The Cognitive Changes Among Patients over 65 Years of Age in a Rural Area—The Preliminary Report of Protective and Predisposing Factors
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Amyloid 1-42 High Sensitive ELISA Kit for Amyloid Beta Peptide 1-42 (Ab1-42) HEA946Hu
- (2)
- Amyloid 1-40 ELISA Kit for Amyloid Beta Peptide 1-40 (Ab1-40) CEA864Hu
- (3)
- APOE SEA704Hu
- MMSE (Mini–Mental State Examination)—a screening tool for cognitive assessment. Scores range from 0 (severe cognitive decline) to 30 (normal cognition) [61].
- ADL (Basic Activities of Daily Living)—an instrument assessing functional capacity in basic activities, for example, eating, dressing, and continence. Scores range from 0 (dependent patient) to 6 (independent patient) [36].
- IADL (Instrumental Activities of Daily Living)—evaluation of functional capacity in more complex activities, such as independent shopping and economic management. Scores range from 8 (dependent daily living functioning) to 24 (independent daily living functioning) [62].
- Beck Depression Inventory—used for depression diagnosis. The version with 21 questions was used. Scores range from 0 (no depression symptoms) to 63 (severe depression symptoms) [63].
- M-ACE (The Mini–Addenbrooke’s Cognitive Examination)—a screening tool for dementia diagnosis. The minimum score is 0 (indicating dementia), and the maximum score is 30 (normal cognitive level). The recommended cut-off used was 25 and 21 points [64]. We used 25 points to divide the study group into the NC group (normal cognition) and the DC group (deteriorated cognition).
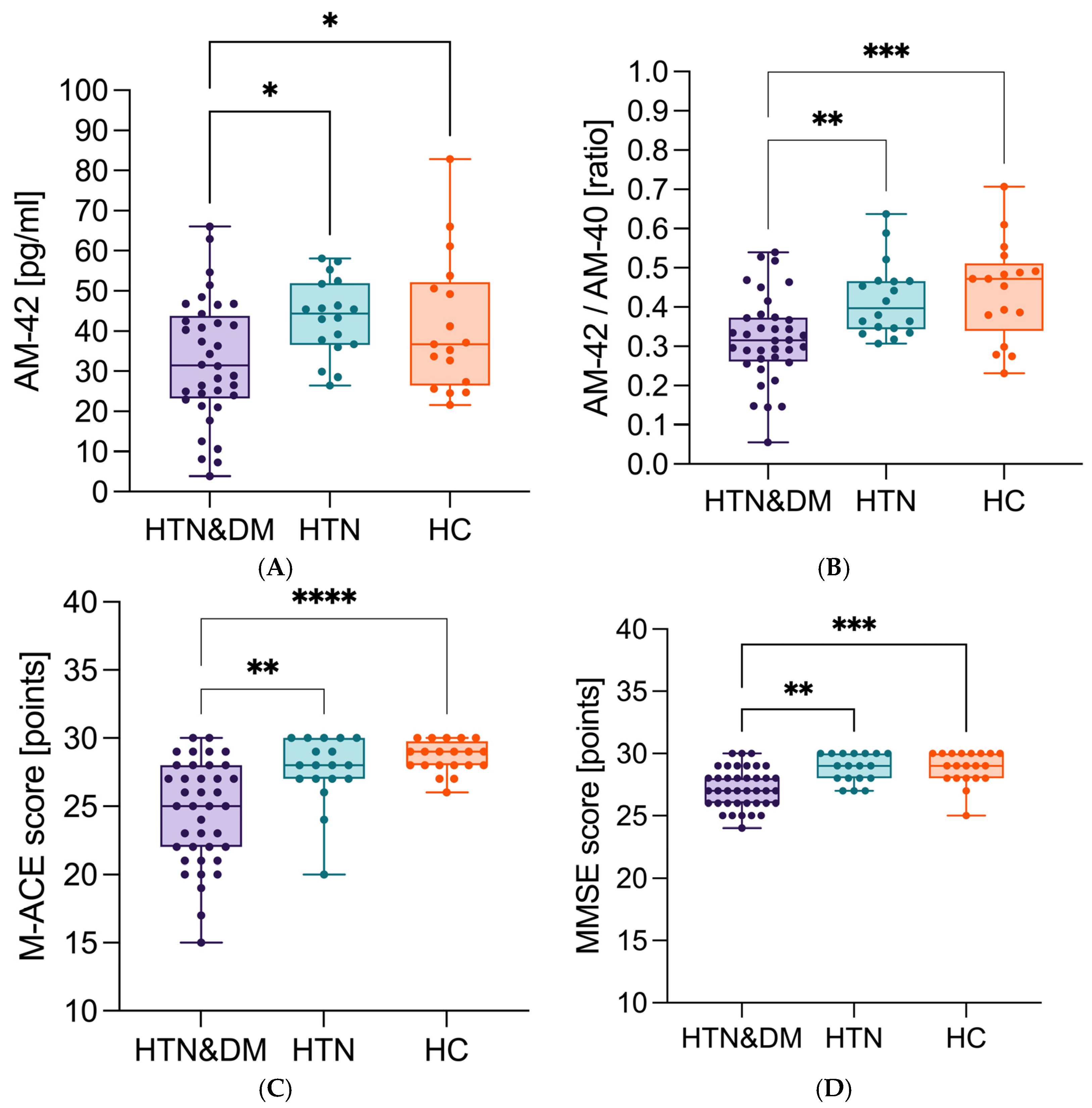
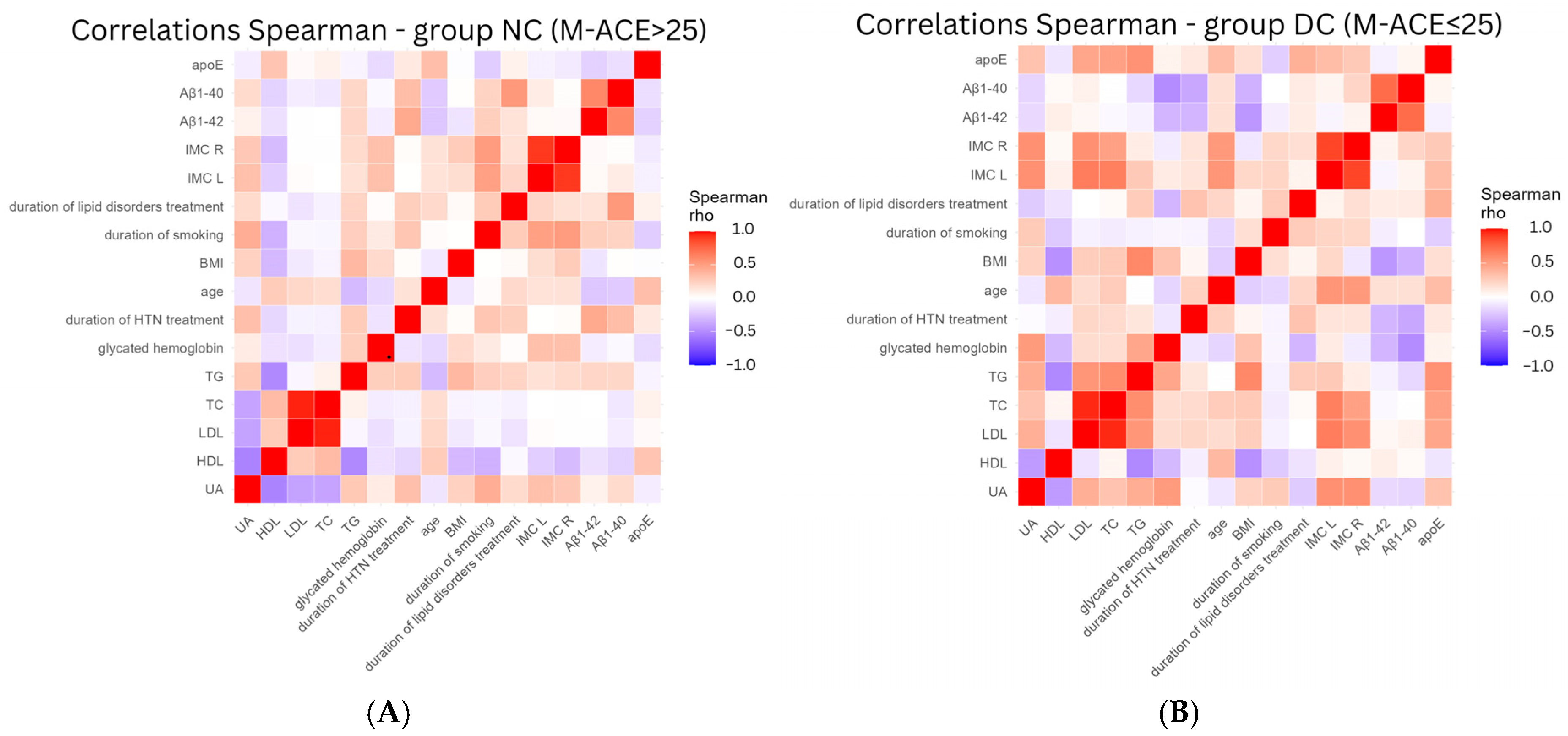
3. Results
4. Discussion
5. Conclusions
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABI L | ankle-brachial index left |
| ABI R | ankle-brachial index right |
| AD | Alzheimer’s disease |
| ADL | Activities of Daily Living |
| AGEs | advanced glycation end products |
| apoE | apolipoprotein E |
| APP | amyloid precursor protein |
| Aβ | beta-amyloid |
| Aβ42/40 | plasma β-amyloid 1-42-to-plasma β-amyloid 1-40 ratio |
| BBB | Blood–brain barrier |
| BMI | Body Mass Index |
| CSF | cerebrospinal fluid |
| DC | deteriorated cognition group |
| DM | diabetes mellitus |
| DSST | Digit Symbol Substitution Test |
| EDTA | ethylenediaminetetraacetic acid |
| FDG-PET | 18F-fluorodeoxyglucose positron emission tomography |
| HC | healthy controls |
| HDL | high-density lipoproteins |
| HTN | arterial hypertension |
| IADL | Instrumental Activities of Daily Living |
| IMC L | intima–media complex thickness left side |
| IMC R | intima–media complex thickness right side |
| LDL | low-density lipoproteins |
| M-ACE | Mini–Addenbrooke’s Cognitive Examination |
| MMSE | Mini–Mental State Examination |
| N | quantity |
| NC | normal cognition group |
| RAGE | advanced glycation end products receptor |
| RAS | renin–angiotensin system |
| ROS | reactive oxygen species |
| TC | total cholesterol |
| TG | triglycerides |
| UA | uric acid |
References
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Dumas, A.; Destrebecq, F.; Esposito, G.; Suchonova, D.; Steen Frederiksen, K. Rethinking the Detection and Diagnosis of Alzheimer’s Disease: Outcomes of a European Brain Council Project. Aging Brain 2023, 4, 100093. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J. Amyloid Beta in Aging and Alzheimer ’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. The Duality of Amyloid-β: Its Role in Normal and Alzheimer’s Disease States. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral Amyloid Angiopathy and Alzheimer Disease—One Peptide, Two Pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, L.M.; Khan, A.F.; Adewale, Q.; Bezgin, G.; Therriault, J.; Fernandez-Arias, J.; Servaes, S.; Rahmouni, N.; Tissot, C.; Stevenson, J.; et al. In-Vivo Neuronal Dysfunction by Aβ and Tau Overlaps with Brain-Wide Inflammatory Mechanisms in Alzheimer’s Disease. Front. Aging Neurosci. 2024, 16, 1383163. [Google Scholar] [CrossRef] [PubMed]
- Nagele, R.G.; D’Andrea, M.R.; Anderson, W.J.; Wang, H.-Y. Intracellular Accumulation of β-Amyloid1–42 in Neurons Is Facilitated by the α7 Nicotinic Acetylcholine Receptor in Alzheimer’s Disease. Neuroscience 2002, 110, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-Based Therapy for Alzheimer’s Disease: Challenges, Successes and Future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Huynh, T.P.V.; Davis, A.A.; Ulrich, J.D.; Holtzman, D.M. Apolipoprotein E and Alzheimer’s Disease: The Influence of Apolipoprotein E on Amyloid-β and Other Amyloidogenic Proteins. J. Lipid Res. 2017, 58, 824–836. [Google Scholar] [CrossRef]
- Liu, C.-C.; Zhao, N.; Fu, Y.; Wang, N.; Linares, C.; Tsai, C.-W.; Bu, G. ApoE4 Accelerates Early Seeding of Amyloid Pathology. Neuron 2017, 96, 1024–1032.e3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jiang, H.; Park, S.; Eltorai, A.E.M.; Stewart, F.R.; Yoon, H.; Basak, J.M.; Finn, M.B.; Holtzman, D.M. Haploinsufficiency of Human APOE Reduces Amyloid Deposition in a Mouse Model of Amyloid-β Amyloidosis. J. Neurosci. 2011, 31, 18007–18012. [Google Scholar] [CrossRef] [PubMed]
- Giannisis, A.; Al-Grety, A.; Carlsson, H.; Patra, K.; Twohig, D.; Sando, S.B.; Lauridsen, C.; Berge, G.; Grøntvedt, G.R.; Bråthen, G.; et al. Plasma Apolipoprotein E Levels in Longitudinally Followed Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Alzheimer’s Res. Ther. 2022, 14, 115. [Google Scholar] [CrossRef]
- Jackson, R.J.; Meltzer, J.C.; Nguyen, H.; Commins, C.; Bennett, R.E.; Hudry, E.; Hyman, B.T. APOE4 Derived from Astrocytes Leads to Blood-Brain Barrier Impairment. Brain 2022, 145, 3582–3593. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Huang, N.; Luo, Y. The Role of TREM2 in Alzheimer’s Disease: From the Perspective of Tau. Front. Cell Dev. Biol. 2023, 11, 1280257. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R. The Role of Astrocytes in Amyloid β-Protein Toxicity and Clearance. Exp. Neurol. 2012, 236, 1–5. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, Y.; Peng, L.-J.; Shao, H.; Menon, N.K.; Yang, J.; Salomon, A.R.; Freidland, R.P.; Zagorski, M.G. Nicotine and Amyloid Formation. Biol. Psychiatry 2001, 49, 248–257. [Google Scholar] [CrossRef]
- Heo, C.E.; Choi, T.S.; Kim, H.I. Competitive Homo- and Hetero- Self-Assembly of Amyloid-β 1–42 and 1–40 in the Early Stage of Fibrillation. Int. J. Mass Spectrom. 2018, 428, 15–21. [Google Scholar] [CrossRef]
- Vogelgsang, J.; Hansen, N.; Stark, M.; Wagner, M.; Klafki, H.; Morgado, B.M.; Jahn-Brodmann, A.; Schott, B.; Esselmann, H.; Bauer, C.; et al. Plasma Amyloid Beta X-42/X-40 Ratio and Cognitive Decline in Suspected Early and Preclinical Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, 5132–5142. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Teunissen, C.E.; Zetterberg, H.; Allué, J.A.; Sarasa, L.; Eichenlaub, U.; Bittner, T.; Ovod, V.; Verberk, I.M.W.; Toba, K.; et al. Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021, 78, 1375–1382. [Google Scholar] [CrossRef]
- Wolters, F.J.; Ikram, M.A. Epidemiology of Vascular Dementia. Arter. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef]
- OECD. Health at a Glance: Europe 2022; OECD: Paris, France, 2022; ISBN 9789264462113. [Google Scholar]
- Haring, B.; Wu, C.; Coker, L.H.; Seth, A.; Snetselaar, L.; Manson, J.E.; Rossouw, J.E.; Wassertheil-Smoller, S. Hypertension, Dietary Sodium, and Cognitive Decline: Results from the Women’s Health Initiative Memory Study. Am. J. Hypertens. 2016, 29, 202–216. [Google Scholar] [CrossRef]
- Heizhati, M.; Wang, L.; Li, N.; Li, M.; Pan, F.; Yang, Z.; Wang, Z.; Abudereyimu, R. Prevalence of Mild Cognitive Impairment Is Higher in Hypertensive Population: A Cross-Sectional Study in Less Developed Northwest China. Medicine 2020, 99, e19891. [Google Scholar] [CrossRef]
- Xue, H.P.; Hou, P.; Li, Y.N.; Mao, X.; Wu, L.F.; Liu, Y.B. Factors for Predicting Reversion from Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Int. J. Geriatr. Psychiatry 2019, 34, 1361–1368. [Google Scholar] [CrossRef]
- Cheon, E.J. Hypertension and Cognitive Dysfunction: A Narrative Review. J. Yeungnam Med. Sci. 2023, 40, 225–232. [Google Scholar] [CrossRef]
- Qin, J.; He, Z.; Wu, L.; Wang, W.; Lin, Q.; Lin, Y.; Zheng, L. Prevalence of Mild Cognitive Impairment in Patients with Hypertension: A Systematic Review and Meta-Analysis. Hypertens. Res. 2021, 44, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Yan, L.; Yang, Z.; Zhong, B.; Xie, W. HbA1c, Diabetes and Cognitive Decline: The English Longitudinal Study of Ageing. Diabetologia 2018, 61, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ding, W.; Zhang, L.; Liu, X. A Nomogram for Predicting Mild Cognitive Impairment in Older Adults with Hypertension. BMC Neurol. 2023, 23, 363. [Google Scholar] [CrossRef]
- Gelber, R.P.; Ross, G.W.; Petrovitch, H.; Masaki, K.H.; Launer, L.J.; White, L.R. Antihypertensive Medication Use and Risk of Cognitive Impairment the Honolulu-Asia Aging Study. Neurology 2013, 81, 888–895. [Google Scholar] [CrossRef]
- Su, C.; Xue, J.; Ye, C.; Chen, A. Role of the Central Renin-angiotensin System in Hypertension (Review). Int. J. Mol. Med. 2021, 47, 95. [Google Scholar] [CrossRef]
- Tsuda, K. Renin-Angiotensin System and Sympathetic Neurotransmitter Release in the Central Nervous System of Hypertension. Int. J. Hypertens. 2012, 2012, 474870. [Google Scholar] [CrossRef]
- Loperena, R.; Harrison, D.G. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef]
- Di Chiara, T.; Del Cuore, A.; Daidone, M.; Scaglione, S.; Norrito, R.L.; Puleo, M.G.; Scaglione, R.; Pinto, A.; Tuttolomondo, A. Pathogenetic Mechanisms of Hypertension—Brain-Induced Complications: Focus on Molecular Mediators. Int. J. Mol. Sci. 2022, 23, 2445. [Google Scholar] [CrossRef] [PubMed]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating Biologic Markers of Endothelial Dysfunction in Cerebral Small Vessel Disease: A Review. J. Cereb. Blood Flow Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef]
- Veglio, F.; Paglieri, C.; Rabbia, F.; Bisbocci, D.; Bergui, M.; Cerrato, P. Hypertension and Cerebrovascular Damage. Atherosclerosis 2009, 205, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.Y.; Snyder, P.J.; Wu, W.-C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic Relationship between Alzheimer’s Disease, Cerebrovascular Disease, and Cardiovascular Risk: A Review and Synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Skoog, I. Vascular Aspects in Alzheimer’s Disease. In Advances in Dementia Research; Jellinger, K., Schmidt, R., Windisch, M., Eds.; Springer: Vienna, Austria, 2000; pp. 37–43. [Google Scholar]
- Tatsumi, Y.; Ohkubo, T. Hypertension with Diabetes Mellitus: Significance from an Epidemiological Perspective for Japanese. Hypertens. Res. 2017, 40, 795–806. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Li, W.; Sun, L.; Li, G.; Xiao, S. Prevalence, Influence Factors and Cognitive Characteristics of Mild Cognitive Impairment in Type 2 Diabetes Mellitus. Front. Aging Neurosci. 2019, 10, 180. [Google Scholar] [CrossRef]
- Husain, K.H.; Sarhan, S.F.; AlKhalifa, H.K.A.A.; Buhasan, A.; Moin, A.S.M.; Butler, A.E. Dementia in Diabetes: The Role of Hypoglycemia. Int. J. Mol. Sci. 2023, 24, 9846. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive Decline and Dementia in Diabetes Mellitus: Mechanisms and Clinical Implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Xu, W.L.; Von Strauss, E.; Qiu, C.X.; Winblad, B.; Fratiglioni, L. Uncontrolled Diabetes Increases the Risk of Alzheimer’s Disease: A Population-Based Cohort Study. Diabetologia 2009, 52, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Peters, S.A.E.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2015, 39, 300–307. [Google Scholar] [CrossRef]
- Gao, L.; Matthews, F.E.; Sargeant, L.A.; Brayne, C. An Investigation of the Population Impact of Variation in HbA 1clevels in Older People in England and Wales: From a Population Based Multi-Centre Longitudinal Study. BMC Public Health 2008, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimäki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640–1649. [Google Scholar] [CrossRef]
- Gungabissoon, U.; Broadbent, M.; Perera, G.; Ashworth, M.; Galwey, N.; Stewart, R. The Impact of Dementia on Diabetes Control: An Evaluation of HbA1c Trajectories and Care Outcomes in Linked Primary and Specialist Care Data. J. Am. Med. Dir. Assoc. 2022, 23, 1555–1563.e4. [Google Scholar] [CrossRef]
- Crane, P.K.; Walker, R.; Hubbard, R.A.; Li, G.; Nathan, D.M.; Zheng, H.; Haneuse, S.; Craft, S.; Montine, T.J.; Kahn, S.E.; et al. Glucose Levels and Risk of Dementia. N. Engl. J. Med. 2013, 369, 540–548. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, X.; Zhuo, X.; Zhang, P. Annual Total Medical Expenditures Associated with Hypertension by Diabetes Status in U.S. Adults. Am. J. Prev. Med. 2017, 53, S182–S189. [Google Scholar] [CrossRef] [PubMed]
- Ruthirakuhan, M.; Swardfager, W.; Xiong, L.; MacIntosh, B.J.; Rabin, J.S.; Lanctôt, K.L.; Ottoy, J.; Ramirez, J.; Keith, J.; Black, S.E. Investigating the Impact of Hypertension with and without Diabetes on Alzheimer’s Disease Risk: A Clinico-Pathological Study. Alzheimer’s Dement. 2024, 20, 2766–2778. [Google Scholar] [CrossRef]
- Solomon, A.; Kåreholt, I.; Ngandu, T.; Wolozin, B.; MacDonald, S.W.S.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Serum Total Cholesterol, Statins and Cognition in Non-Demented Elderly. Neurobiol. Aging 2009, 30, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Kivipelto, M.; Wolozin, B.; Zhou, J.; Whitmer, R.A. Midlife Serum Cholesterol and Increased Risk of Alzheimer’s and Vascular Dementia Three Decades Later. Dement. Geriatr. Cogn. Disord. 2009, 28, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Liu, C.; Tong, J.; Ouyang, W.; Hu, S.; Tang, Y. Higher Total Cholesterol Concentration May Be Associated with Better Cognitive Performance among Elderly Females. Nutrients 2022, 14, 4198. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Ticinesi, A.; Prati, B.; Nouvenne, A.; Meschi, T. Uric Acid and Cognitive Function in Older Individuals. Nutrients 2018, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Soumaré, A.; Bardin, T.; Perez-Ruiz, F.; Debette, S.; Richette, P. Uric Acid and Incident Dementia over 12 Years of Follow-up: A Population-Based Cohort Study. Ann. Rheum. Dis. 2018, 77, 328–335. [Google Scholar] [CrossRef]
- Mollinedo-Cardalda, I.; Ferreira, M.; Bezerra, P.; Cancela-Carral, J.M. Health-Related Functional Fitness within the Elderly Communities of Five European Countries: The in Common Sports Study. Int. J. Environ. Res. Public Health 2021, 18, 12810. [Google Scholar] [CrossRef]
- Statistics Poland. Demographic Situation of Małopolskie Voivodship in 2024; Statistical Office in Kraków: Kraków, Poland, 2024. Available online: https://krakow.stat.gov.pl/en/publications/population/demographic-situation-of-malopolskie-voivodship-in-2024,2,19.html (accessed on 15 August 2025).
- Gallegos, M.; Morgan, M.L.; Cervigni, M.; Martino, P.; Murray, J.; Calandra, M.; Razumovskiy, A.; Caycho-Rodríguez, T.; Gallegos, W.L.A. 45 Years of the Mini-Mental State Examination (MMSE): A Perspective from Ibero-America. Dement. Neuropsychol. 2022, 16, 384–387. [Google Scholar] [CrossRef]
- Edemekong, P.F.; Bomgaars, D.L.; Sukumaran, S.; Schoo, C. Activities of Daily Living; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Aalto, A.-M.; Elovainio, M.; Kivimäki, M.; Uutela, A.; Pirkola, S. The Beck Depression Inventory and General Health Questionnaire as Measures of Depression in the General Population: A Validation Study Using the Composite International Diagnostic Interview as the Gold Standard. Psychiatry Res. 2012, 197, 163–171. [Google Scholar] [CrossRef]
- Hsieh, S.; McGrory, S.; Leslie, F.; Dawson, K.; Ahmed, S.; Butler, C.R.; Rowe, J.B.; Mioshi, E.; Hodges, J.R. The Mini-Addenbrooke’s Cognitive Examination: A New Assessment Tool for Dementia. Dement. Geriatr. Cogn. Disord. 2015, 39, 1–11. [Google Scholar] [CrossRef]
- Homza, M.; Machaczka, O.; Porzer, M.; Kozak, M.; Plasek, J.; Sipula, D. Comparison of Different Methods of ABI Acquisition for Detection of Peripheral Artery Disease in Diabetic Patients. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2019, 163, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Day, T.G.; Park, M.; Kinra, S. The Association between Blood Pressure and Carotid Intima-Media Thickness in Children: A Systematic Review. Cardiol. Young 2017, 27, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Plavnik, F.L.; Ajzen, S.; Kohlmann, O.J.; Tavares, A.; Zanella, M.T.; Ribeiro, A.B.; Ramos, O.L. Intima-Media Thickness Evaluation by B-Mode Ultrasound. Correlation with Blood Pressure Levels and Cardiac Structures. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2000, 33, 55–64. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Shah, N.S.; Vidal, J.-S.; Masaki, K.; Petrovitch, H.; Ross, G.W.; Tilley, C.; DeMattos, R.B.; Tracy, R.P.; White, L.R.; Launer, L.J. Midlife Blood Pressure, Plasma β-Amyloid, and the Risk for Alzheimer Disease. Hypertension 2012, 59, 780–786. [Google Scholar] [CrossRef]
- Mizoi, M.; Yoshida, M.; Saiki, R.; Waragai, M.; Uemura, K.; Akatsu, H.; Kashiwagi, K.; Igarashi, K. Distinction between Mild Cognitive Impairment and Alzheimer’s Disease by CSF Amyloid Β40 and Β42, and Protein-Conjugated Acrolein. Clin. Chim. Acta 2014, 430, 150–155. [Google Scholar] [CrossRef]
- Lopez, O.L.; Klunk, W.E.; Mathis, C.A.; Snitz, B.E.; Chang, Y.; Tracy, R.P.; Kuller, L.H. Relationship of Amyloid-Β1-42 in Blood and Brain Amyloid: Ginkgo Evaluation of Memory Study. Brain Commun. 2020, 2, fcz038. [Google Scholar] [CrossRef]
- Gallardo, G.; Holtzman, D.M. Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease. In Tau Biology; Takashima, A., Wolozin, B., Buee, L., Eds.; Springer: Singapore, 2019; pp. 187–203. ISBN 978-981-32-9358-8. [Google Scholar]
- Iqbal, G.; Braidy, N.; Ahmed, T. Blood-Based Biomarkers for Predictive Diagnosis of Cognitive Impairment in a Pakistani Population. Front. Aging Neurosci. 2020, 12, 223. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Doecke, J.D.; Thota, R.; Villemagne, V.L.; Doré, V.; Singh, A.K.; Wang, P.; Rainey-Smith, S.; Fowler, C.; et al. Plasma Aβ42/40 Ratio, p-Tau181, GFAP, and NfL across the Alzheimer’s Disease Continuum: A Cross-Sectional and Longitudinal Study in the AIBL Cohort. Alzheimer’s Dement. 2023, 19, 1117–1134. [Google Scholar] [CrossRef]
- Pérez-Grijalba, V.; Romero, J.; Pesini, P.; Sarasa, L.; Monleón, I.; San-José, I.; Arbizu, J.; Martínez-Lage, P.; Munuera, J.; Ruiz, A.; et al. Plasma Aβ42/40 Ratio Detects Early Stages of Alzheimer’s Disease and Correlates with CSF and Neuroimaging Biomarkers in the AB255 Study. J. Prev. Alzheimer’s Dis. 2019, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Doecke, J.D.; Pérez-Grijalba, V.; Fandos, N.; Fowler, C.; Villemagne, V.L.; Masters, C.L.; Pesini, P.; Sarasa, M. Total Aβ(42)/Aβ(40) Ratio in Plasma Predicts Amyloid-PET Status, Independent of Clinical AD Diagnosis. Neurology 2020, 94, e1580–e1591. [Google Scholar] [CrossRef]
- Jang, H.; Kim, J.S.; Lee, H.J.; Kim, C.-H.; Na, D.L.; Kim, H.J.; Allué, J.A.; Sarasa, L.; Castillo, S.; Pesini, P.; et al. Performance of the Plasma Aβ42/Aβ40 Ratio, Measured with a Novel HPLC-MS/MS Method, as a Biomarker of Amyloid PET Status in a DPUK-KOREAN Cohort. Alzheimer’s Res. Ther. 2021, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.-F.; Pai, M.-C.; Chen, T.-F.; Hu, C.-J.; Huang, L.-K.; Lin, W.-C.; Wu, C.-C.; Jeng, J.-S.; Blennow, K.; Sabbagh, M.N.; et al. Age-Dependent Relationship Between Plasma Aβ40 and Aβ42 and Total Tau Levels in Cognitively Normal Subjects. Front. Aging Neurosci. 2019, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Zecca, C.; Pasculli, G.; Tortelli, R.; Dell’Abate, M.T.; Capozzo, R.; Barulli, M.R.; Barone, R.; Accogli, M.; Arima, S.; Pollice, A.; et al. The Role of Age on Beta-Amyloid1–42 Plasma Levels in Healthy Subjects. Front. Aging Neurosci. 2021, 13, 698571. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhou, X.; Liu, Y.; Li, D.; Li, Y.; Zhang, T.; Fu, C.; Li, L.; Hu, Y.; Jiang, L. Serum Amyloid Beta 42 Levels Correlated with Metabolic Syndrome and Its Components. Front. Endocrinol. 2024, 15, 1278477. [Google Scholar] [CrossRef]
- Hermesdorf, M.; Esselmann, H.; Morgado, B.; Jahn-Brodmann, A.; Herrera-Rivero, M.; Wiltfang, J.; Berger, K. The Association of Body Mass Index and Body Composition with Plasma Amyloid Beta Levels. Brain Commun. 2023, 5, fcad263. [Google Scholar] [CrossRef]
- Meakin, P.J.; Coull, B.M.; Tuharska, Z.; McCaffery, C.; Akoumianakis, I.; Antoniades, C.; Brown, J.; Griffin, K.J.; Platt, F.; Ozber, C.H.; et al. Elevated Circulating Amyloid Concentrations in Obesity and Diabetes Promote Vascular Dysfunction. J. Clin. Investig. 2020, 130, 4104–4117. [Google Scholar] [CrossRef]
- Hall, L.G.; Czeczor, J.K.; Connor, T.; Botella, J.; De Jong, K.A.; Renton, M.C.; Genders, A.J.; Venardos, K.; Martin, S.D.; Bond, S.T.; et al. Amyloid Beta 42 Alters Cardiac Metabolism and Impairs Cardiac Function in Male Mice with Obesity. Nat. Commun. 2024, 15, 258. [Google Scholar] [CrossRef]
- Prendecki, M.; Florczak-Wyspianska, J.; Kowalska, M.; Ilkowski, J.; Grzelak, T.; Bialas, K.; Kozubski, W.; Dorszewska, J. APOE Genetic Variants and ApoE, MiR-107 and MiR-650 Levels in Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 106–116. [Google Scholar] [CrossRef]
- Juul Rasmussen, I.; Luo, J.; Frikke-Schmidt, R. Lipids, Lipoproteins, and Apolipoproteins: Associations with Cognition and Dementia. Atherosclerosis 2024, 398, 118614. [Google Scholar] [CrossRef]
- Alosco, M.L.; Gunstad, J.; Xu, X.; Clark, I.S.; Labbe, D.R.; Riskin-Jones, H.H.; Terrero, G.; Schwarz, N.F.; Walsh, E.G.; Poppas, A.; et al. The Impact of Hypertension on Cerebral Perfusion and Cortical Thickness in Older Adults. J. Am. Soc. Hypertens. 2015, 8, 561–570. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.R.; Seshadri, S. Blood Pressure from Mid- to Late Life and Risk of Incident Dementia. Neurology 2018, 91, 149. [Google Scholar] [CrossRef]
- Dolui, S.; Detre, J.A.; Gaussoin, S.A.; Herrick, J.S.; Wang, D.J.J.; Tamura, M.K.; Cho, M.E.; Haley, W.E.; Launer, L.J.; Punzi, H.A.; et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral Blood Flow: Secondary Analysis of the SPRINT MIND Randomized Clinical Trial. JAMA Neurol. 2022, 79, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Kivipelto, M.; Helkala, E.L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissien, A. Midlife Vascular Risk Factors and Alzheimer’s Disease in Later Life: Longitudinal, Population Based Study. Br. Med. J. 2001, 322, 1447–1451. [Google Scholar] [CrossRef]
- Israeli-Korn, S.D.; Masarwa, M.; Schechtman, E.; Abuful, A.; Strugatsky, R.; Avni, S.; Farrer, L.A.; Friedland, R.P.; Inzelberg, R. Hypertension Increases the Probability of Alzheimer’s Disease and of Mild Cognitive Impairment in an Arab Community in Northern Israel. Neuroepidemiology 2010, 34, 99–105. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Jiang, K.; Lin, F.; Zhu, T.; Khan, N.H.; Jiang, E. Pathophysiological Association of Alzheimer’s Disease and Hypertension: A Clinical Concern for Elderly Population. Clin. Interv. Aging 2023, 18, 713–728. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Amado, J.; Vidal, X.; Romero-Ortuno, R. Angiotensin-Receptor Blockers and the Risk of Alzheimer’s Disease: A Meta-Analysis. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 73–78. [Google Scholar] [CrossRef]
- Csiszar, A.; Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Koller, A.; Deak, F.; Sonntag, W.E.; Ungvari, Z. Synergistic Effects of Hypertension and Aging on Cognitive Function and Hippocampal Expression of Genes Involved in β-Amyloid Generation and Alzheimer’s Disease. Am. J. Physiol.—Hear. Circ. Physiol. 2013, 305, 1120–1130. [Google Scholar] [CrossRef]
- Ryan, L.; Hay, M.; Huentelman, M.J.; Duarte, A.; Rundek, T.; Levin, B.; Soldan, A.; Pettigrew, C.; Mehl, M.R.; Barnes, C.A. Precision Aging: Applying Precision Medicine to the Field of Cognitive Aging. Front. Aging Neurosci. 2019, 11, 128. [Google Scholar] [CrossRef]
- Carnevale, D.; Mascio, G.; D’Andrea, I.; Fardella, V.; Bell, R.D.; Branchi, I.; Pallante, F.; Zlokovic, B.; Yan, S.S.; Lembo, G. Hypertension Induces Brain β-Amyloid Accumulation, Cognitive Impairment, and Memory Deterioration Through Activation of Receptor for Advanced Glycation End Products in Brain Vasculature. Hypertension 2012, 60, 188–197. [Google Scholar] [CrossRef]
- Yanai, H. Causative Anti-Diabetic Drugs and the Underlying Clinical Factors for Hypoglycemia in Patients with Diabetes. World J. Diabetes 2015, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Falvey, C.M.; Hamilton, N.; Harris, T.B.; Simonsick, E.M.; Strotmeyer, E.S.; Shorr, R.I.; Metti, A.; Schwartz, A.V. Association between Hypoglycemia and Dementia in a Biracial Cohort of Older Adults with Diabetes Mellitus. JAMA Intern. Med. 2013, 173, 1300–1306. [Google Scholar] [CrossRef]
- Kawamura, T.; Umemura, T.; Hotta, N. Cognitive Impairment in Diabetic Patients: Can Diabetic Control Prevent Cognitive Decline? J. Diabetes Investig. 2012, 3, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced Glycation End-Products Decreases Expression of Endothelial Nitric Oxide Synthase through Oxidative Stress in Human Coronary Artery Endothelial Cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Barnes, C.; Huentelman, M.; Brinton, R.; Ryan, L. Hypertension and Age-Related Cognitive Impairment: Common Risk Factors and a Role for Precision Aging. Curr. Hypertens. Rep. 2020, 22, 80. [Google Scholar] [CrossRef]
- Zheng, T.; Qin, L.; Chen, B.; Hu, X.; Zhang, X.; Liu, Y.; Liu, H.; Qin, S.; Li, G.; Li, Q. Association of Plasma DPP4 Activity with Mild Cognitive Impairment in Elderly Patients with Type 2 Diabetes: Results from the GDMD Study in China. Diabetes Care 2016, 39, 1594–1601. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Boyd, G.W. The Role of Insulin-Like Growth Factors and Insulin-Like Growth Factor–Binding Proteins in the Nervous System. Biochem. Insights 2019, 12, 117862641984217. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Ding, G.; Davoodi-Bojd, E.; Li, Q.; Li, L.; Sadry, N.; Nedergaard, M.; Chopp, M.; Zhang, Z. Impairment of the Glymphatic System after Diabetes. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017, 37, 1326–1337. [Google Scholar] [CrossRef]
- Moloney, A.M.; Griffin, R.J.; Timmons, S.; O’Connor, R.; Ravid, R.; O’Neill, C. Defects in IGF-1 Receptor, Insulin Receptor and IRS-1/2 in Alzheimer’s Disease Indicate Possible Resistance to IGF-1 and Insulin Signalling. Neurobiol. Aging 2010, 31, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Gontier, G.; George, C.; Chaker, Z.; Holzenberger, M.; Aïd, S. Blocking IGF Signaling in Adult Neurons Alleviates Alzheimer’s Disease Pathology through Amyloid-β Clearance. J. Neurosci. 2015, 35, 11500–11513. [Google Scholar] [CrossRef] [PubMed]
- Pelimanni, E.; Jehkonen, M. Type 2 Diabetes and Cognitive Functions in Middle Age: A Meta-Analysis. J. Int. Neuropsychol. Soc. 2019, 25, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gong, Z.; Ma, C.; Wang, Z.; Wang, K. Relationship between Glycemic Control and Cognitive Impairment: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2023, 15, 1126183. [Google Scholar] [CrossRef]
- Cukierman-Yaffe, T.; Gerstein, H.C.; Williamson, J.D.; Lazar, R.M.; Lovato, L.; Miller, M.E.; Coker, L.H.; Murray, A.; Sullivan, M.D.; Marcovina, S.M.; et al. Relationship Between Baseline Glycemic Control and Cognitive Function in Individuals With Type 2 Diabetes and Other Cardiovascular Risk Factors: The Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Trial. Diabetes Care 2009, 32, 221–226. [Google Scholar] [CrossRef]
- Cintoli, S.; Favilli, L.; Morganti, R.; Siciliano, G.; Ceravolo, R.; Tognoni, G. Verbal Fluency Patterns Associated with the Amnestic Conversion from Mild Cognitive Impairment to Dementia. Sci. Rep. 2024, 14, 2029. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Zhou, R.; Shang, S.; Dang, L.; Gao, L.; Chen, C.; Huo, K.; Wang, J.; Wang, J.; et al. The Relationships Between Lipid Accumulation Product Levels and Cognitive Decline Over 4 Years in a Rural Area of Xi’an, China. Front. Aging Neurosci. 2021, 13, 761886. [Google Scholar] [CrossRef]
- Sparks, D.L.; Kryscio, R.J.; Connor, D.J.; Sabbagh, M.N.; Sparks, L.M.; Lin, Y.; Liebsack, C. Cholesterol and Cognitive Performance in Normal Controls and the Influence of Elective Statin Use after Conversion to Mild Cognitive Impairment: Results in a Clinical Trial Cohort. Neurodegener. Dis. 2010, 7, 183–186. [Google Scholar] [CrossRef]
- Iwagami, M.; Qizilbash, N.; Gregson, J.; Douglas, I.; Johnson, M.; Pearce, N.; Evans, S.; Pocock, S. Blood Cholesterol and Risk of Dementia in More than 1·8 Million People over Two Decades: A Retrospective Cohort Study. Lancet Health Longev. 2021, 2, e498–e506. [Google Scholar] [CrossRef]
- Lipnicki, D.M.; Makkar, S.R.; Crawford, J.D.; Thalamuthu, A.; Kochan, N.A.; Lima-Costa, M.F.; Castro-Costa, E.; Ferri, C.P.; Brayne, C.; Stephan, B.; et al. Determinants of Cognitive Performance and Decline in 20 Diverse Ethno-Regional Groups: A COSMIC Collaboration Cohort Study. PLoS Med. 2019, 16, e1002853. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Robb, C.; Tonkin, A.M.; Lacaze, P.; Chong, T.T.-J.; Beilin, L.J.; Yu, C.; Watts, G.F.; Ryan, J.; Ernst, M.E.; et al. Association of Plasma High-Density Lipoprotein Cholesterol Level with Risk of Incident Dementia: A Cohort Study of Healthy Older Adults. Lancet Reg. Health—West. Pac. 2024, 43, 100963. [Google Scholar] [CrossRef]
- Dhakal, S.; Subhan, M.; Fraser, J.M.; Gardiner, K.; Macreadie, I. Simvastatin Efficiently Reduces Levels of Alzheimer’s Amyloid Beta in Yeast. Int. J. Mol. Sci. 2019, 20, 3531. [Google Scholar] [CrossRef]
- Höglund, K.; Wiklund, O.; Vanderstichele, H.; Eikenberg, O.; Vanmechelen, E.; Blennow, K. Plasma Levels of β-Amyloid(1-40), β-Amyloid(1-42), and Total β-Amyloid Remain Unaffected in Adult Patients With Hypercholesterolemia After Treatment With Statins. Arch. Neurol. 2004, 61, 333–337. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, M.; Yang, T.; Si, G. Uric Acid Levels and Risk of Cognitive Impairment: Dose-Response Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2023, 18, e0293832. [Google Scholar] [CrossRef]
- Shahin, L.; Patel, K.M.; Heydari, M.K.; Kesselman, M.M. Hyperuricemia and Cardiovascular Risk. Cureus 2021, 13, e14855. [Google Scholar] [CrossRef]
- Wang, L.; Tan, Z.; Wang, F.-Y.; Wu, W.-P.; Wu, J.-C. Gout/Hyperuricemia Reduces the Risk of Alzheimer’s Disease: A Meta-Analysis Based on Latest Evidence. Brain Behav. 2023, 13, e3207. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Shannon, J.; Frei, B.; Kaye, J.A.; Quinn, J.F. Uric Acid as a CNS Antioxidant. J. Alzheimer’s Dis. 2010, 19, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Cheng, R.-J.; Xia, Z.-J.; Zhang, Q.-P.; Liu, Y. Risk of Dementia in Gout and Hyperuricaemia: A Meta-Analysis of Cohort Studies. BMJ Open 2021, 11, e041680. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, S.; Liang, Y.; Zhang, X.; Zhang, R.; Kang, K.; Qu, H.; Xu, Y.; Zhao, C.; Zhao, M. Serum Uric Acid and the Risk of Dementia: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 625690. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C. High Plasma Uric Acid Concentration: Causes and Consequences. Diabetol. Metab. Syndr. 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Church, T.S.; Meriwether, R.A.; Lobelo, F.; Blair, S.N. Uric Acid and the Development of Metabolic Syndrome in Women and Men. Metabolism 2008, 57, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Razmi, M.; Rafiee, M.; Watts, G.F.; Sahebkar, A. The Effect of Statin Therapy on Serum Uric Acid Levels: A Systematic Review and Meta-Analysis. Curr. Med. Chem. 2024, 31, 1726–1739. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Noda, Y.; Shinagawa, S.; Chung, J.K.; Sawada, K.; Ogyu, K.; Tarumi, R.; Tsugawa, S.; Miyazaki, T.; Yamagata, B.; et al. Effect of Education on Alzheimer’s Disease-Related Neuroimaging Biomarkers in Healthy Controls, and Participants with Mild Cognitive Impairment and Alzheimer’s Disease: A Cross-Sectional Study. J. Alzheimer’s Dis. 2018, 63, 861–869. [Google Scholar] [CrossRef]
- Mukadam, N.; Wolters, F.J.; Walsh, S.; Wallace, L.; Brayne, C.; Matthews, F.E.; Sacuiu, S.; Skoog, I.; Seshadri, S.; Beiser, A.; et al. Changes in Prevalence and Incidence of Dementia and Risk Factors for Dementia: An Analysis from Cohort Studies. Lancet Public Health 2024, 9, e443–e460. [Google Scholar] [CrossRef]
- Mollalo, A.; Kramer, M.; Cutty, M.; Hoseini, B. Systematic Review and Meta-Analysis of Rural-Urban Disparities in Alzheimer’s Disease Dementia Prevalence. J. Prev. Alzheimer’s Dis. 2025, 12, 100305. [Google Scholar] [CrossRef]
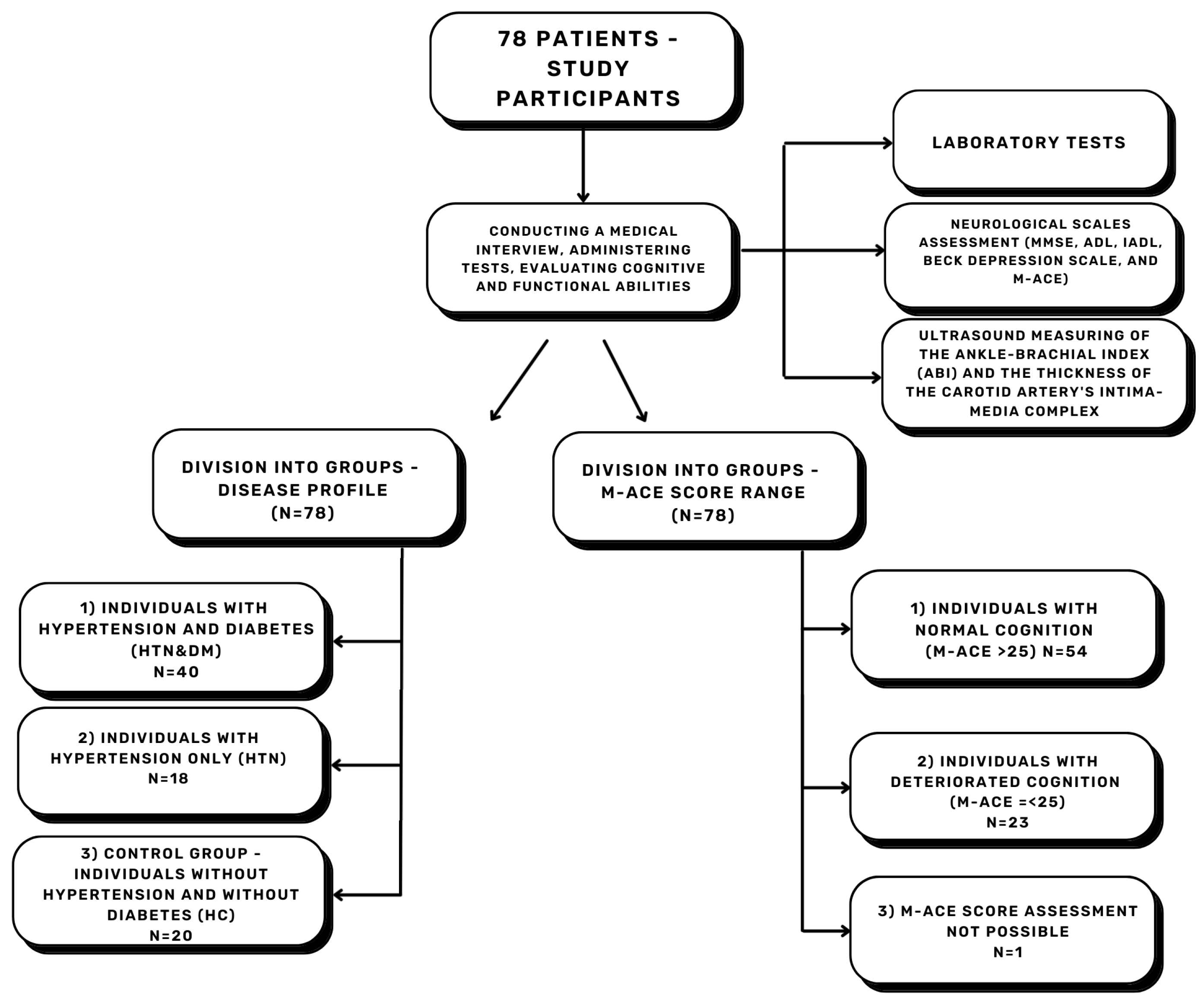

| Parameter | Description | Total (%) | HTN&DM (%) | HTN (%) | HC (%) |
|---|---|---|---|---|---|
| N | Total | 78 | 40 (47.1%) | 18(21.2%) | 20 (23.5%) |
| women | 50 (64.1%) | 26 (65%) | 11 (61.1%) | 13 (65%) | |
| Median age [years] | 71 | 72.5 | 69 | 70.5 | |
| Professional activity | physical | 53 (67.9%) | 30 (75%) | 11 (61.1%) | 12 (60%) |
| mental | 25 (32.1%) | 10 (25%) | 7 (38.9%) | 8 (40%) | |
| Diet | none | 21 (26.9%) | 6 (15%) | 3 (16.7%) | 12 (60%) |
| limited simple sugars | 5 (6.41%) | 3 (7.5%) | 0 | 2 (10%) | |
| limited animal fats | 11 (14.1%) | 0 | 0 | 5 (25%) | |
| limited simple sugars and animal fats | 41 (52.6%) | 31 (77.5%) | 9 (50%) | 1 (5%) | |
| Body mass | underweight | 0 | 0 | 0 | 0 |
| normal range | 9 (11.5%) | 4 (10%) | 0 | 5 (25%) | |
| overweight | 31 (39.74%) | 15 (37.5%) | 9 (50%) | 7 (35%) | |
| obese class I (BMI 30.0–34.9) | 27 (34.6%) | 13 (32.5%) | 9 (50%) | 5 (25%) | |
| obese class II (BMI 35.0–39.9) | 9 (11.5%) | 6 (15%) | 0 | 3 (15%) | |
| obese class III (BMI ≥ 40) | 2 (2.6%) | 2 (5%) | 0 | 0 | |
| Addictions | present tobacco smoking | 13 (16.7%) | 4 (10%) | 4 (22.2%) | 5 (25%) |
| history of tobacco smoking | 36 (46.15%) | 19 (47.5%) | 9 (50%) | 8 (40%) | |
| regular alcohol consumption | 20 (25.6%) | 10 (25%) | 5 (27.8%) | 5 (25%) | |
| Social conditions | good | 78 (100%) | 40 (100%) | 18 (100%) | 20 (100%) |
| Contact with close relatives | more than 3 times a week | 72 (92.3%) | 37 (92.5%) | 16 (88.9%) | 19 (95%) |
| maximum 3 times a week | 5 (6.4%) | 2 (5%) | 2 (11.1%) | 1 (5%) | |
| loneliness | 1 (1.3%) | 1 (2.5%) | 0 | 0 | |
| Accommodation | with close relative | 69 (88.5%) | 35 (87.5%) | 15 (83.3%) | 19 (95%) |
| alone | 9 (11.5%) | 5 (12.5%) | 3 (16.7%) | 1 (5%) | |
| closed care facility | 0 | 0 | 0 | 0 | |
| Education | basic | 23 (29.5%) | 13 (32.5%) | 3 (16.7%) | 7 (35%) |
| professional | 31 (39.7%) | 18 (45%) | 8 (44.4%) | 5 (25%) | |
| medium | 14 (17.9%) | 4 (10%) | 5 (27.8%) | 5 (25%) | |
| post-secondary | 5 (6.4%) | 3 (7.5%) | 1 (5.6%) | 1 (5%) | |
| higher | 5 (6.4%) | 2 (5%) | 1 (5.6%) | 2 (10%) | |
| High school certificate | yes | 21 (26.9%) | 8 (20%) | 6 (33.3%) | 7 (35%) |
| Parameter | Study Group | n | Median Value | Lower Quartile | Upper Quartile | 95% CI Lower | 95% CI Upper | Effect Size (Cohen) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Years of education | NC | 54 | 11 | 10 | 13 | 0 | 3 | 0.717 | 0.015 |
| DC | 23 | 10 | 8 | 11 | |||||
| Duration of lipid disorders treatment [years] | NC | 28 | 10 | 4 | 13 | −9.500 | 2 | −0.491 | 0.04 |
| DC | 19 | 14 | 7 | 19 | |||||
| IMC L [mm] | NC | 54 | 0.9 | 0.8 | 1.1 | −0.300 | 0 | −0.691 | 0.005 |
| DC | 22 | 1.1 | 1.0 | 1.2 | |||||
| IMC R [mm] | NC | 54 | 0.9 | 0.8 | 1.1 | −0.300 | 0 | −0.671 | 0.01 |
| DC | 22 | 1.05 | 1.0 | 1.2 | |||||
| Aβ1-42 [pg/mL] | NC | 50 | 38.52 | 28.90 | 48.43 | −4.561 | −21.542 | 0.738 | 0.02 |
| DC | 20 | 27.35 | 15.13 | 44.97 | |||||
| Aβ42/40 | NC | 50 | 0.39 | 0.33 | 0.48 | 0.033 | 0.184 | 1.122 | <0.000 |
| DC | 20 | 0.29 | 0.25 | 0.36 | |||||
| apoE [μg/mL] | NC | 50 | 125.0 | 94.15 | 125.0 | 24.35 | 77.600 | 0.805 | 0.002 |
| DC | 20 | 65.73 | 44.28 | 104.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zachara, R.; Gendosz de Carrillo, D.; Wlaszczuk, A.; Gorzkowska, A.; Mazur, W.; Jedrzejowska-Szypulka, H. The Cognitive Changes Among Patients over 65 Years of Age in a Rural Area—The Preliminary Report of Protective and Predisposing Factors. Neurol. Int. 2025, 17, 180. https://doi.org/10.3390/neurolint17110180
Zachara R, Gendosz de Carrillo D, Wlaszczuk A, Gorzkowska A, Mazur W, Jedrzejowska-Szypulka H. The Cognitive Changes Among Patients over 65 Years of Age in a Rural Area—The Preliminary Report of Protective and Predisposing Factors. Neurology International. 2025; 17(11):180. https://doi.org/10.3390/neurolint17110180
Chicago/Turabian StyleZachara, Radoslaw, Daria Gendosz de Carrillo, Adam Wlaszczuk, Agnieszka Gorzkowska, Wiktoria Mazur, and Halina Jedrzejowska-Szypulka. 2025. "The Cognitive Changes Among Patients over 65 Years of Age in a Rural Area—The Preliminary Report of Protective and Predisposing Factors" Neurology International 17, no. 11: 180. https://doi.org/10.3390/neurolint17110180
APA StyleZachara, R., Gendosz de Carrillo, D., Wlaszczuk, A., Gorzkowska, A., Mazur, W., & Jedrzejowska-Szypulka, H. (2025). The Cognitive Changes Among Patients over 65 Years of Age in a Rural Area—The Preliminary Report of Protective and Predisposing Factors. Neurology International, 17(11), 180. https://doi.org/10.3390/neurolint17110180






