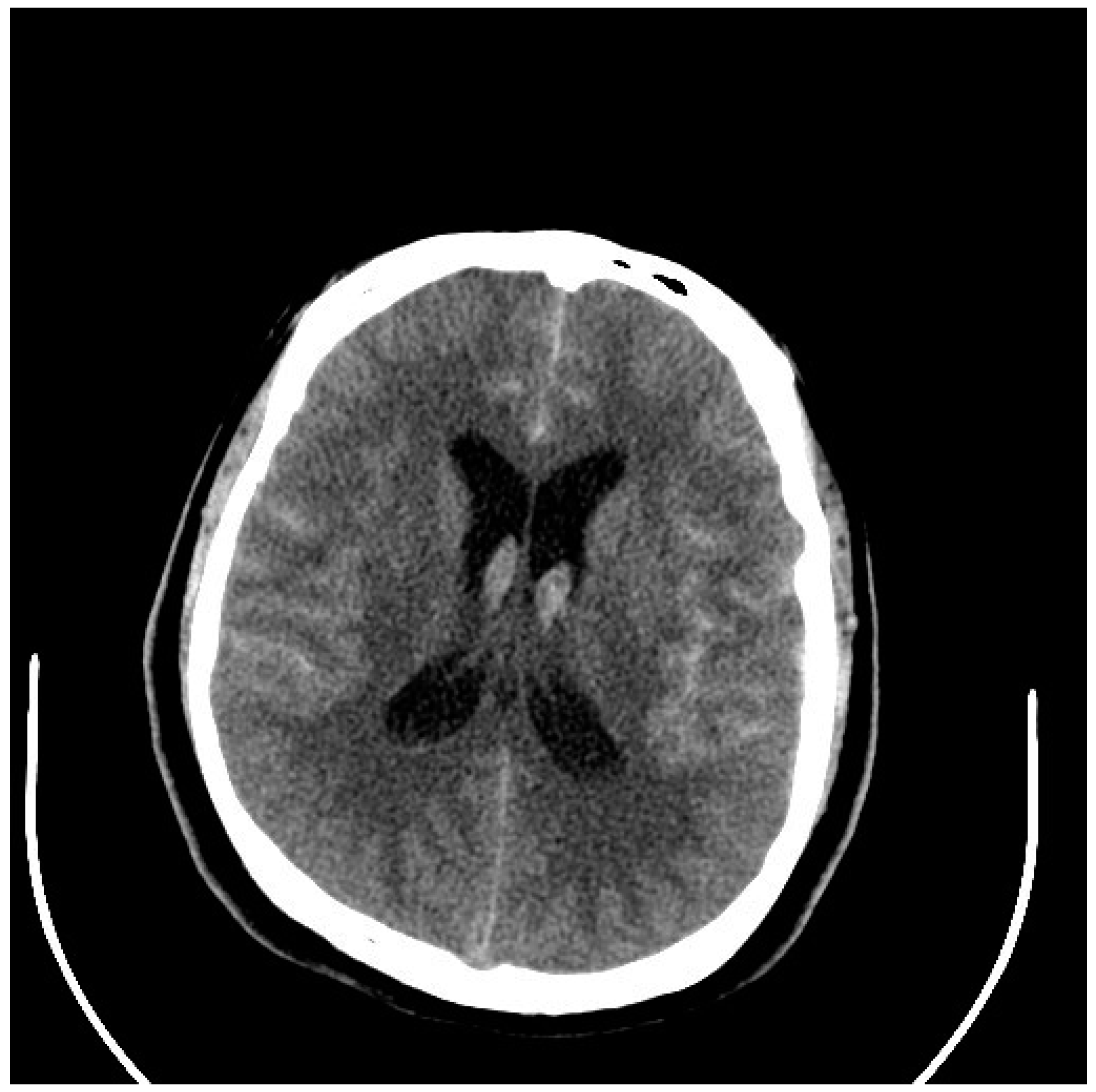
5. Diagnosis
6. Immediate Management of aSAH
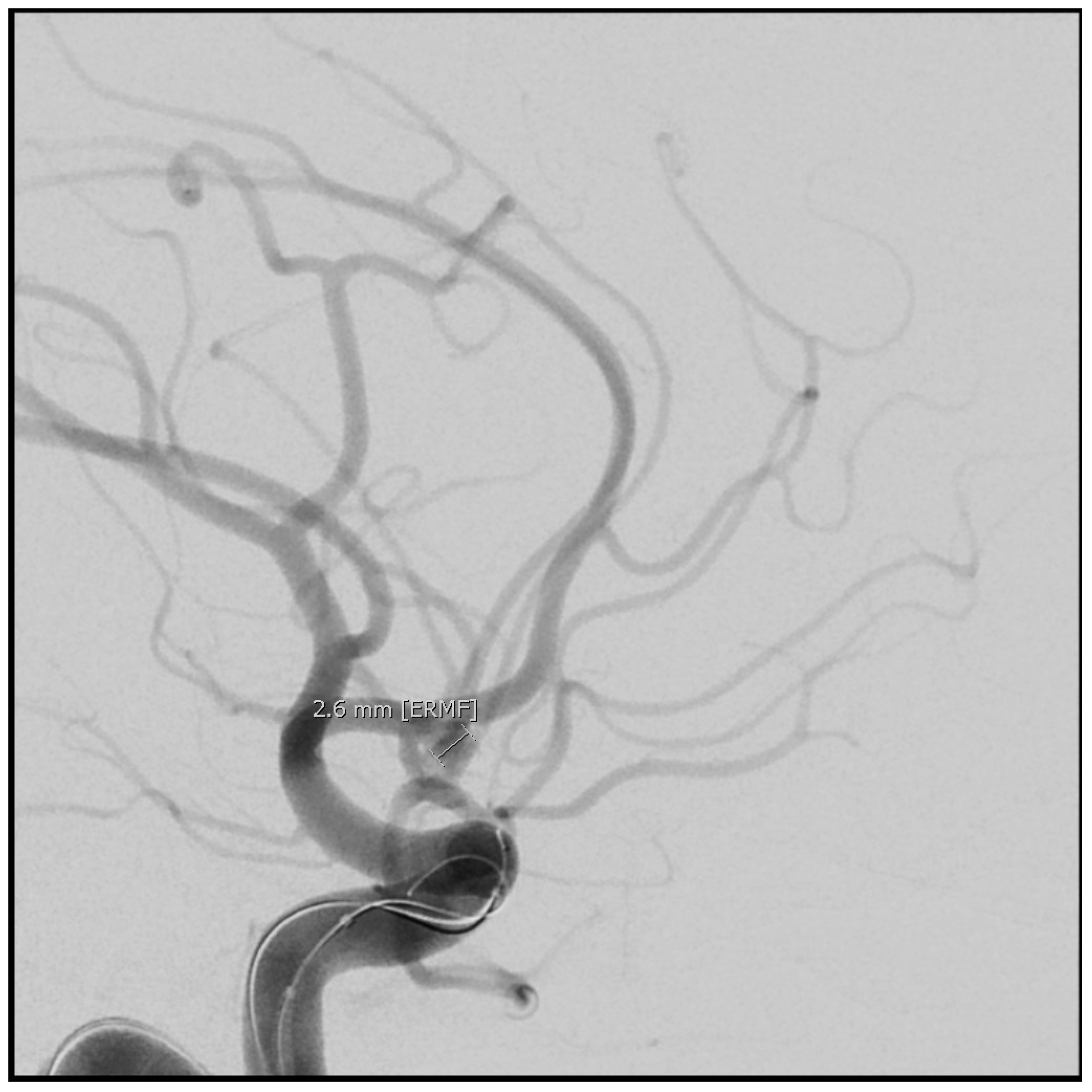
7. Aneurysm Repair
8. Complications of aSAH and Their Management
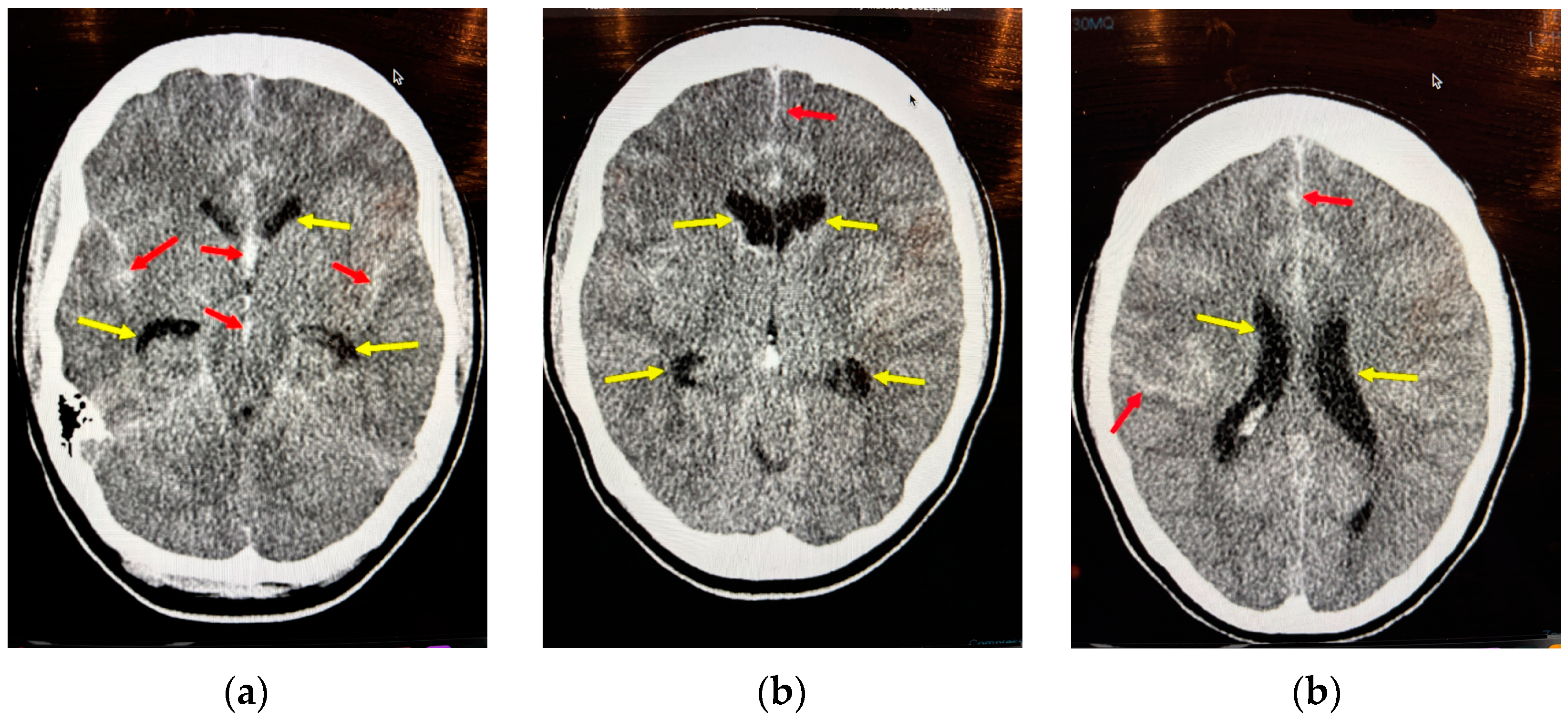
8.1. Vasospasm
8.2. Acute Hydrocephalus
8.3. Raised Intracranial Pressure
8.4. Seizures
8.5. Electrolyte, Hemoglobin, and General Measures
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Diagbouga, M.R.; Morel, S.; Bijlenga, P.; Kwak, B.R. Role of hemodynamics in initiation/growth of intracranial aneurysms. Eur. J. Clin. Investig. 2018, 48, e12992. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. Classification of Stroke Subtypes. Cerebrovasc. Dis. 2009, 5, 493–501. [Google Scholar] [CrossRef]
- Flaherty, M.I.; Haverbusch, M.; Kissela, B.; Kleindorfer, D.; Schneider, A.; Sekar, P.; Moomaw, C.J.; Sauerback, L.; Broderick, J.P.; Woo, D. Perimesencephalic Subarachnoid Hemorrhage: Incidence, Risk Factors, and Outcome. J. Stroke Cerebrovasc. Dis. 2005, 14, 267–271. [Google Scholar] [CrossRef]
- Classen, J.; Park, S. Spontaneous subarachnoid hemorrhage. Lancet 2022, 400, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Vlak, M.H.; Algra, A.; Brandenburg Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Karhunem, V.; Bakker, M.K.; Ruigrok, Y.M.; Gill, D.; Larsson, S.C. Modifiable risk factors for intracranial aneurysmal subrarachnoid hemorrhage: A mendelian randomization study. J. Am. Heart Assoc. 2021, 10, E022277. [Google Scholar] [CrossRef] [PubMed]
- Kleinloog, R.; de Mul, N.; Verweij, B.H.; Post, J.A.; Rinkel, G.J.; Ruigrok, Y.M. Risk Factors for Intracranial Aneurysm Rupture: A Systematic Review. Neurosurgery 2018, 82, 431–440. [Google Scholar] [CrossRef]
- Botterell, E.H.; Lougheed, W.M.; Scott, J.W.; Vandewater, S.L. Hypothermia and interruption of carotid and vertebral circulation in the surgical management of intracranial aneurysms. J. Neurosurg. 1956, 13, 1–42. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Diepinigaitis, A.J.; Bowers, C.A.; Classen, J.; Park, S.; Khandelwal PQureshi, A.I.; Majidi, S.; Fifi, J.T.; Lee, S.K. Prognostication Following Aneurysmal Subarachnoid Hemorrhage: The Modified Hunt and Hess Grading Scale. Stroke Vasc. Interv. Neurol. 2024, 4, e001349. [Google Scholar] [CrossRef]
- Sano, H.; Satoh, A.; Murayama, Y.; Kato, Y.; Origasa, H.; Inamasu, J.; Nouri, M.; Cherian, I.; Saito, N. Modified World Federation of Neurosurgical Societies subarachnoid hemorrhage grading system. World Neurosurg. 2015, 83, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S., Jr.; MacDonald, R.L.; Mayer, S.A. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery 2006, 59, 21–27. [Google Scholar]
- Cortnum, S.; Sorensen, P.; Jorgensen, J. Determining the sensitivity of computed tomography scanning in early detecrtion of subarachnoid hemorrhage. Neurosurgery 2010, 55, 900–902. [Google Scholar]
- McCormack, R.F.; Hudson, A. Can computed angiography replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontraast cranial computed tomography scan? Acad. Emerg. Med. 2010, 17, 444–451. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Hyssain, A.M.; Ward, M.J.; Zipfel, G.J.; Fowler, S.; Pines, J.M.; Sivilotti, M.L. Spontaneous Subarachnoid Hemorrhage: A Systematic Review and Meta-analysis Describing the Diagnostic Accuracy of History, Physical Examination, Imaging and Lumbar Puncture With an Exploration of Test Thresholds. Acad. Emerg. Med. 2016, 23, 963–1003. [Google Scholar] [CrossRef] [PubMed]
- Jabbarli, R.; Dinger, T.F.; Oppong, M.D.; Pierscianek, D.; Dammann, P.; Wrede, K.H.; Kaier, K.; Kohrmann, M.; Forsting, M.; Kleinschnitz, C.; et al. Risk Facotors for and Clinical Consequences of Multiple Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Stroke 2018, 49, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Vethuis, B.K.; Rinkel, G.J.E.; Ramos, L.M.P.; Witkamp, T.D.; van Leewen, M.S. Perimesencephalic Hemorrage: Exclusion of Vertebrobasilar Aneurysms with CT Angiography. Stroke 1999, 30, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Wilkinson, I.D.; Horgaard, N.; Paley, M.N.; Jellinek, D.A.; Powell, T.; Romanowski, C.; Hodgson, T.; Griffiths, P.D. Detection of subarachnoid haemorrhage with magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 2001, 70, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ducros, A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012, 11, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Hillman, J.; Fridriksson, S.; Nilsson, O.; Yu, Z.; Saveland, H.; Jakobsson, K.-E. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: A prospective randomized study. J. Neurosurg. 2002, 97, 771–778. [Google Scholar] [CrossRef]
- Denneman, N.; Post, R.; Coert, B.A.; van den Berg Verbaan, D.; Vandertop, P. Resilience after High-Grade subarachnoid Hemorrhage: A Prospective Cohort study on Quality of Life. Neurosurgery 2025, 96, 96–103. [Google Scholar] [CrossRef]
- Darsaut, T.E.; Roy, D.; Weill, A.; Bojanowski, M.W.; Chaalala, C.; Biolocq, A.; Findlay, J.M.; Rempel, J.L.; Chow, M.M.; O’Kelly, C.; et al. A randomized trial of endovascular versus surgical management of intracranial aneurysms: Interim results from ISAT2. Neurochirurgie 2019, 65, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Loan, J.J.M.; Wiggins, A.N.; Brennan, P.M. Medically induced hypretension, hypervolaemia and haemodilution for the treatment and prophylaxis of vasospasm following aneurysmal subarachnoid haemorrhage: Systemic review. Br. J. Neurosurg. 2018, 32, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.H.Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guidelines for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, T.; Zhai, Q.; Zhang, X.; Jing, H.; Li, K.; Liu, S.; Guojun, G.; Wang, L.; Li, L.; et al. Impacts of Statin Therapy Strategies on Incidence of Ischemic Cerebrovascular Events in Patients with Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Bayesian Network Met-Analysis. Neurosurgery 2023, 93, 24–32. [Google Scholar] [CrossRef]
- Findlay, J.M.; Chen, V. Chapter 46—Medical Management of Cerebral Vasospasm. In Youmans Neurological Surgery, 8th ed.; Winn, H.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 4, pp. 3647–3658. [Google Scholar]
- Chen, S.; Luo, J.; Reis, C.; Manaenko, A.; Zhang, J. Hydrocephalus after Subarachnoid Hemorrhage: Pathophysiology, Diagnosis and Treatment. Biomed. Res. Int. 2017, 2017, e8584753. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.B.; Jahromi, B.S.; Batra, A.; Potts, M.B.; Maidech, A.M.; Liotta, E.M. Magnesium and Risk of Bleeding Complications from Ventriculostomy Insertion. Stroke 2020, 51, 2795–2800. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Korenfeld, Y.; Crisman, C.; Badjatia, N.; Mayer, S.A.; Connolly Jr, E.S. Prevention of ventriculostomy-related infections with prophylactic antibiotic and antibiotic-coated external ventricular drains: A systematic review. Neurosurgery 2011, 68, 996–1005. [Google Scholar] [CrossRef]
- Capion, T.; Lilja-Cyron, A.; Juhler, M.; Mathiesen, T.I.; Wetterslev, J. Prompt closure versus gradual weaning of external ventricular drainage for hydrocephalus in adult patients with aneurysmal subarachnoid haemorrhage: A systematic review. BMJ Open 2020, 10, e040722. [Google Scholar] [CrossRef]
- Findlay, J.M.; Jacka, M.J. Cohort study of intraventricular thrombolysis with recombinant tissue plasminogen activator for aneurysmal intraventricular hemorrhage. Neurosurgery 2004, 55, 532–537. [Google Scholar] [CrossRef]
- Ropper, A.H. Management of raised intracranial pressure and hyperosmolar therapy. Pract. Neurol. 2014, 14, 152–158. [Google Scholar] [CrossRef]
- O’Connor, K.L.; Westover, M.B.; Phillips, M.T.; Iftimia, N.A.; Buckley, D.A.; Ogilvy, C.S.; Shafi, M.M.; Rosenthal, E.S. High Risk for Seizures Following Subarachnoid Hemorrhage Regardless of Referral Bias. Neurocrit. Care 2014, 21, 476–482. [Google Scholar] [CrossRef]
- Alshekhlee, A.; Mehta, S.; Willmore, L. Seizures in Subarachnoid Hemorrhage. In Seizures in Cerebrovascular Disorders; Koubeissi, M., Alshekhlee, A., Mehndiratta, P., Eds.; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Naidech, A.M.; Kreiter, K.T.; Janjua, N.; Ostapkovich, N.; Parra, A.; Commichau, C.; Connolly, E.S.; Mayer, S.A.; Fitzsimons, B.F.M. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke 2005, 36, 583–587. [Google Scholar] [CrossRef] [PubMed]
- English, S.W.; Delaney, A.; Fergusson, D.A.; Chassé, M.; Turgeon, A.F.; Lauzier, F.; Tuttle, A.; Sadan, O.; Griesdale, D.E.; Redekop, G.; et al. Liberal or Restrictive Transfusion Strategy in Aneurysmal Subarachnoid Hemorrhage. N. Eng. J. Med. 2024. [Google Scholar] [CrossRef]
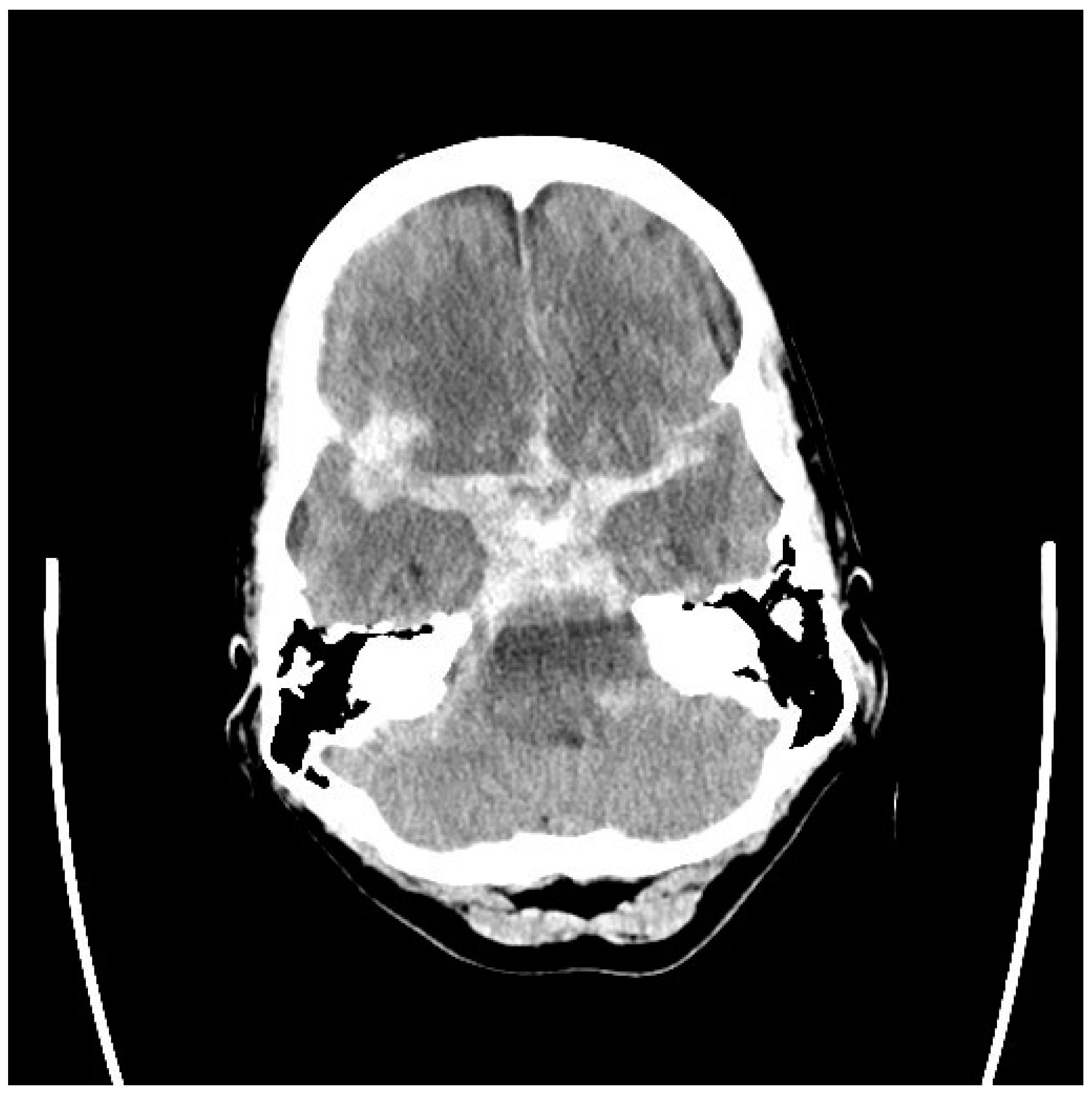
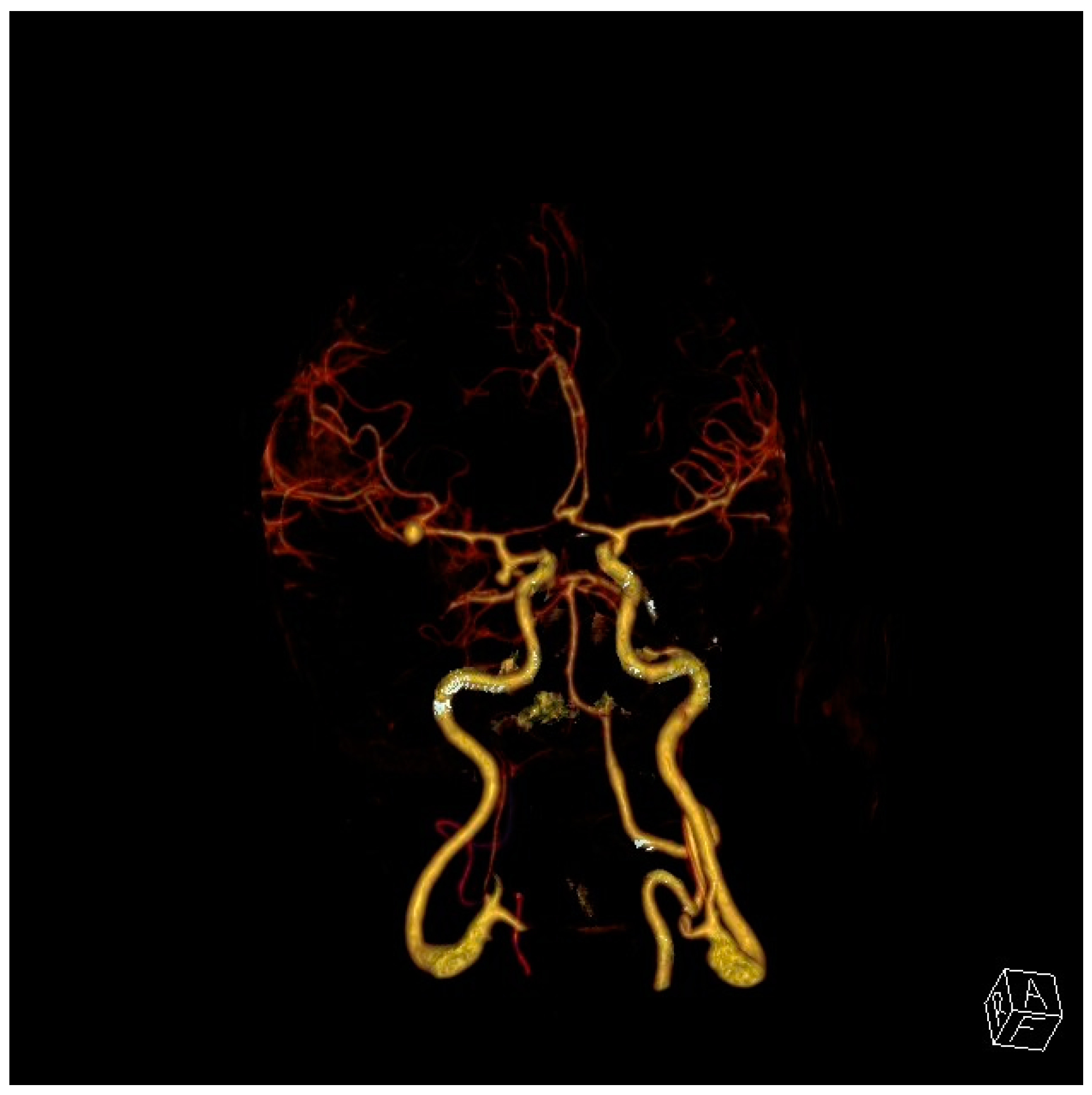

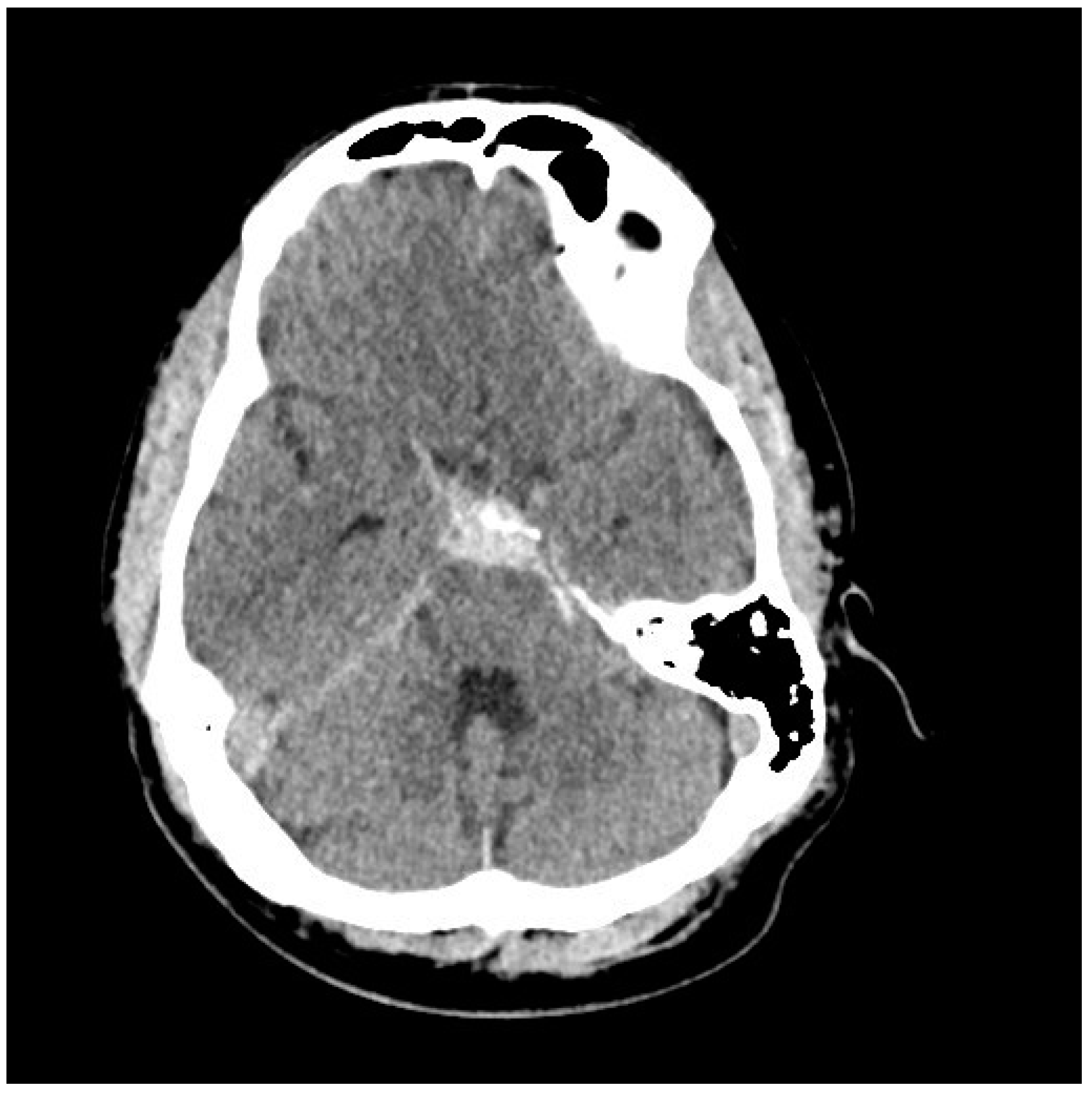
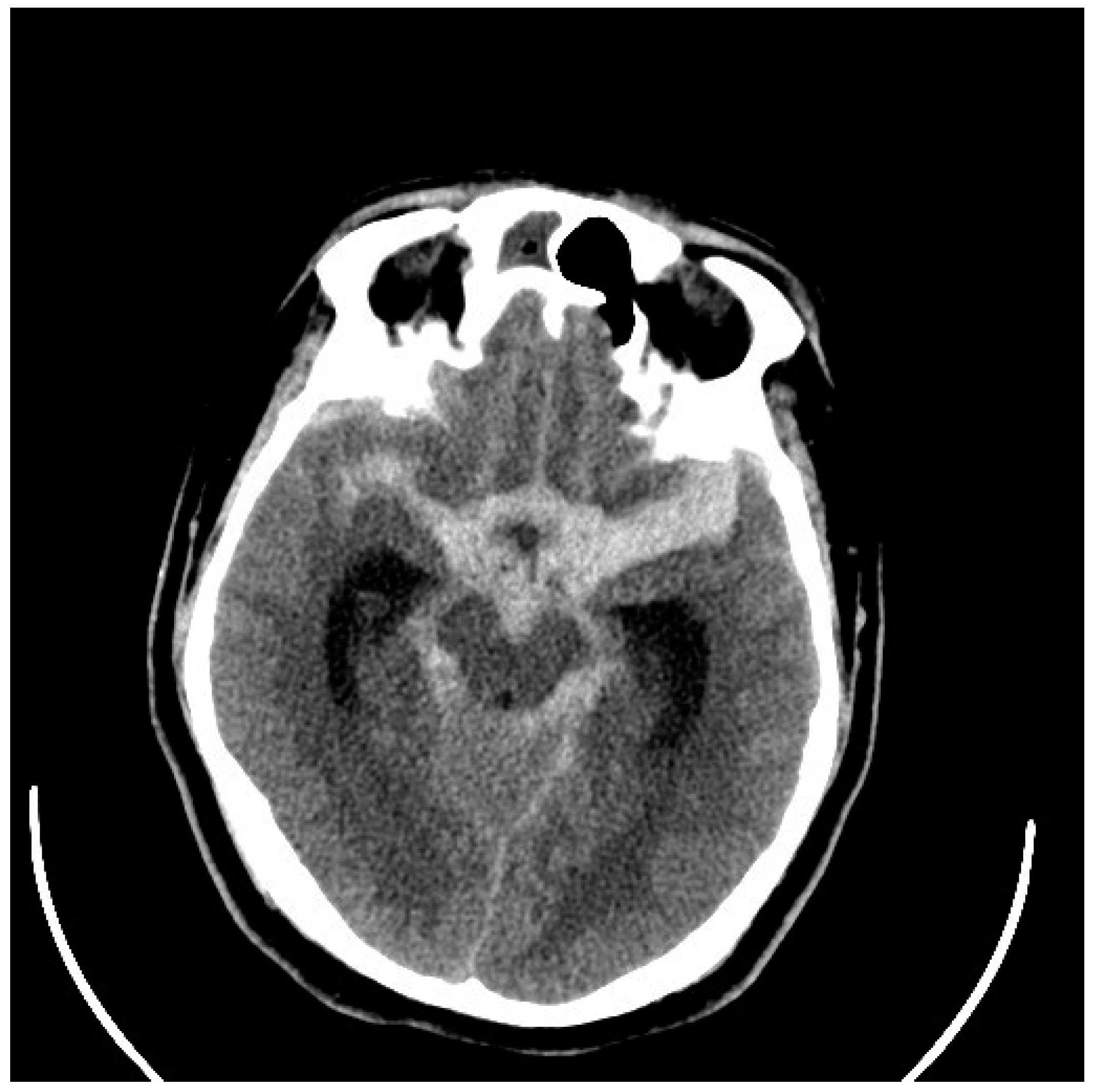


| Age at Diagnosis | Remaining Years to Age 80 | Risk of Hemorrhage 1% per Year | Risk of Hemorrhage 2% per Year | Risk of Hemorrhage 3% per Year |
|---|---|---|---|---|
| 35 | 45 | 35 | 60 | 75 |
| 45 | 35 | 29 | 51 | 66 |
| 55 | 25 | 22 | 40 | 53 |
| 65 | 15 | 18 | 26 | 37 |
| 75 | 5 | 10 | 10 | 14 |
| Hunt and Hess SAH Grading Scale [8] | Glasgow Coma Scale (GCS, out of 15) | WFNS SAH Grading Scale (Modified) [9] | Fisher SAH Scale (Modified) for Vasospasm Prediction [10] |
|---|---|---|---|
| Grade 1: Headache Grade 2: Severe headache but alert Grade 3: Drowsy Grade 4: Coma Grade 5: Coma with abnormal brainstem findings | Eye opening: 4. spontaneous 3. to speech 2. to pain 1. none Speech: 5. alert 4. confused 3. inappropriate 2. incomprehensive 1. none Motor response: 6. normal 5. localizing 4. withdrawal 3. decorticate 2. decerebrate 1. none | 1. GCS 15 2. GCS 13–14, no focal deficit or meningismus 3. GCS 13–14, mild deficit or meningismus present 4. GCS 7–12 5. GCS <7 |
thin SAH is <1 mm VSP = symptomatic vasospasm |
| Five-Step Management of Symptomatic Vasospasm Complicating aSAH |
|---|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Findlay, J.M. Current Management of Aneurysmal Subarachnoid Hemorrhage. Neurol. Int. 2025, 17, 36. https://doi.org/10.3390/neurolint17030036
Findlay JM. Current Management of Aneurysmal Subarachnoid Hemorrhage. Neurology International. 2025; 17(3):36. https://doi.org/10.3390/neurolint17030036
Chicago/Turabian StyleFindlay, Jay Max. 2025. "Current Management of Aneurysmal Subarachnoid Hemorrhage" Neurology International 17, no. 3: 36. https://doi.org/10.3390/neurolint17030036
APA StyleFindlay, J. M. (2025). Current Management of Aneurysmal Subarachnoid Hemorrhage. Neurology International, 17(3), 36. https://doi.org/10.3390/neurolint17030036




