Autism Spectrum Disorder: The Cerebellum, Genes, and Pathways
Abstract
1. Autism Spectrum Disorder (ASD)
2. Cerebellar Structure and Function
3. Cerebellum in Human Studies of Autism
4. Cerebellar Development in ASD
5. The Genetic Basis of ASD
6. Non-Genetic Factors That Combine with Genetic Susceptibility in ASD
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Volpe, J.J. Cerebellum of the premature infant: Rapidly developing, vulnerable, clinically important. J. Child Neurol. 2009, 24, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Commentary—Cerebellar underdevelopment in the very preterm infant: Important and underestimated source of cognitive deficits. J. Neonatal Perinat. Med. 2021, 14, 451–456. [Google Scholar] [CrossRef]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.E.; Mandell, D.S.; Schultz, R.T. Autism. Lancet 2009, 374, 1627–1638. [Google Scholar] [CrossRef]
- Nimbley, E.; Golds, L.; Sharpe, H.; Gillespie-Smith, K.; Duffy, F. Sensory processing and eating behaviours in autism: A systematic review. Eur. Eat. Disord. Rev. 2022, 30, 538–559. [Google Scholar] [CrossRef]
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Marco, E.J.; Hinkley, L.B.N.; Hill, S.S.; Nagarajan, S.S. Sensory processing in autism: A review of neurophysiologic findings. Pediatr. Res. 2011, 69, 48R–54R. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- Lainhart, J.E.; Bigler, E.D.; Bocian, M.; Coon, H.; Dinh, E.; Dawson, G.; Deutsch, C.K.; Dunn, M.; Estes, A.; Tager-Flusberg, H.; et al. Head circumference and height in autism: A study by the Collaborative Program of Excellence in Autism. Am. J. Med. Genet. A 2006, 140, 2257–2274. [Google Scholar] [CrossRef]
- Sacco, R.; Gabriele, S.; Persico, A.M. Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Res. 2015, 234, 239–251. [Google Scholar] [CrossRef]
- Courchesne, E.; Mouton, P.R.; Calhoun, M.E.; Semendeferi, K.; Ahrens-Barbeau, C.; Hallet, M.J.; Barnes, C.C.; Pierce, K. Neuron number and size in prefrontal cortex of children with autism. J. Am. Med. Assoc. 2011, 306, 2001–2010. [Google Scholar] [CrossRef]
- Shaw, K.A.; Williams, S.; Patrick, M.E.; Valencia-Prado, M.; Durkin, M.S.; Howerton, E.M.; Ladd-Acosta, C.M.; Pas, E.T.; Bakian, A.V.; Bartholomew, P.; et al. Prevalence and Early Identification of Autism Spectrum Disorder Among Children Aged 4 and 8 Years—Autism and Developmental Disabilities Monitoring Network, 16 Sites, United States, 2022. MMWR Surveill. Summ. 2025, 74, 1–22. [Google Scholar] [CrossRef]
- Rakic, P.; Bourgeois, J.P.; Eckenhoff, M.F.; Zecevic, N.; Goldman-Rakic, P.S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 1986, 232, 232–235. [Google Scholar] [CrossRef]
- Petanjek, Z.; Judaš, M.; Šimic, G.; Rasin, M.R.; Uylings, H.B.M.; Rakic, P.; Kostovic, I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 13281–13286. [Google Scholar] [CrossRef]
- Kilpatrick, S.; Irwin, C.; Singh, K.K. Human pluripotent stem cell (hPSC) and organoid models of autism: Opportunities and limitations. Transl. Psychiatry 2023, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Kanupriya, K.; Pal Verma, S.; Sharma, V.; Mishra, I.; Mishra, R. Advances in Human Brain Organoids: Methodological Innovations and Future Directions for Drug Discovery. Curr. Drug Res. Rev. 2025, 17, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, J.; Bhattacharya, A. The Evolving Landscape of Functional Models of Autism Spectrum Disorder. Cells 2025, 14, 908. [Google Scholar] [CrossRef]
- Steinberg, Y.; Zoghbi, H.Y. Lessons learned from studying syndromic autism spectrum disorders. Nat. Neurosci. 2016, 19, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Weuring, W.; Geerligs, J.; Koeleman, B.P.C. Gene Therapies for Monogenic Autism Spectrum Disorders. Genes 2021, 12, 1667. [Google Scholar] [CrossRef]
- Moss, J.; Howlin, P. Autism spectrum disorders in genetic syndromes: Implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J. Intellect. Disabil. Res. 2009, 53, 852–873. [Google Scholar] [CrossRef]
- Bourgeron, T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus paper: Pathological role of the cerebellum in autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef]
- Becker, E.B.E.; Stoodley, C.J. Autism spectrum disorder and the cerebellum. Int. Rev. Neurobiol. 2013, 113, 1–34. [Google Scholar] [CrossRef]
- D’Mello, A.M.; Stoodley, C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci. 2015, 9, 408. [Google Scholar] [CrossRef]
- D’Mello, A.M.; Moore, D.M.; Crocetti, D.; Mostofsky, S.H.; Stoodley, C.J. Cerebellar gray matter differentiates children with early language delay in autism. Autism Res. 2016, 9, 1191–1204. [Google Scholar] [CrossRef]
- Stoodley, C.J.; D’Mello, A.M.; Ellegood, J.; Jakkamsetti, V.; Liu, P.; Nebel, M.B.; Gibson, J.M.; Kelly, E.; Meng, F.; Cano, C.A.; et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci. 2017, 20, 1744–1751. [Google Scholar] [CrossRef]
- Kelly, E.; Meng, F.; Fujita, H.; Morgado, F.; Kazemi, Y.; Rice, L.C.; Ren, C.; Escamilla, C.O.; Gibson, J.M.; Sajadi, S.; et al. Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits. Nat. Neurosci. 2020, 23, 1102–1110. [Google Scholar] [CrossRef]
- Roostaei, T.; Nazeri, A.; Sahraian, M.A.; Minagar, A. The Human Cerebellum: A Review of Physiologic Neuroanatomy. Neurol. Clin. 2014, 32, 859–869. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E. Physiology of the cerebellum. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 154, pp. 85–108. [Google Scholar] [CrossRef]
- Farini, D.; Cesari, E.; Weatheritt, R.J.; La Sala, G.; Naro, C.; Pagliarini, V.; Bonvissuto, D.; Medici, V.; Guerra, M.; Di Pietro, C.; et al. A Dynamic Splicing Program Ensures Proper Synaptic Connections in the Developing Cerebellum. Cell Rep. 2020, 31, 107703. [Google Scholar] [CrossRef] [PubMed]
- Farini, D.; Marazziti, D.; Geloso, M.C.; Sette, C. Transcriptome programs involved in the development and structure of the cerebellum. Cell. Mol. Life Sci. 2021, 78, 6431–6451. [Google Scholar] [CrossRef] [PubMed]
- Frosch, I.R.; Mittal, V.A.; D’Mello, A.M. Cerebellar Contributions to Social Cognition in ASD: A Predictive Processing Framework. Front. Integr. Neurosci. 2022, 16, 810425. [Google Scholar] [CrossRef]
- Dickson, P.E.; Cairns, J.; Goldowitz, D.; Mittleman, G. Cerebellar contribution to higher and lower order rule learning and cognitive flexibility in mice. Neuroscience 2017, 345, 99–109. [Google Scholar] [CrossRef]
- Carta, I.; Chen, C.H.; Schott, A.L.; Dorizan, S.; Khodakhah, K. Cerebellar modulation of the reward circuitry and social behavior. Science 2019, 363, eaav0581. [Google Scholar] [CrossRef]
- Chabrol, F.P.; Blot, A.; Mrsic-Flogel, T.D. Cerebellar Contribution to Preparatory Activity in Motor Neocortex. Neuron 2019, 103, 506–519.e4. [Google Scholar] [CrossRef]
- Deverett, B.; Kislin, M.; Tank, D.W.; Wang, S.S.-H. Cerebellar disruption impairs working memory during evidence accumulation. Nat. Commun. 2019, 10, 3128. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Guell, X.; Stoodley, C.J.; Halko, M.A. The Theory and Neuroscience of Cerebellar Cognition. Annu. Rev. Neurosci. 2019, 42, 337–364. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Broussard, G.J.; Kislin, M.; Yuede, C.M.; Zhang, S.; Dietmann, S.; Gabel, H.; Zhao, G.; Wang, S.S.-H.; et al. Transcriptomic mapping uncovers Purkinje neuron plasticity driving learning. Nature 2022, 605, 722–727. [Google Scholar] [CrossRef]
- Olson, I.R.; Hoffman, L.J.; Jobson, K.R.; Popal, H.S.; Wang, Y. Little brain, little minds: The big role of the cerebellum in social development. Dev. Cogn. Neurosci. 2023, 60, 101238. [Google Scholar] [CrossRef] [PubMed]
- Jobson, K.R.; Hoffman, L.J.; Metoki, A.; Popal, H.; Dick, A.S.; Reilly, J.; Olson, I.R. Language and the Cerebellum: Structural Connectivity to the Eloquent Brain. Neurobiol. Lang. 2024, 5, 652–675. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, M.; Doron, K. Cerebellum: Connections and Functions. Cerebellum 2008, 7, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009, 32, 413–434. [Google Scholar] [CrossRef]
- Sivalingam, A.M.; Pandian, A. Cerebellar Roles in Motor and Social Functions and Implications for ASD. Cerebellum 2024, 23, 2564–2574. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010, 46, 831–844. [Google Scholar] [CrossRef]
- Ciricugno, A.; Ferrari, C.; Battelli, L.; Cattaneo, Z. A chronometric study of the posterior cerebellum’s function in emotional processing. Curr. Biol. 2024, 34, 1844–1852.e3. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Pu, M.; Ma, Q.; Li, M.; Haihambo, N.; Baetens, K.; Deroost, N.; Baeken, C.; Heleven, E. The Involvement of the Posterior Cerebellum in Reconstructing and Predicting Social Action Sequences. Cerebellum 2022, 21, 733–741. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, S.; Cao, Y.; Cheng, P.; Lin, Y.; Sun, Z.; Jiang, W.; Du, Y. Abnormalities of Gray Matter Volume and Its Correlation with Clinical Symptoms in Adolescents with High-Functioning Autism Spectrum Disorder. Neuropsychiatr. Dis. Treat. 2022, 18, 717–730. [Google Scholar] [CrossRef]
- Christensen, Z.P.; Freedman, E.G.; Foxe, J.J. Autism is associated with in vivo changes in gray matter neurite architecture. Autism Res. 2024, 17, 2261–2277. [Google Scholar] [CrossRef]
- Verly, M.; Verhoeven, J.; Zink, I.; Mantini, D.; Peeters, R.; Deprez, S.; Emsell, L.; Boets, B.; Noens, I.; Steyaert, J.; et al. Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. Neuroimage Clin. 2014, 4, 374–382. [Google Scholar] [CrossRef]
- Khan, A.J.; Nair, A.; Keown, C.L.; Datko, M.C.; Lincoln, A.J.; Müller, R.-A. Cerebro-cerebellar Resting-State Functional Connectivity in Children and Adolescents with Autism Spectrum Disorder. Biol. Psychiatry 2015, 78, 625–634. [Google Scholar] [CrossRef]
- Alho, J.; Samuelsson, J.G.; Khan, S.; Mamashli, F.; Bharadwaj, H.; Losh, A.; McGuiggan, N.M.; Graham, S.; Nayal, Z.; Perrachione, T.K.; et al. Both stronger and weaker cerebro-cerebellar functional connectivity patterns during processing of spoken sentences in autism spectrum disorder. Hum. Brain Mapp. 2023, 44, 5810–5827. [Google Scholar] [CrossRef] [PubMed]
- Cakar, M.E.; Okada, N.J.; Cummings, K.K.; Jung, J.; Bookheimer, S.Y.; Dapretto, M.; Green, S.A. Functional connectivity of the sensorimotor cerebellum in autism: Associations with sensory over-responsivity. Front. Psychiatry 2024, 15, 1337921. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.J.; Liu, J.; Tsang, T.; Nosco, E.; McDonald, N.M.; Cummings, K.K.; Jung, J.; Patterson, G.; Bookheimer, S.Y.; Green, S.A.; et al. Atypical cerebellar functional connectivity at 9 months of age predicts delayed socio-communicative profiles in infants at high and low risk for autism. J. Child Psychol. Psychiatry 2022, 63, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- ten Donkelaar, H.J.; Lammens, M.; Wesseling, P.; Thijssen, H.O.M.; Renier, W.O. Development and developmental disorders of the human cerebellum. J. Neurol. 2003, 250, 1025–1036. [Google Scholar] [CrossRef]
- Leto, K.; Arancillo, M.; Becker, E.B.E.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef]
- Stoodley, C.J. The Cerebellum and Neurodevelopmental Disorders. Cerebellum 2016, 15, 34–37. [Google Scholar] [CrossRef]
- Courchesne, E. Neuroanatomic imaging in autism. Pediatrics 1991, 87, 781–790. [Google Scholar] [CrossRef]
- Ellegood, J.; Crawley, J.N. Behavioral and Neuroanatomical Phenotypes in Mouse Models of Autism. Neurotherapeutics 2015, 12, 521–533. [Google Scholar] [CrossRef] [PubMed]
- McKinney, W.S.; Kelly, S.E.; Unruh, K.E.; Shafer, R.L.; Sweeney, J.A.; Styner, M.; Mosconi, M.W. Cerebellar Volumes and Sensorimotor Behavior in Autism Spectrum Disorder. Front. Integr. Neurosci. 2022, 16, 821109. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.D.; Dickson, P.E.; McKimm, E.; Heck, D.H.; Goldowitz, D.; Blaha, C.D.; Mittleman, G. Reorganization of circuits underlying cerebellar modulation of prefrontal cortical dopamine in mouse models of autism spectrum disorder. Cerebellum 2013, 12, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Piochon, C.; Kloth, A.D.; Grasselli, G.; Titley, H.K.; Nakayama, H.; Hashimoto, K.; Wan, V.; Simmons, D.H.; Eissa, T.; Nakatani, J.; et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun. 2014, 5, 5586. [Google Scholar] [CrossRef]
- Kloth, A.D.; Badura, A.; Li, A.; Cherskov, A.; Connolly, S.G.; Giovannucci, A.; Bangash, M.A.; Grasselli, G.; Peñagarikano, O.; Piochon, C.; et al. Cerebellar associative sensory learning defects in five mouse autism models. eLife 2015, 4, e06085. [Google Scholar] [CrossRef]
- Fernández, M.; Sánchez-León, C.A.; Llorente, J.; Sierra-Arregui, T.; Knafo, S.; Márquez-Ruiz, J.; Peñagarikano, O. Altered Cerebellar Response to Somatosensory Stimuli in the Cntnap2 Mouse Model of Autism. eNeuro 2021, 8, ENEURO.0333-21.2021. [Google Scholar] [CrossRef]
- Hanzel, M.; Fernando, K.; Maloney, S.E.; Horn, Z.; Gong, S.; Mätlik, K.; Zhao, J.; Pasolli, H.A.; Heissel, S.; Dougherty, J.D.; et al. Mice lacking Astn2 have ASD-like behaviors and altered cerebellar circuit properties. Proc. Natl. Acad. Sci. USA 2024, 121, e2405901121. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- Minami, T.; Miyata, E.; Sakamoto, Y.; Yamazaki, H.; Ichida, S. Induction of metallothionein in mouse cerebellum and cerebrum with low-dose thimerosal injection. Cell Biol. Toxicol. 2010, 26, 143–152. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; Audhya, T.; King, P.G.; Sykes, L.K.; Geier, M.R. Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiol. Exp. 2012, 72, 113–153. [Google Scholar] [CrossRef]
- Kumar, A.; LaVoie, H.A.; DiPette, D.J.; Singh, U.S. Ethanol neurotoxicity in the developing cerebellum: Underlying mechanisms and implications. Brain Sci. 2013, 3, 941–963. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Tsai, P.T. Adaptive Prediction for Social Contexts: The Cerebellar Contribution to Typical and Atypical Social Behaviors. Annu. Rev. Neurosci. 2021, 44, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.; Medici, V.; Weatheritt, R.; Corvino, V.; Palacios, D.; Geloso, M.C.; Farini, D.; Sette, C. Fetal exposure to valproic acid dysregulates the expression of autism-linked genes in the developing cerebellum. Transl. Psychiatry 2023, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Manto, M.; Shaikh, A.G. Alcohol Toxicity in the Developing Cerebellum. Diagnostics 2024, 14, 1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.-H.; Kloth, A.D.; Badura, A. The cerebellum, sensitive periods, and autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Stevens, H.E.; Jones, K.L. Prenatal Stress, Maternal Immune Dysregulation, and Their Association With Autism Spectrum Disorders. Curr. Psychiatry Rep. 2018, 20, 76. [Google Scholar] [CrossRef]
- Zawadzka, A.; Cieślik, M.; Adamczyk, A. The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 11516. [Google Scholar] [CrossRef]
- Hampson, D.R.; Blatt, G.J. Autism spectrum disorders and neuropathology of the cerebellum. Front. Neurosci. 2015, 9, 420. [Google Scholar] [CrossRef]
- Tanaka, M. Dendrite formation of cerebellar Purkinje cells. Neurochem. Res. 2009, 34, 2078–2088. [Google Scholar] [CrossRef]
- Nishiyama, H. Learning-induced structural plasticity in the cerebellum. Int. Rev. Neurobiol. 2014, 117, 1–19. [Google Scholar] [CrossRef]
- Fernández Santoro, E.M.; Karim, A.; Warnaar, P.; De Zeeuw, C.I.; Badura, A.; Negrello, M. Purkinje cell models: Past, present and future. Front. Comput. Neurosci. 2024, 18, 1426653. [Google Scholar] [CrossRef]
- Bauman, M.; Kemper, T.L. Histoanatomic observations of the brain in early infantile autism. Neurology 1985, 35, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.; Luthert, P.; Dean, A.; Harding, B.; Janota, I.; Montgomery, M.; Rutter, M.; Lantos, P. A clinicopathological study of autism. Brain 1998, 121 Pt 5, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Halt, A.R.; Realmuto, G.; Earle, J.; Kist, D.A.; Thuras, P.; Merz, A. Purkinje cell size is reduced in cerebellum of patients with autism. Cell. Mol. Neurobiol. 2002, 22, 171–175. [Google Scholar] [CrossRef]
- Whitney, E.R.; Kemper, T.L.; Rosene, D.L.; Bauman, M.L.; Blatt, G.J. Density of cerebellar basket and stellate cells in autism: Evidence for a late developmental loss of Purkinje cells. J. Neurosci. Res. 2009, 87, 2245–2254. [Google Scholar] [CrossRef]
- Skefos, J.; Cummings, C.; Enzer, K.; Holiday, J.; Weed, K.; Levy, E.; Yuce, T.; Kemper, T.; Bauman, M. Regional alterations in purkinje cell density in patients with autism. PLoS ONE 2014, 9, e81255. [Google Scholar] [CrossRef]
- Wang, R.; Tan, J.; Guo, J.; Zheng, Y.; Han, Q.; So, K.-F.; Yu, J.; Zhang, L. Aberrant Development and Synaptic Transmission of Cerebellar Cortex in a VPA Induced Mouse Autism Model. Front. Cell. Neurosci. 2018, 12, 500. [Google Scholar] [CrossRef]
- Ma, S.Y.; Kwan, K.M. Size anomaly and alteration of GABAergic enzymes expressions in cerebellum of a valproic acid mouse model of autism. Behav. Brain Res. 2022, 428, 113896. [Google Scholar] [CrossRef]
- Martin, L.A.; Goldowitz, D.; Mittleman, G. Repetitive behavior and increased activity in mice with Purkinje cell loss: A model for understanding the role of cerebellar pathology in autism. Eur. J. Neurosci. 2010, 31, 544–555. [Google Scholar] [CrossRef]
- Xing, Y.; Sapuan, A.; Dineen, R.A.; Auer, D.P. Life span pigmentation changes of the substantia nigra detected by neuromelanin-sensitive MRI. Mov. Disord. 2018, 33, 1792–1799. [Google Scholar] [CrossRef]
- Yang, X.; Yin, H.; Wang, X.; Sun, Y.; Bian, X.; Zhang, G.; Li, A.; Cao, A.; Li, B.; Ebrahimi-Fakhari, D.; et al. Social Deficits and Cerebellar Degeneration in Purkinje Cell Scn8a Knockout Mice. Front. Mol. Neurosci. 2022, 15, 822129. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-Y.; Wang, X.-T.; Guo, J.-W.; Xu, F.-X.; Ma, K.-Y.; Wang, Z.-X.; Zhao, Y.; Xie, W.; Schonewille, M.; De Zeeuw, C.; et al. Aberrant outputs of cerebellar nuclei and targeted rescue of social deficits in an autism mouse model. Protein Cell 2024, 15, pwae040. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M. Genetic and environmental mouse models of autism reproduce the spectrum of the disease. J. Neural Transm. 2023, 130, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Chua, S.E.; Cheung, V.; Khong, P.L.; Tai, K.S.; Wong, T.K.W.; Ho, T.P.; McAlonan, G.M. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J. Child Psychol. Psychiatry 2009, 50, 1102–1112. [Google Scholar] [CrossRef]
- Hatten, M.E. Adding cognitive connections to the cerebellum. Science 2020, 370, 1411–1412. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Tseng, R.-Y.; Ni, H.-C.; Cocchi, L.; Chang, J.-C.; Hsu, M.-Y.; Tu, E.-N.; Wu, Y.-Y.; Chou, T.-L.; Gau, S.S.-F.; et al. White matter microstructural and morphometric alterations in autism: Implications for intellectual capabilities. Mol. Autism 2022, 13, 21. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- DiLiberti, J.H.; Farndon, P.A.; Dennis, N.R.; Curry, C.J. The fetal valproate syndrome. Am. J. Med. Genet. 1984, 19, 473–481. [Google Scholar] [CrossRef]
- Clayton-Smith, J.; Donnai, D. Fetal valproate syndrome. J. Med. Genet. 1995, 32, 724–727. [Google Scholar] [CrossRef]
- Nicolai, J.; Vles, J.S.H.; Aldenkamp, A.P. Neurodevelopmental delay in children exposed to antiepileptic drugs in utero: A critical review directed at structural study-bias. J. Neurol. Sci. 2008, 271, 1–14. [Google Scholar] [CrossRef]
- Bromley, R.L.; Mawer, G.E.; Briggs, M.; Cheyne, C.; Clayton-Smith, J.; García-Fiñana, M.; Kneen, R.; Lucas, S.B.; Shallcross, R.; Baker, G.A.; et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J. Neurol. Neurosurg. Psychiatry 2013, 84, 637–643. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Halt, A.R.; Stary, J.M.; Kanodia, R.; Schulz, S.C.; Realmuto, G.R. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol. Psychiatry 2002, 52, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.; Soghomonian, J.-J.; Blatt, G.J. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: Pathophysiological implications. Acta Neuropathol. 2007, 113, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, C.; Griswold, A.J.; Van Booven, D.J.; Kilander, M.B.C.; Frei, J.A.; Nestor, M.W.; Dykxhoorn, D.M.; Pericak-Vance, M.A.; Blatt, G.J. Transcriptomic analysis of isolated and pooled human postmortem cerebellar Purkinje cells in autism spectrum disorders. Front. Genet. 2022, 13, 944837. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Ruiz, A.; Leichnetz, G.R.; Hardy, S.G. Projections of the medial cerebellar nucleus to oculomotor-related midbrain areas in the rat: An anterograde and retrograde HRP study. J. Comp. Neurol. 1990, 296, 427–436. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshida, K.; Yoshikawa, H.; Kishimoto, Y.; Oka, H. The medial dorsal nucleus is one of the thalamic relays of the cerebellocerebral responses to the frontal association cortex in the monkey: Horseradish peroxidase and fluorescent dye double staining study. Brain Res. 1992, 579, 315–320. [Google Scholar] [CrossRef]
- Middleton, F.A.; Strick, P.L. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 2001, 21, 700–712. [Google Scholar] [CrossRef]
- Cusmano, D.M.; Mong, J.A. In utero exposure to valproic acid changes sleep in juvenile rats: A model for sleep disturbances in autism. Sleep 2014, 37, 1489–1499. [Google Scholar] [CrossRef]
- Olexová, L.; Štefánik, P.; Kršková, L. Increased anxiety-like behaviour and altered GABAergic system in the amygdala and cerebellum of VPA rats—An animal model of autism. Neurosci. Lett. 2016, 629, 9–14. [Google Scholar] [CrossRef]
- Win-Shwe, T.-T.; Nway, N.C.; Imai, M.; Lwin, T.-T.; Mar, O.; Watanabe, H. Social behavior, neuroimmune markers and glutamic acid decarboxylase levels in a rat model of valproic acid-induced autism. J. Toxicol. Sci. 2018, 43, 631–643. [Google Scholar] [CrossRef]
- Baizer, J.S. Neuroanatomy of autism: What is the role of the cerebellum? Cereb. Cortex 2024, 34, 94–103. [Google Scholar] [CrossRef]
- d’Oleire Uquillas, F.; Sefik, E.; Li, B.; Trotter, M.A.; Steele, K.A.; Seidlitz, J.; Gesue, R.; Latif, M.; Fasulo, T.; Zhang, V.; et al. Multimodal evidence for cerebellar influence on cortical development in autism: Structural growth amidst functional disruption. Mol. Psychiatry 2025, 30, 1558–1572. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, E.; Barlas, Y.; Ulgen, K.O. Circadian clock crosstalks with autism. Brain Behav. 2023, 13, e3273. [Google Scholar] [CrossRef] [PubMed]
- Gerhant, A.; Olajossy, M.; Olajossy-Hilkesberger, L. [Neuroanatomical, genetic and neurochemical aspects of infantile autism]. Psychiatr. Pol. 2013, 47, 1101–1111. [Google Scholar] [PubMed]
- Dusart, I.; Guenet, J.L.; Sotelo, C. Purkinje cell death: Differences between developmental cell death and neurodegenerative death in mutant mice. Cerebellum 2006, 5, 163–173. [Google Scholar] [CrossRef]
- Wang, T.; Morgan, J.I. The Purkinje cell degeneration (pcd) mouse: An unexpected molecular link between neuronal degeneration and regeneration. Brain Res. 2007, 1140, 26–40. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Duffin, C.A.; McFarland, R.; Vogel, M.W. Mechanisms of compartmental purkinje cell death and survival in the lurcher mutant mouse. Cerebellum 2011, 10, 504–514. [Google Scholar] [CrossRef]
- Cendelin, J. From mice to men: Lessons from mutant ataxic mice. Cerebellum Ataxias 2014, 1, 4. [Google Scholar] [CrossRef]
- King, M.; Hernandez-Castillo, C.R.; Poldrack, R.A.; Ivry, R.B.; Diedrichsen, J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 2019, 22, 1371–1378. [Google Scholar] [CrossRef]
- Kebschull, J.M.; Richman, E.B.; Ringach, N.; Friedmann, D.; Albarran, E.; Kolluru, S.S.; Jones, R.C.; Allen, W.E.; Wang, Y.; Cho, S.W.; et al. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 2020, 370, eabd5059. [Google Scholar] [CrossRef]
- Daskalakis, Z.J.; Christensen, B.K.; Fitzgerald, P.B.; Fountain, S.I.; Chen, R. Reduced cerebellar inhibition in schizophrenia: A preliminary study. Am. J. Psychiatry 2005, 162, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Q.; Zuo, C.; Zhao, L.; Hao, L. Longitudinal Changes of Cerebellar Thickness in Autism Spectrum Disorder. Neurosci. Lett. 2020, 728, 134949. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.; Escott-Price, V.; Legge, S.; Baker, E.; Singh, K.D.; Walters, J.T.R.; Caseras, X.; Anney, R.J.L. Genetic common variants associated with cerebellar volume and their overlap with mental disorders: A study on 33,265 individuals from the UK-Biobank. Mol. Psychiatry 2022, 27, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Castillo, A.; Boeckx, C. Insights into the genetic architecture of cerebellar lobules derived from the UK Biobank. Sci. Rep. 2024, 14, 9488. [Google Scholar] [CrossRef]
- Abdul, F.; Sreenivas, N.; Kommu, J.V.S.; Banerjee, M.; Berk, M.; Maes, M.; Leboyer, M.; Debnath, M. Disruption of circadian rhythm and risk of autism spectrum disorder: Role of immune-inflammatory, oxidative stress, metabolic and neurotransmitter pathways. Rev. Neurosci. 2022, 33, 93–109. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Schibler, U.; Gotic, I.; Saini, C.; Gos, P.; Curie, T.; Emmenegger, Y.; Sinturel, F.; Gosselin, P.; Gerber, A.; Fleury-Olela, F.; et al. Clock-Talk: Interactions between Central and Peripheral Circadian Oscillators in Mammals. Cold Spring Harb. Symp. Quant. Biol. 2015, 80, 223–232. [Google Scholar] [CrossRef]
- Geoffray, M.-M.; Nicolas, A.; Speranza, M.; Georgieff, N. Are circadian rhythms new pathways to understand Autism Spectrum Disorder? J. Physiol. Paris 2016, 110, 434–438. [Google Scholar] [CrossRef]
- Mendoza, J.; Pévet, P.; Felder-Schmittbuhl, M.-P.; Bailly, Y.; Challet, E. The cerebellum harbors a circadian oscillator involved in food anticipation. J. Neurosci. 2010, 30, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.F.; Rohde, K.; Møller, M. Circadian oscillations of molecular clock components in the cerebellar cortex of the rat. Chronobiol. Int. 2012, 29, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Bering, T.; Hertz, H.; Rath, M.F. The Circadian Oscillator of the Cerebellum: Triiodothyronine Regulates Clock Gene Expression in Granule Cells in vitro and in the Cerebellum of Neonatal Rats in vivo. Front. Physiol. 2021, 12, 706433. [Google Scholar] [CrossRef] [PubMed]
- Mordel, J.; Karnas, D.; Pévet, P.; Isope, P.; Challet, E.; Meissl, H. The Output Signal of Purkinje Cells of the Cerebellum and Circadian Rhythmicity. PLoS ONE 2013, 8, e58457. [Google Scholar] [CrossRef]
- Petrelli, F.; Pucci, L.; Bezzi, P. Astrocytes and Microglia and Their Potential Link with Autism Spectrum Disorders. Front. Cell. Neurosci. 2016, 10, 21. [Google Scholar] [CrossRef]
- Scuderi, C.; Verkhratsky, A. The role of neuroglia in autism spectrum disorders. Prog. Mol. Biol. Transl. Sci. 2020, 173, 301–330. [Google Scholar] [CrossRef]
- Gzielo, K.; Nikiforuk, A. Astroglia in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 11544. [Google Scholar] [CrossRef]
- Cantando, I.; Centofanti, C.; D’Alessandro, G.; Limatola, C.; Bezzi, P. Metabolic dynamics in astrocytes and microglia during post-natal development and their implications for autism spectrum disorders. Front. Cell. Neurosci. 2024, 18, 1354259. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Koyama, R.; Ikegaya, Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 2015, 100, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Talavera, Y.; Pérez-Rodríguez, M.; Prius-Mengual, J.; Rodríguez-Moreno, A. Neuronal and astrocyte determinants of critical periods of plasticity. Trends Neurosci. 2023, 46, 566–580. [Google Scholar] [CrossRef]
- Valles, S.L.; Singh, S.K.; Campos-Campos, J.; Colmena, C.; Campo-Palacio, I.; Alvarez-Gamez, K.; Caballero, O.; Jorda, A. Functions of Astrocytes under Normal Conditions and after a Brain Disease. Int. J. Mol. Sci. 2023, 24, 8434. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Park, J.; Chung, W.-S. Astrocyte-dependent circuit remodeling by synapse phagocytosis. Curr. Opin. Neurobiol. 2023, 81, 102732. [Google Scholar] [CrossRef]
- Sun, M.; You, H.; Hu, X.; Luo, Y.; Zhang, Z.; Song, Y.; An, J.; Lu, H. Microglia-Astrocyte Interaction in Neural Development and Neural Pathogenesis. Cells 2023, 12, 1942. [Google Scholar] [CrossRef]
- Edmonson, C.; Ziats, M.N.; Rennert, O.M. Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol. Autism 2014, 5, 3. [Google Scholar] [CrossRef]
- Bellamy, T.C. Interactions between Purkinje neurones and Bergmann glia. Cerebellum 2006, 5, 116–126. [Google Scholar] [CrossRef]
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Chrobak, A.A.; Soltys, Z. Bergmann Glia, Long-Term Depression, and Autism Spectrum Disorder. Mol. Neurobiol. 2017, 54, 1156–1166. [Google Scholar] [CrossRef]
- Yamada, K.; Fukaya, M.; Shibata, T.; Kurihara, H.; Tanaka, K.; Inoue, Y.; Watanabe, M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J. Comp. Neurol. 2000, 418, 106–120. [Google Scholar] [CrossRef]
- Lordkipanidze, T.; Dunaevsky, A. Purkinje cell dendrites grow in alignment with Bergmann glia. Glia 2005, 51, 229–234. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Q.; Wang, W.; Takano, T.; Nedergaard, M. Bergmann glia modulate cerebellar Purkinje cell bistability via Ca2+-dependent K+ uptake. Proc. Natl. Acad. Sci. USA 2012, 109, 7911–7916. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Sajdel-Sulkowska, E.M.; Xu, M.; Koibuchi, N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum 2009, 8, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Sajdel-Sulkowska, E.M.; Xu, M.; McGinnis, W.; Koibuchi, N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD). Cerebellum 2011, 10, 43–48. [Google Scholar] [CrossRef]
- Dong, D.; Zielke, H.R.; Yeh, D.; Yang, P. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism Res. 2018, 11, 1076–1090. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Rosenberg, R.E.; Law, J.K.; Yenokyan, G.; McGready, J.; Kaufmann, W.E.; Law, P.A. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch. Pediatr. Adolesc. Med. 2009, 163, 907–914. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Carlström, E.; Råstam, M.; Gillberg, C.; Anckarsäter, H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am. J. Psychiatry 2010, 167, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Towheed, A.; Luong, S.; Zucker, S.; Fethke, E. Clinical Discordance in Monozygotic Twins With Autism Spectrum Disorder. Cureus 2022, 14, e24813. [Google Scholar] [CrossRef] [PubMed]
- Vorstman, J.A.S.; Freitag, C.M.; Persico, A.M. From Genes to Therapy in Autism Spectrum Disorder. Genes 2022, 13, 1377. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors With Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef]
- Cheroni, C.; Caporale, N.; Testa, G. Autism spectrum disorder at the crossroad between genes and environment: Contributions, convergences, and interactions in ASD developmental pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef]
- Andrade, C. Maternal Cannabis Use in Pregnancy and Autism Spectrum Disorder or Attention-Deficit/Hyperactivity Disorder in Offspring. J. Clin. Psychiatry 2024, 86, 24f15717. [Google Scholar] [CrossRef]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef]
- Havdahl, A.; Niarchou, M.; Starnawska, A.; Uddin, M.; van der Merwe, C.; Warrier, V. Genetic contributions to autism spectrum disorder. Psychol. Med. 2021, 51, 2260–2273. [Google Scholar] [CrossRef]
- Chahrour, M.; O’Roak, B.J.; Santini, E.; Samaco, R.C.; Kleiman, R.J.; Manzini, M.C. Current Perspectives in Autism Spectrum Disorder: From Genes to Therapy. J. Neurosci. 2016, 36, 11402–11410. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, S.; Chahrour, M.H. Genomic strategies to untangle the etiology of autism: A primer. Autism Res. 2023, 16, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Salenius, K.; Väljä, N.; Thusberg, S.; Iris, F.; Ladd-Acosta, C.; Roos, C.; Nykter, M.; Fasano, A.; Autio, R.; Lin, J.; et al. Exploring autism spectrum disorder and co-occurring trait associations to elucidate multivariate genetic mechanisms and insights. BMC Psychiatry 2024, 24, 934. [Google Scholar] [CrossRef]
- Harrison, A.J.; Gamsiz, E.D.; Berkowitz, I.C.; Nagpal, S.; Jerskey, B.A. Genetic variation in the oxytocin receptor gene is associated with a social phenotype in autism spectrum disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168, 720–729. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, R.; Zhou, J.; Sun, S.; Di, Y.; Ren, W.; Tian, Y. RNA-Seq data on prefrontal cortex in valproic acid model of autism and control rats. Data Brief 2018, 18, 787–789. [Google Scholar] [CrossRef]
- Jin, X.; Simmons, S.K.; Guo, A.; Shetty, A.S.; Ko, M.; Nguyen, L.; Jokhi, V.; Robinson, E.; Oyler, P.; Curry, N.; et al. In vivo Perturb-Seq reveals neuronal and glial abnormalities associated with autism risk genes. Science 2020, 370, eaaz6063. [Google Scholar] [CrossRef]
- Morabito, S.; Reese, F.; Rahimzadeh, N.; Miyoshi, E.; Swarup, V. hdWGCNA identifies co-expression networks in high-dimensional transcriptomics data. Cell Rep. Methods 2023, 3, 100498. [Google Scholar] [CrossRef]
- Wamsley, B.; Bicks, L.; Cheng, Y.; Kawaguchi, R.; Quintero, D.; Margolis, M.; Grundman, J.; Liu, J.; Xiao, S.; Hawken, N.; et al. Molecular cascades and cell type-specific signatures in ASD revealed by single-cell genomics. Science 2024, 384, eadh2602. [Google Scholar] [CrossRef]
- van der Heijden, M.E.; Gill, J.S.; Sillitoe, R.V. Abnormal Cerebellar Development in Autism Spectrum Disorders. Dev. Neurosci. 2021, 43, 181–190. [Google Scholar] [CrossRef]
- Zoghbi, H.Y. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science 2003, 302, 826–830. [Google Scholar] [CrossRef]
- Garber, K. Neuroscience. Autism’s cause may reside in abnormalities at the synapse. Science 2007, 317, 190–191. [Google Scholar] [CrossRef]
- Nagappan-Chettiar, S.; Yasuda, M.; Johnson-Venkatesh, E.M.; Umemori, H. The molecular signals that regulate activity-dependent synapse refinement in the brain. Curr. Opin. Neurobiol. 2023, 79, 102692. [Google Scholar] [CrossRef]
- Monteiro, P.; Feng, G. SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017, 18, 147–157. [Google Scholar] [CrossRef]
- Wan, L.; Ai, J.-Q.; Yang, C.; Jiang, J.; Zhang, Q.-L.; Luo, Z.-H.; Huang, R.-J.; Tu, T.; Pan, A.; Tu, E.; et al. Expression of the Excitatory Postsynaptic Scaffolding Protein, Shank3, in Human Brain: Effect of Age and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 717263. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, S.; Pedro, M.T.; Wagner, J.; Schön, M.; Boeckers, T.M. Expression profiles of the autism-related SHANK proteins in the human brain. BMC Biol. 2023, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Böckers, T.M.; Segger-Junius, M.; Iglauer, P.; Bockmann, J.; Gundelfinger, E.D.; Kreutz, M.R.; Richter, D.; Kindler, S.; Kreienkamp, H.-J. Differential expression and dendritic transcript localization of Shank family members: Identification of a dendritic targeting element in the 3’ untranslated region of Shank1 mRNA. Mol. Cell. Neurosci. 2004, 26, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef]
- Sato, D.; Lionel, A.C.; Leblond, C.S.; Prasad, A.; Pinto, D.; Walker, S.; O’Connor, I.; Russell, C.; Drmic, I.E.; Hamdan, F.F.; et al. SHANK1 Deletions in Males with Autism Spectrum Disorder. Am. J. Hum. Genet. 2012, 90, 879–887. [Google Scholar] [CrossRef]
- Gong, X.; Wang, H. SHANK1 and autism spectrum disorders. Sci. China Life Sci. 2015, 58, 985–990. [Google Scholar] [CrossRef]
- Qiu, S.; Li, Y.; Bai, Y.; Shi, J.; Cui, H.; Gu, Y.; Ren, Y.; Zhao, Q.; Zhang, K.; Lu, M.; et al. SHANK1 polymorphisms and SNP-SNP interactions among SHANK family: A possible cue for recognition to autism spectrum disorder in infant age. Autism Res. 2019, 12, 375–383. [Google Scholar] [CrossRef]
- Sungur, A.Ö.; Vörckel, K.J.; Schwarting, R.K.W.; Wöhr, M. Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. J. Neurosci. Methods 2014, 234, 92–100. [Google Scholar] [CrossRef]
- Sungur, A.Ö.; Schwarting, R.K.W.; Wöhr, M. Early communication deficits in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. Autism Res. 2016, 9, 696–709. [Google Scholar] [CrossRef]
- Sungur, A.Ö.; Schwarting, R.K.W.; Wöhr, M. Behavioral phenotypes and neurobiological mechanisms in the Shank1 mouse model for autism spectrum disorder: A translational perspective. Behav. Brain Res. 2018, 352, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Du, Y.; Chen, L.; Liu, Y.; Xu, W.; Liu, Y.; Li, Y.; Leng, J.; Wang, Y.; Zhang, X.-Y.; et al. A recurrent SHANK1 mutation implicated in autism spectrum disorder causes autistic-like core behaviors in mice via downregulation of mGluR1-IP3R1-calcium signaling. Mol. Psychiatry 2022, 27, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, X.-Y.; Liu, Y.; Ma, Z.; Tao, S.; Li, Y.; Peng, R.; Wang, F.; Wang, J.; Feng, J.; et al. Downregulation of mGluR1-mediated signaling underlying autistic-like core symptoms in Shank1 P1812L-knock-in mice. Transl. Psychiatry 2023, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Ten Brinke, M.M.; Stedehouder, J.; Reinelt, C.M.; Wu, B.; Zhou, H.; Zhou, K.; Boele, H.-J.; Kushner, S.A.; Lee, M.G.; et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat. Commun. 2016, 7, 12627. [Google Scholar] [CrossRef]
- Nardi, L.; Chhabra, S.; Leukel, P.; Krueger-Burg, D.; Sommer, C.J.; Schmeisser, M.J. Neuroanatomical changes of ionotropic glutamatergic and GABAergic receptor densities in male mice modeling idiopathic and syndromic autism spectrum disorder. Front. Psychiatry 2023, 14, 1199097. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef]
- Uchino, S.; Waga, C. SHANK3 as an autism spectrum disorder-associated gene. Brain Dev. 2013, 35, 106–110. [Google Scholar] [CrossRef]
- Mashayekhi, F.; Mizban, N.; Bidabadi, E.; Salehi, Z. The association of SHANK3 gene polymorphism and autism. Minerva Pediatr. 2021, 73, 251–255. [Google Scholar] [CrossRef]
- Phelan, K.; Rogers, R.C.; Boccuto, L. Phelan-McDermid Syndrome-SHANK3 Related. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Mitz, A.R.; Boccuto, L.; Thurm, A. Evidence for common mechanisms of pathology between SHANK3 and other genes of Phelan-McDermid syndrome. Clin. Genet. 2024, 105, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.M.; Betancur, C.; Boeckers, T.M.; Bockmann, J.; Chaste, P.; Fauchereau, F.; Nygren, G.; Rastam, M.; Gillberg, I.C.; Anckarsäter, H.; et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007, 39, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Moessner, R.; Marshall, C.R.; Sutcliffe, J.S.; Skaug, J.; Pinto, D.; Vincent, J.; Zwaigenbaum, L.; Fernandez, B.; Roberts, W.; Szatmari, P.; et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am. J. Hum. Genet. 2007, 81, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Boccuto, L.; Lauri, M.; Sarasua, S.M.; Skinner, C.D.; Buccella, D.; Dwivedi, A.; Orteschi, D.; Collins, J.S.; Zollino, M.; Visconti, P.; et al. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur. J. Hum. Genet. 2013, 21, 310–316. [Google Scholar] [CrossRef]
- Jacot-Descombes, S.; Keshav, N.U.; Dickstein, D.L.; Wicinski, B.; Janssen, W.G.M.; Hiester, L.L.; Sarfo, E.K.; Warda, T.; Fam, M.M.; Harony-Nicolas, H.; et al. Altered synaptic ultrastructure in the prefrontal cortex of Shank3-deficient rats. Mol. Autism 2020, 11, 89. [Google Scholar] [CrossRef]
- Durand, C.M.; Perroy, J.; Loll, F.; Perrais, D.; Fagni, L.; Bourgeron, T.; Montcouquiol, M.; Sans, N. SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol. Psychiatry 2012, 17, 71–84. [Google Scholar] [CrossRef]
- Lee, J.; Chung, C.; Ha, S.; Lee, D.; Kim, D.-Y.; Kim, H.; Kim, E. Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front. Cell. Neurosci. 2015, 9, 94. [Google Scholar] [CrossRef]
- Kareklas, K.; Teles, M.C.; Dreosti, E.; Oliveira, R.F. Autism-associated gene shank3 is necessary for social contagion in zebrafish. Mol. Autism 2023, 14, 23. [Google Scholar] [CrossRef]
- Tian, R.; Li, Y.; Zhao, H.; Lyu, W.; Zhao, J.; Wang, X.; Lu, H.; Xu, H.; Ren, W.; Tan, Q.-Q.; et al. Modeling SHANK3-associated autism spectrum disorder in Beagle dogs via CRISPR/Cas9 gene editing. Mol. Psychiatry 2023, 28, 3739–3750. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, J.; Ke, Q.; Landman, R.; Yuan, J.; Chen, H.; Hayden, D.S.; Fisher, J.W.; Jiang, M.; Menegas, W.; et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature 2019, 570, 326–331. [Google Scholar] [CrossRef]
- Kshetri, R.; Beavers, J.O.; Hyde, R.; Ewa, R.; Schwertman, A.; Porcayo, S.; Richardson, B.D. Behavioral decline in Shank3Δex4-22 mice during early adulthood parallels cerebellar granule cell glutamatergic synaptic changes. Mol. Autism 2024, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Lehr, A.W.; Roche, K.W. Neuroligins and Neurodevelopmental Disorders: X-Linked Genetics. Front. Synaptic Neurosci. 2020, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Marth, L.; Krueger-Burg, D. Neuroligin-2 as a central organizer of inhibitory synapses in health and disease. Sci. Signal. 2020, 13, eabd8379. [Google Scholar] [CrossRef]
- Chen, J.; Yu, S.; Fu, Y.; Li, X. Synaptic proteins and receptors defects in autism spectrum disorders. Front. Cell. Neurosci. 2014, 8, 276. [Google Scholar] [CrossRef]
- Chih, B.; Engelman, H.; Scheiffele, P. Control of excitatory and inhibitory synapse formation by neuroligins. Science 2005, 307, 1324–1328. [Google Scholar] [CrossRef]
- Varoqueaux, F.; Aramuni, G.; Rawson, R.L.; Mohrmann, R.; Missler, M.; Gottmann, K.; Zhang, W.; Südhof, T.C.; Brose, N. Neuroligins determine synapse maturation and function. Neuron 2006, 51, 741–754. [Google Scholar] [CrossRef]
- Craig, A.M.; Kang, Y. Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 2007, 17, 43–52. [Google Scholar] [CrossRef]
- Bolliger, M.F.; Pei, J.; Maxeiner, S.; Boucard, A.A.; Grishin, N.V.; Südhof, T.C. Unusually rapid evolution of Neuroligin-4 in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 6421–6426. [Google Scholar] [CrossRef]
- Reissner, C.; Klose, M.; Fairless, R.; Missler, M. Mutational analysis of the neurexin/neuroligin complex reveals essential and regulatory components. Proc. Natl. Acad. Sci. USA 2008, 105, 15124–15129. [Google Scholar] [CrossRef]
- Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef]
- Koehnke, J.; Katsamba, P.S.; Ahlsen, G.; Bahna, F.; Vendome, J.; Honig, B.; Shapiro, L.; Jin, X. Splice form dependence of beta-neurexin/neuroligin binding interactions. Neuron 2010, 67, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M. Neuregulins and the shaping of synapses. Neuroscientist 2001, 7, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Marchot, P. The Neuroligins and Their Ligands: From Structure to Function at the Synapse. J. Mol. Neurosci. 2014, 53, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, J.J.; Kuzniewska, B.; Milek, J.; Urbanska, K.; Dziembowska, M. Neuroligin 1, 2, and 3 Regulation at the Synapse: FMRP-Dependent Translation and Activity-Induced Proteolytic Cleavage. Mol. Neurobiol. 2019, 56, 2741–2759. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.Y.; Liu, X.; Maxeiner, S.; Lee, S.-J.; Gokce, O.; Südhof, T.C. Neuroligins Sculpt Cerebellar Purkinje-Cell Circuits by Differential Control of Distinct Classes of Synapses. Neuron 2015, 87, 781–796. [Google Scholar] [CrossRef]
- Zhang, B.; Südhof, T.C. Neuroligins Are Selectively Essential for NMDAR Signaling in Cerebellar Stellate Interneurons. J. Neurosci. 2016, 36, 9070–9083. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Z.; Guo, S.; Han, Y.; Wang, X.; Ren, W.; Chen, J.; Zhen, H.; Nie, C.; Xing, K.-K.; et al. Astrocytic Neuroligin-3 influences gene expression and social behavior, but is dispensable for synapse number. Mol. Psychiatry 2024, 30, 84–96. [Google Scholar] [CrossRef]
- Fu, Z.; Washbourne, P.; Ortinski, P.; Vicini, S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J. Neurophysiol. 2003, 90, 3950–3957. [Google Scholar] [CrossRef]
- Blundell, J.; Tabuchi, K.; Bolliger, M.F.; Blaiss, C.A.; Brose, N.; Liu, X.; Südhof, T.C.; Powell, C.M. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009, 8, 114–126. [Google Scholar] [CrossRef]
- Blundell, J.; Blaiss, C.A.; Etherton, M.R.; Espinosa, F.; Tabuchi, K.; Walz, C.; Bolliger, M.F.; Südhof, T.C.; Powell, C.M. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J. Neurosci. 2010, 30, 2115–2129. [Google Scholar] [CrossRef]
- Dahlhaus, R.; El-Husseini, A. Altered neuroligin expression is involved in social deficits in a mouse model of the fragile X syndrome. Behav. Brain Res. 2010, 208, 96–105. [Google Scholar] [CrossRef]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Oliveira, G.; Coutinho, A.; Yang, C.; Feng, J.; Katz, C.; Sram, J.; Bockholt, A.; Jones, I.R.; Craddock, N.; et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol. Psychiatry 2005, 10, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Speed, H.E.; Masiulis, I.; Gibson, J.R.; Powell, C.M. Increased Cortical Inhibition in Autism-Linked Neuroligin-3R451C Mice Is Due in Part to Loss of Endocannabinoid Signaling. PLoS ONE 2015, 10, e0140638. [Google Scholar] [CrossRef] [PubMed]
- Ellegood, J.; Lerch, J.P.; Henkelman, R.M. Brain abnormalities in a Neuroligin3 R451C knockin mouse model associated with autism. Autism Res. 2011, 4, 368–376. [Google Scholar] [CrossRef]
- Etherton, M.; Földy, C.; Sharma, M.; Tabuchi, K.; Liu, X.; Shamloo, M.; Malenka, R.C.; Südhof, T.C. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. USA 2011, 108, 13764–13769. [Google Scholar] [CrossRef]
- Lai, E.S.K.; Nakayama, H.; Miyazaki, T.; Nakazawa, T.; Tabuchi, K.; Hashimoto, K.; Watanabe, M.; Kano, M. An Autism-Associated Neuroligin-3 Mutation Affects Developmental Synapse Elimination in the Cerebellum. Front. Neural Circuits 2021, 15, 676891. [Google Scholar] [CrossRef]
- Watanabe, T.; Kano, M. Molecular and cellular mechanisms of developmental synapse elimination in the cerebellum: Involvement of autism spectrum disorder-related genes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2024, 100, 508–523. [Google Scholar] [CrossRef]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef]
- Jaramillo, T.C.; Escamilla, C.O.; Liu, S.; Peca, L.; Birnbaum, S.G.; Powell, C.M. Genetic background effects in Neuroligin-3 mutant mice: Minimal behavioral abnormalities on C57 background. Autism Res. 2018, 11, 234–244. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.H.; Baldwin, K.T.; Enustun, E.; Ejikeme, T.; Ji, R.-R.; Eroglu, C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Veenstra-VanderWeele, J.; Cook, E.H. Molecular genetics of autism spectrum disorder. Mol. Psychiatry 2004, 9, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Christian, S.L. Genetics of autism spectrum disorders. Curr. Neurol. Neurosci. Rep. 2009, 9, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.; Soykan, T.; Falkenburger, B.; Hammer, M.; Patrizi, A.; Schmidt, K.-F.; Sassoè-Pognetto, M.; Löwel, S.; Moser, T.; Taschenberger, H.; et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc. Natl. Acad. Sci. USA 2011, 108, 3053–3058. [Google Scholar] [CrossRef]
- Jamain, S.; Radyushkin, K.; Hammerschmidt, K.; Granon, S.; Boretius, S.; Varoqueaux, F.; Ramanantsoa, N.; Gallego, J.; Ronnenberg, A.; Winter, D.; et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA 2008, 105, 1710–1715. [Google Scholar] [CrossRef]
- Zhang, B.; Gokce, O.; Hale, W.D.; Brose, N.; Südhof, T.C. Autism-associated neuroligin-4 mutation selectively impairs glycinergic synaptic transmission in mouse brainstem synapses. J. Exp. Med. 2018, 215, 1543–1553. [Google Scholar] [CrossRef]
- Marro, S.G.; Chanda, S.; Yang, N.; Janas, J.A.; Valperga, G.; Trotter, J.; Zhou, B.; Merrill, S.; Yousif, I.; Shelby, H.; et al. Neuroligin-4 Regulates Excitatory Synaptic Transmission in Human Neurons. Neuron 2019, 103, 617–626.e6. [Google Scholar] [CrossRef]
- Cast, T.P.; Boesch, D.J.; Smyth, K.; Shaw, A.E.; Ghebrial, M.; Chanda, S. An Autism-Associated Mutation Impairs Neuroligin-4 Glycosylation and Enhances Excitatory Synaptic Transmission in Human Neurons. J. Neurosci. 2021, 41, 392–407. [Google Scholar] [CrossRef]
- El-Kordi, A.; Winkler, D.; Hammerschmidt, K.; Kästner, A.; Krueger, D.; Ronnenberg, A.; Ritter, C.; Jatho, J.; Radyushkin, K.; Bourgeron, T.; et al. Development of an autism severity score for mice using Nlgn4 null mutants as a construct-valid model of heritable monogenic autism. Behav. Brain Res. 2013, 251, 41–49. [Google Scholar] [CrossRef]
- Nakanishi, M.; Nomura, J.; Ji, X.; Tamada, K.; Arai, T.; Takahashi, E.; Bućan, M.; Takumi, T. Functional significance of rare neuroligin 1 variants found in autism. PLoS Genet. 2017, 13, e1006940. [Google Scholar] [CrossRef]
- Chanda, S.; Aoto, J.; Lee, S.-J.; Wernig, M.; Südhof, T.C. Pathogenic mechanism of an autism-associated neuroligin mutation involves altered AMPA-receptor trafficking. Mol. Psychiatry 2016, 21, 169–177. [Google Scholar] [CrossRef]
- Zhang, C.; Milunsky, J.M.; Newton, S.; Ko, J.; Zhao, G.; Maher, T.A.; Tager-Flusberg, H.; Bolliger, M.F.; Carter, A.S.; Boucard, A.A.; et al. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J. Neurosci. 2009, 29, 10843–10854. [Google Scholar] [CrossRef]
- Missler, M.; Fernandez-Chacon, R.; Südhof, T.C. The making of neurexins. J. Neurochem. 1998, 71, 1339–1347. [Google Scholar] [CrossRef]
- Reissner, C.; Runkel, F.; Missler, M. Neurexins. Genome Biol. 2013, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Khoja, S.; Haile, M.T.; Chen, L.Y. Advances in neurexin studies and the emerging role of neurexin-2 in autism spectrum disorder. Front. Mol. Neurosci. 2023, 16, 1125087. [Google Scholar] [CrossRef] [PubMed]
- Vaags, A.K.; Lionel, A.C.; Sato, D.; Goodenberger, M.; Stein, Q.P.; Curran, S.; Ogilvie, C.; Ahn, J.W.; Drmic, I.; Senman, L.; et al. Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am. J. Hum. Genet. 2012, 90, 133–141. [Google Scholar] [CrossRef]
- Cooper, J.N.; Mittal, J.; Sangadi, A.; Klassen, D.L.; King, A.M.; Zalta, M.; Mittal, R.; Eshraghi, A.A. Landscape of NRXN1 Gene Variants in Phenotypic Manifestations of Autism Spectrum Disorder: A Systematic Review. J. Clin. Med. 2024, 13, 2067. [Google Scholar] [CrossRef]
- Harkin, L.F.; Lindsay, S.J.; Xu, Y.; Alzu’bi, A.; Ferrara, A.; Gullon, E.A.; James, O.G.; Clowry, G.J. Neurexins 1-3 Each Have a Distinct Pattern of Expression in the Early Developing Human Cerebral Cortex. Cereb. Cortex 2017, 27, 216–232. [Google Scholar] [CrossRef]
- Uemura, T.; Lee, S.-J.; Yasumura, M.; Takeuchi, T.; Yoshida, T.; Ra, M.; Taguchi, R.; Sakimura, K.; Mishina, M. Trans-Synaptic Interaction of GluRδ2 and Neurexin through Cbln1 Mediates Synapse Formation in the Cerebellum. Cell 2010, 141, 1068–1079. [Google Scholar] [CrossRef]
- Wang, J.; Gong, J.; Li, L.; Chen, Y.; Liu, L.; Gu, H.; Luo, X.; Hou, F.; Zhang, J.; Song, R. Neurexin gene family variants as risk factors for autism spectrum disorder. Autism Res. 2018, 11, 37–43. [Google Scholar] [CrossRef]
- Gauthier, J.; Siddiqui, T.J.; Huashan, P.; Yokomaku, D.; Hamdan, F.F.; Champagne, N.; Lapointe, M.; Spiegelman, D.; Noreau, A.; Lafrenière, R.G.; et al. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum. Genet. 2011, 130, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kasem, E.; Kurihara, T.; Tabuchi, K. Neurexins and neuropsychiatric disorders. Neurosci. Res. 2018, 127, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gerik-Celebi, H.B.; Bolat, H.; Unsel-Bolat, G. Rare heterozygous genetic variants of NRXN and NLGN gene families involved in synaptic function and their association with neurodevelopmental disorders. Dev. Neurobiol. 2024, 84, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Song, Y.; Zhang, Y.; Ho, C.W.; Xia, W.; Li, Z.; Ge, F.; Ou, Q.; Dai, Z.; Dai, Z. Neurexin dysfunction in neurodevelopmental and neuropsychiatric disorders: A PRIMSA-based systematic review through iPSC and animal models. Front. Behav. Neurosci. 2024, 18, 1297374. [Google Scholar] [CrossRef]
- Etherton, M.R.; Blaiss, C.A.; Powell, C.M.; Südhof, T.C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc. Natl. Acad. Sci. USA 2009, 106, 17998–18003. [Google Scholar] [CrossRef]
- Grayton, H.M.; Missler, M.; Collier, D.A.; Fernandes, C. Altered social behaviours in neurexin 1α knockout mice resemble core symptoms in neurodevelopmental disorders. PLoS ONE 2013, 8, e67114. [Google Scholar] [CrossRef]
- Armstrong, E.C.; Caruso, A.; Servadio, M.; Andreae, L.C.; Trezza, V.; Scattoni, M.L.; Fernandes, C. Assessing the developmental trajectory of mouse models of neurodevelopmental disorders: Social and communication deficits in mice with Neurexin 1α deletion. Genes Brain Behav. 2020, 19, e12630. [Google Scholar] [CrossRef]
- Born, G.; Grayton, H.M.; Langhorst, H.; Dudanova, I.; Rohlmann, A.; Woodward, B.W.; Collier, D.A.; Fernandes, C.; Missler, M. Genetic targeting of NRXN2 in mice unveils role in excitatory cortical synapse function and social behaviors. Front. Synaptic Neurosci. 2015, 7, 3. [Google Scholar] [CrossRef]
- Aoto, J.; Földy, C.; Ilcus, S.M.C.; Tabuchi, K.; Südhof, T.C. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat. Neurosci. 2015, 18, 997–1007. [Google Scholar] [CrossRef]
- Hauser, D.; Behr, K.; Konno, K.; Schreiner, D.; Schmidt, A.; Watanabe, M.; Bischofberger, J.; Scheiffele, P. Targeted proteoform mapping uncovers specific Neurexin-3 variants required for dendritic inhibition. Neuron 2022, 110, 2094–2109.e10. [Google Scholar] [CrossRef] [PubMed]
- Peñagarikano, O.; Geschwind, D.H. What does CNTNAP2 reveal about autism spectrum disorder? Trends Mol. Med. 2012, 18, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Rodenas-Cuadrado, P.; Ho, J.; Vernes, S.C. Shining a light on CNTNAP2: Complex functions to complex disorders. Eur. J. Hum. Genet. 2014, 22, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Gollan, L.; Martinez, R.; Custer, A.; Einheber, S.; Salzer, J.L.; Trimmer, J.S.; Shrager, P.; Peles, E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 1999, 24, 1037–1047. [Google Scholar] [CrossRef]
- Arking, D.E.; Cutler, D.J.; Brune, C.W.; Teslovich, T.M.; West, K.; Ikeda, M.; Rea, A.; Guy, M.; Lin, S.; Cook, E.H.; et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am. J. Hum. Genet. 2008, 82, 160–164. [Google Scholar] [CrossRef]
- Bakkaloglu, B.; O’Roak, B.J.; Louvi, A.; Gupta, A.R.; Abelson, J.F.; Morgan, T.M.; Chawarska, K.; Klin, A.; Ercan-Sencicek, A.G.; Stillman, A.A.; et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am. J. Hum. Genet. 2008, 82, 165–173. [Google Scholar] [CrossRef]
- Nascimento, P.P.; Bossolani-Martins, A.L.; Rosan, D.B.A.; Mattos, L.C.; Brandão-Mattos, C.; Fett-Conte, A.C. Single nucleotide polymorphisms in the CNTNAP2 gene in Brazilian patients with autistic spectrum disorder. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Chiocchetti, A.G.; Kopp, M.; Waltes, R.; Haslinger, D.; Duketis, E.; Jarczok, T.A.; Poustka, F.; Voran, A.; Graab, U.; Meyer, J.; et al. Variants of the CNTNAP2 5’ promoter as risk factors for autism spectrum disorders: A genetic and functional approach. Mol. Psychiatry 2015, 20, 839–849. [Google Scholar] [CrossRef]
- Peñagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Gordon, A.; Salomon, D.; Barak, N.; Pen, Y.; Tsoory, M.; Kimchi, T.; Peles, E. Expression of Cntnap2 (Caspr2) in multiple levels of sensory systems. Mol. Cell. Neurosci. 2016, 70, 42–53. [Google Scholar] [CrossRef]
- Tan, G.C.Y.; Doke, T.F.; Ashburner, J.; Wood, N.W.; Frackowiak, R.S.J. Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. Neuroimage 2010, 53, 1030–1042. [Google Scholar] [CrossRef]
- Crawley, J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Argent, L.; Winter, F.; Prickett, I.; Carrasquero-Ordaz, M.; Olsen, A.L.; Kramer, H.; Lancaster, E.; Becker, E.B.E. Caspr2 interacts with type 1 inositol 1,4,5-trisphosphate receptor in the developing cerebellum and regulates Purkinje cell morphology. J. Biol. Chem. 2020, 295, 12716–12726. [Google Scholar] [CrossRef] [PubMed]
- Otazu, G.H.; Li, Y.; Lodato, Z.; Elnasher, A.; Keever, K.M.; Li, Y.; Ramos, R.L. Neurodevelopmental malformations of the cerebellum and neocortex in the Shank3 and Cntnap2 mouse models of autism. Neurosci. Lett. 2021, 765, 136257. [Google Scholar] [CrossRef] [PubMed]
- St George-Hyslop, F.; Haneklaus, M.; Kivisild, T.; Livesey, F.J. Loss of CNTNAP2 Alters Human Cortical Excitatory Neuron Differentiation and Neural Network Development. Biol. Psychiatry 2023, 94, 780–791. [Google Scholar] [CrossRef]
- Anderson, G.R.; Galfin, T.; Xu, W.; Aoto, J.; Malenka, R.C.; Südhof, T.C. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc. Natl. Acad. Sci. USA 2012, 109, 18120–18125. [Google Scholar] [CrossRef]
- Varea, O.; Martin-de-Saavedra, M.D.; Kopeikina, K.J.; Schürmann, B.; Fleming, H.J.; Fawcett-Patel, J.M.; Bach, A.; Jang, S.; Peles, E.; Kim, E.; et al. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 6176–6181. [Google Scholar] [CrossRef]
- Obst-Pernberg, K.; Redies, C. Cadherins and synaptic specificity. J. Neurosci. Res. 1999, 58, 130–138. [Google Scholar] [CrossRef]
- Huntley, G.W.; Gil, O.; Bozdagi, O. The cadherin family of cell adhesion molecules: Multiple roles in synaptic plasticity. Neuroscientist 2002, 8, 221–233. [Google Scholar] [CrossRef]
- Basu, R.; Taylor, M.R.; Williams, M.E. The classic cadherins in synaptic specificity. Cell Adhes. Migr. 2015, 9, 193–201. [Google Scholar] [CrossRef]
- de Agustín-Durán, D.; Mateos-White, I.; Fabra-Beser, J.; Gil-Sanz, C. Stick around: Cell-Cell Adhesion Molecules during Neocortical Development. Cells 2021, 10, 118. [Google Scholar] [CrossRef]
- Redies, C.; Neudert, F.; Lin, J. Cadherins in cerebellar development: Translation of embryonic patterning into mature functional compartmentalization. Cerebellum 2011, 10, 393–408. [Google Scholar] [CrossRef]
- Redies, C.; Hertel, N.; Hübner, C.A. Cadherins and neuropsychiatric disorders. Brain Res. 2012, 1470, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Takeichi, M. Cadherin superfamily genes: Functions, genomic organization, and neurologic diversity. Genes Dev. 2000, 14, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K.; Nakagawa, S.; Takeichi, M.; Redies, C. Cadherin-Defined Segments and Parasagittal Cell Ribbons in the Developing Chicken Cerebellum. Mol. Cell. Neurosci. 1998, 10, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Treubert-Zimmermann, U.; Redies, C. Cadherins guide migrating Purkinje cells to specific parasagittal domains during cerebellar development. Mol. Cell. Neurosci. 2004, 25, 138–152. [Google Scholar] [CrossRef]
- Betancur, C.; Sakurai, T.; Buxbaum, J.D. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009, 32, 402–412. [Google Scholar] [CrossRef]
- Hussman, J.P.; Chung, R.-H.; Griswold, A.J.; Jaworski, J.M.; Salyakina, D.; Ma, D.; Konidari, I.; Whitehead, P.L.; Vance, J.M.; Martin, E.R.; et al. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. Mol. Autism 2011, 2, 1. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Frei, J.A.; Kilander, M.B.C.; Shen, W.; Blatt, G.J. A Subset of Autism-Associated Genes Regulate the Structural Stability of Neurons. Front. Cell. Neurosci. 2016, 10, 263. [Google Scholar] [CrossRef]
- Costa, C.I.S.; da Silva Montenegro, E.M.; Zarrei, M.; de Sá Moreira, E.; Silva, I.M.W.; de Oliveira Scliar, M.; Wang, J.Y.T.; Zachi, E.C.; Branco, E.V.; da Costa, S.S.; et al. Copy number variations in a Brazilian cohort with autism spectrum disorders highlight the contribution of cell adhesion genes. Clin. Genet. 2022, 101, 134–141. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.-H.; Wang, Y.; Blatt, G.; Yuan, X.-B. Segregated expressions of autism risk genes Cdh11 and Cdh9 in autism-relevant regions of developing cerebellum. Mol. Brain 2019, 12, 40. [Google Scholar] [CrossRef]
- Crepel, A.; De Wolf, V.; Brison, N.; Ceulemans, B.; Walleghem, D.; Peuteman, G.; Lambrechts, D.; Steyaert, J.; Noens, I.; Devriendt, K.; et al. Association of CDH11 with non-syndromic ASD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014, 165B, 391–398. [Google Scholar] [CrossRef]
- Frei, J.A.; Niescier, R.F.; Bridi, M.S.; Durens, M.; Nestor, J.E.; Kilander, M.B.C.; Yuan, X.; Dykxhoorn, D.M.; Nestor, M.W.; Huang, S.; et al. Regulation of Neural Circuit Development by Cadherin-11 Provides Implications for Autism. eNeuro 2021, 8, ENEURO.0066-21.2021. [Google Scholar] [CrossRef]
- Chapman, N.H.; Estes, A.; Munson, J.; Bernier, R.; Webb, S.J.; Rothstein, J.H.; Minshew, N.J.; Dawson, G.; Schellenberg, G.D.; Wijsman, E.M. Genome-scan for IQ discrepancy in autism: Evidence for loci on chromosomes 10 and 16. Hum. Genet. 2011, 129, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Tantra, M.; Guo, L.; Kim, J.; Zainolabidin, N.; Eulenburg, V.; Augustine, G.J.; Chen, A.I. Conditional deletion of Cadherin 13 perturbs Golgi cells and disrupts social and cognitive behaviors. Genes Brain Behav. 2018, 17, e12466. [Google Scholar] [CrossRef] [PubMed]
- Kiser, D.P.; Popp, S.; Schmitt-Böhrer, A.G.; Strekalova, T.; van den Hove, D.L.; Lesch, K.-P.; Rivero, O. Early-life stress impairs developmental programming in Cadherin 13 (CDH13)-deficient mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Neale, B.M.; Kou, Y.; Liu, L.; Ma’ayan, A.; Samocha, K.E.; Sabo, A.; Lin, C.-F.; Stevens, C.; Wang, L.-S.; Makarov, V.; et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 2012, 485, 242–245. [Google Scholar] [CrossRef]
- Cukier, H.N.; Dueker, N.D.; Slifer, S.H.; Lee, J.M.; Whitehead, P.L.; Lalanne, E.; Leyva, N.; Konidari, I.; Gentry, R.C.; Hulme, W.F.; et al. Exome sequencing of extended families with autism reveals genes shared across neurodevelopmental and neuropsychiatric disorders. Mol. Autism 2014, 5, 1. [Google Scholar] [CrossRef]
- Frei, J.A.; Brandenburg, C.; Nestor, J.E.; Hodzic, D.M.; Plachez, C.; McNeill, H.; Dykxhoorn, D.M.; Nestor, M.W.; Blatt, G.J.; Lin, Y.-C. Postnatal expression profiles of atypical cadherin FAT1 suggest its role in autism. Biol. Open 2021, 10, bio056457. [Google Scholar] [CrossRef]
- Boucherie, C.; Boutin, C.; Jossin, Y.; Schakman, O.; Goffinet, A.M.; Ris, L.; Gailly, P.; Tissir, F. Neural progenitor fate decision defects, cortical hypoplasia and behavioral impairment in Celsr1-deficient mice. Mol. Psychiatry 2018, 23, 723–734. [Google Scholar] [CrossRef]
- Goffinet, A.M.; Tissir, F. Seven pass Cadherins CELSR1-3. Semin. Cell Dev. Biol. 2017, 69, 102–110. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, J.; Liang, Y.; Zhang, W.; He, S.; Tissir, F.; Qu, Y.; Zhou, L. Celsr3 is required for Purkinje cell maturation and regulates cerebellar postsynaptic plasticity. iScience 2021, 24, 102812. [Google Scholar] [CrossRef]
- Shimizu, A.; Asakawa, S.; Sasaki, T.; Yamazaki, S.; Yamagata, H.; Kudoh, J.; Minoshima, S.; Kondo, I.; Shimizu, N. A novel giant gene CSMD3 encoding a protein with CUB and sushi multiple domains: A candidate gene for benign adult familial myoclonic epilepsy on human chromosome 8q23.3-q24.1. Biochem. Biophys. Res. Commun. 2003, 309, 143–154. [Google Scholar] [CrossRef]
- Floris, C.; Rassu, S.; Boccone, L.; Gasperini, D.; Cao, A.; Crisponi, L. Two patients with balanced translocations and autistic disorder: CSMD3 as a candidate gene for autism found in their common 8q23 breakpoint area. Eur. J. Hum. Genet. 2008, 16, 696–704. [Google Scholar] [CrossRef]
- Mizukami, T.; Kohno, T.; Hattori, M. CUB and Sushi multiple domains 3 regulates dendrite development. Neurosci. Res. 2016, 110, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Cai, S.-Q.; Yan, H.-F.; Tian, Y.; Cai, J.; Yang, X.-M.; Wang, J.-M.; Xing, G.-G. CSMD3 Deficiency Leads to Motor Impairments and Autism-Like Behaviors via Dysfunction of Cerebellar Purkinje Cells in Mice. J. Neurosci. 2023, 43, 3949–3969. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, Q.; Wang, T.; Li, Y.; Fan, T.; Zhang, J.; Wang, Q.; Pan, J.; Dong, Q.; Sun, Z.S.; et al. Putative complement control protein CSMD3 dysfunction impairs synaptogenesis and induces neurodevelopmental disorders. Brain Behav. Immun. 2022, 102, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Joyner, A.L.; Herrup, K.; Auerbach, B.A.; Davis, C.A.; Rossant, J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science 1991, 251, 1239–1243. [Google Scholar] [CrossRef]
- Vogel, M.W.; Ji, Z.; Millen, K.; Joyner, A.L. The Engrailed-2 homeobox gene and patterning of spinocerebellar mossy fiber afferents. Brain Res. Dev. Brain Res. 1996, 96, 210–218. [Google Scholar] [CrossRef]
- Kuemerle, B.; Zanjani, H.; Joyner, A.; Herrup, K. Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development. J. Neurosci. 1997, 17, 7881–7889. [Google Scholar] [CrossRef]
- Sudarov, A.; Joyner, A.L. Cerebellum morphogenesis: The foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2007, 2, 26. [Google Scholar] [CrossRef]
- Cheng, Y.; Sudarov, A.; Szulc, K.U.; Sgaier, S.K.; Stephen, D.; Turnbull, D.H.; Joyner, A.L. The Engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum. Development 2010, 137, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, G.; Gatta, A.; Miragliotta, V.; Vaglini, F.; Viaggi, C.; Pirone, A. Glial cells are affected more than interneurons by the loss of Engrailed 2 gene in the mouse cerebellum. J. Anat. 2024, 244, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Lee, A.S.; Bayin, N.S.; Stephen, D.N.; Nasef, O.; Lao, Z.; Joyner, A.L. Engrailed transcription factors direct excitatory cerebellar neuron diversity and survival. Development 2024, 151, dev202502. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Holst, M.I.; Liebig, C.; Oberdick, J.; Baader, S.L. Engrailed-2 negatively regulates the onset of perinatal Purkinje cell differentiation. J. Comp. Neurol. 2004, 472, 87–99. [Google Scholar] [CrossRef]
- Gharani, N.; Benayed, R.; Mancuso, V.; Brzustowicz, L.M.; Millonig, J.H. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Mol. Psychiatry 2004, 9, 474–484. [Google Scholar] [CrossRef]
- Benayed, R.; Gharani, N.; Rossman, I.; Mancuso, V.; Lazar, G.; Kamdar, S.; Bruse, S.E.; Tischfield, S.; Smith, B.J.; Zimmerman, R.A.; et al. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am. J. Hum. Genet. 2005, 77, 851–868. [Google Scholar] [CrossRef]
- Yang, P.; Lung, F.-W.; Jong, Y.-J.; Hsieh, H.-Y.; Liang, C.-L.; Juo, S.-H.H. Association of the homeobox transcription factor gene ENGRAILED 2 with autistic disorder in Chinese children. Neuropsychobiology 2008, 57, 3–8. [Google Scholar] [CrossRef]
- Sen, B.; Singh, A.S.; Sinha, S.; Chatterjee, A.; Ahmed, S.; Ghosh, S.; Usha, R. Family-based studies indicate association of Engrailed 2 gene with autism in an Indian population. Genes Brain Behav. 2010, 9, 248–255. [Google Scholar] [CrossRef]
- Benayed, R.; Choi, J.; Matteson, P.G.; Gharani, N.; Kamdar, S.; Brzustowicz, L.M.; Millonig, J.H. Autism-associated haplotype affects the regulation of the homeobox gene, ENGRAILED 2. Biol. Psychiatry 2009, 66, 911–917. [Google Scholar] [CrossRef]
- Choi, J.; Ababon, M.R.; Soliman, M.; Lin, Y.; Brzustowicz, L.M.; Matteson, P.G.; Millonig, J.H. Autism associated gene, engrailed2, and flanking gene levels are altered in post-mortem cerebellum. PLoS ONE 2014, 9, e87208. [Google Scholar] [CrossRef]
- James, S.J.; Shpyleva, S.; Melnyk, S.; Pavliv, O.; Pogribny, I.P. Complex epigenetic regulation of engrailed-2 (EN-2) homeobox gene in the autism cerebellum. Transl. Psychiatry 2013, 3, e232. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Shpyleva, S.; Melnyk, S.; Pavliv, O.; Pogribny, I.P. Elevated 5-hydroxymethylcytosine in the Engrailed-2 (EN-2) promoter is associated with increased gene expression and decreased MeCP2 binding in autism cerebellum. Transl. Psychiatry 2014, 4, e460. [Google Scholar] [CrossRef] [PubMed]
- Cheh, M.A.; Millonig, J.H.; Roselli, L.M.; Ming, X.; Jacobsen, E.; Kamdar, S.; Wagner, G.C. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006, 1116, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Brielmaier, J.; Matteson, P.G.; Silverman, J.L.; Senerth, J.M.; Kelly, S.; Genestine, M.; Millonig, J.H.; DiCicco-Bloom, E.; Crawley, J.N. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS ONE 2012, 7, e40914. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Genovesi, S.; Balasco, L.; Cerilli, E.; Robol, C.; Zunino, G.; Piazza, S.; Provenzano, G.; Bozzi, Y. Immune dysfunction in the cerebellum of mice lacking the autism candidate gene Engrailed 2. J. Neuroimmunol. 2022, 367, 577870. [Google Scholar] [CrossRef]
- Boer, K.; Troost, D.; Jansen, F.; Nellist, M.; van den Ouweland, A.M.W.; Geurts, J.J.G.; Spliet, W.G.M.; Crino, P.; Aronica, E. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 2008, 28, 577–590. [Google Scholar] [CrossRef]
- Orlova, K.A.; Crino, P.B. The tuberous sclerosis complex. Ann. N. Y. Acad. Sci. 2010, 1184, 87–105. [Google Scholar] [CrossRef]
- McDonald, N.M.; Varcin, K.J.; Bhatt, R.; Wu, J.Y.; Sahin, M.; Nelson, C.A.; Jeste, S.S. Early autism symptoms in infants with tuberous sclerosis complex. Autism Res. 2017, 10, 1981–1990. [Google Scholar] [CrossRef]
- Dickinson, A.; Varcin, K.J.; Sahin, M.; Nelson, C.A.; Jeste, S.S. Early patterns of functional brain development associated with autism spectrum disorder in tuberous sclerosis complex. Autism Res. 2019, 12, 1758–1773. [Google Scholar] [CrossRef]
- Samanta, D. An Updated Review of Tuberous Sclerosis Complex-Associated Autism Spectrum Disorder. Pediatr. Neurol. 2020, 109, 4–11. [Google Scholar] [CrossRef]
- Kashii, H.; Kasai, S.; Sato, A.; Hagino, Y.; Nishito, Y.; Kobayashi, T.; Hino, O.; Mizuguchi, M.; Ikeda, K. Tsc2 mutation rather than Tsc1 mutation dominantly causes a social deficit in a mouse model of tuberous sclerosis complex. Hum. Genom. 2023, 17, 4. [Google Scholar] [CrossRef]
- Tsai, P.T.; Hull, C.; Chu, Y.; Greene-Colozzi, E.; Sadowski, A.R.; Leech, J.M.; Steinberg, J.; Crawley, J.N.; Regehr, W.G.; Sahin, M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 2012, 488, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Reith, R.M.; McKenna, J.; Wu, H.; Hashmi, S.S.; Cho, S.-H.; Dash, P.K.; Gambello, M.J. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2013, 51, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Goorden, S.M.I.; van Woerden, G.M.; van der Weerd, L.; Cheadle, J.P.; Elgersma, Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann. Neurol. 2007, 62, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Capal, J.K.; Jeste, S.S. Autism and Epilepsy. Pediatr. Clin. N. Am. 2024, 71, 241–252. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Battaglioni, S.; Benjamin, D.; Wälchli, M.; Maier, T.; Hall, M.N. mTOR substrate phosphorylation in growth control. Cell 2022, 185, 1814–1836. [Google Scholar] [CrossRef]
- Gaubitz, C.; Prouteau, M.; Kusmider, B.; Loewith, R. TORC2 Structure and Function. Trends Biochem. Sci. 2016, 41, 532–545. [Google Scholar] [CrossRef]
- Xie, J.; Wang, X.; Proud, C.G. Who does TORC2 talk to? Biochem. J. 2018, 475, 1721–1738. [Google Scholar] [CrossRef]
- Tavazoie, S.F.; Alvarez, V.A.; Ridenour, D.A.; Kwiatkowski, D.J.; Sabatini, B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005, 8, 1727–1734. [Google Scholar] [CrossRef]
- Bowling, H.; Klann, E. Shaping dendritic spines in autism spectrum disorder: mTORC1-dependent macroautophagy. Neuron 2014, 83, 994–996. [Google Scholar] [CrossRef]
- Winden, K.D.; Ebrahimi-Fakhari, D.; Sahin, M. Abnormal mTOR Activation in Autism. Annu. Rev. Neurosci. 2018, 41, 1–23. [Google Scholar] [CrossRef]
- Rosina, E.; Battan, B.; Siracusano, M.; Di Criscio, L.; Hollis, F.; Pacini, L.; Curatolo, P.; Bagni, C. Disruption of mTOR and MAPK pathways correlates with severity in idiopathic autism. Transl. Psychiatry 2019, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Gudsnuk, K.; Kuo, S.-H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Barsotti, N.; Bertero, A.; Trakoshis, S.; Ulysse, L.; Locarno, A.; Miseviciute, I.; De Felice, A.; Canella, C.; Supekar, K.; et al. mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat. Commun. 2021, 12, 6084. [Google Scholar] [CrossRef] [PubMed]
- Kútna, V.; O’Leary, V.B.; Hoschl, C.; Ovsepian, S.V. Cerebellar demyelination and neurodegeneration associated with mTORC1 hyperactivity may contribute to the developmental onset of autism-like neurobehavioral phenotype in a rat model. Autism Res. 2022, 15, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kasai, S.; Kobayashi, T.; Takamatsu, Y.; Hino, O.; Ikeda, K.; Mizuguchi, M. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat. Commun. 2012, 3, 1292. [Google Scholar] [CrossRef]
- Alsaqati, M.; Heine, V.M.; Harwood, A.J. Pharmacological intervention to restore connectivity deficits of neuronal networks derived from ASD patient iPSC with a TSC2 mutation. Mol. Autism 2020, 11, 80. [Google Scholar] [CrossRef]
- Sato, A. mTOR, a Potential Target to Treat Autism Spectrum Disorder. CNS Neurol. Disord. Drug Targets 2016, 15, 533–543. [Google Scholar] [CrossRef]
- Angliker, N.; Burri, M.; Zaichuk, M.; Fritschy, J.-M.; Rüegg, M.A. mTORC1 and mTORC2 have largely distinct functions in Purkinje cells. Eur. J. Neurosci. 2015, 42, 2595–2612. [Google Scholar] [CrossRef] [PubMed]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, X.; Hou, Q.; Hu, Z.; Wang, Y.; Wang, Z. Regulation of mTORC1 by amino acids in mammalian cells: A general picture of recent advances. Anim. Nutr. 2021, 7, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Gkogkas, C.G.; Khoutorsky, A.; Ran, I.; Rampakakis, E.; Nevarko, T.; Weatherill, D.B.; Vasuta, C.; Yee, S.; Truitt, M.; Dallaire, P.; et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 2013, 493, 371–377. [Google Scholar] [CrossRef]
- Santini, E.; Huynh, T.N.; MacAskill, A.F.; Carter, A.G.; Pierre, P.; Ruggero, D.; Kaphzan, H.; Klann, E. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature 2013, 493, 411–415. [Google Scholar] [CrossRef]
- Banko, J.L.; Merhav, M.; Stern, E.; Sonenberg, N.; Rosenblum, K.; Klann, E. Behavioral alterations in mice lacking the translation repressor 4E-BP2. Neurobiol. Learn. Mem. 2007, 87, 248–256. [Google Scholar] [CrossRef]
- Banko, J.L.; Poulin, F.; Hou, L.; DeMaria, C.T.; Sonenberg, N.; Klann, E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J. Neurosci. 2005, 25, 9581–9590. [Google Scholar] [CrossRef]
- Wiebe, S.; Nagpal, A.; Truong, V.T.; Park, J.; Skalecka, A.; He, A.J.; Gamache, K.; Khoutorsky, A.; Gantois, I.; Sonenberg, N. Inhibitory interneurons mediate autism-associated behaviors via 4E-BP2. Proc. Natl. Acad. Sci. USA 2019, 116, 18060–18067. [Google Scholar] [CrossRef]
- Hooshmandi, M.; Truong, V.T.; Fields, E.; Thomas, R.E.; Wong, C.; Sharma, V.; Gantois, I.; Soriano Roque, P.; Chalkiadaki, K.; Wu, N.; et al. 4E-BP2-dependent translation in cerebellar Purkinje cells controls spatial memory but not autism-like behaviors. Cell Rep. 2021, 35, 109036. [Google Scholar] [CrossRef]
- Chalkiadaki, K.; Statoulla, E.; Zafeiri, M.; Haji, N.; Lacaille, J.-C.; Powell, C.M.; Jafarnejad, S.M.; Khoutorsky, A.; Gkogkas, C.G. Reversal of memory and autism-related phenotypes in Tsc2+/− mice via inhibition of Nlgn1. Front. Cell Dev. Biol. 2023, 11, 1205112. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Kim, G.H.; Tan, J.-W.; Riso, A.E.; Sun, Y.; Xu, E.Y.; Liao, G.-Y.; Xu, H.; Lee, S.-H.; Do, N.-Y.; et al. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat. Commun. 2020, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Dixon, J.E. PTEN. Annu. Rev. Biochem. 2014, 83, 641–669. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Krimpenfort, P.; Leung, C.; van der Korput, H.A.G.M.; Trapman, J.; Camenisch, I.; Berns, A.; Brandner, S. PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development 2002, 129, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Gambini, D.; Ferrero, S.; Bulfamante, G.; Pisani, L.; Corbo, M.; Kuhn, E. Cerebellar phenotypes in germline PTEN mutation carriers. Neuropathol. Appl. Neurobiol. 2024, 50, e12970. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Choudhury, G.R.; He, R.; Yang, T.; Liu, R.; Jin, K.; Yang, S.-H. Astroglial PTEN Loss Disrupts Neuronal Lamination by Dysregulating Radial Glia-guided Neuronal Migration. Aging Dis. 2013, 4, 113–126. [Google Scholar]
- Frazier, T.W.; Embacher, R.; Tilot, A.K.; Koenig, K.; Mester, J.; Eng, C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol. Psychiatry 2015, 20, 1132–1138. [Google Scholar] [CrossRef]
- Butler, M.G.; Dasouki, M.J.; Zhou, X.-P.; Talebizadeh, Z.; Brown, M.; Takahashi, T.N.; Miles, J.H.; Wang, C.H.; Stratton, R.; Pilarski, R.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005, 42, 318–321. [Google Scholar] [CrossRef]
- Herman, G.E.; Butter, E.; Enrile, B.; Pastore, M.; Prior, T.W.; Sommer, A. Increasing knowledge of PTEN germline mutations: Two additional patients with autism and macrocephaly. Am. J. Med. Genet. A 2007, 143A, 589–593. [Google Scholar] [CrossRef]
- Yehia, L.; Keel, E.; Eng, C. The Clinical Spectrum of PTEN Mutations. Annu. Rev. Med. 2020, 71, 103–116. [Google Scholar] [CrossRef]
- Buxbaum, J.D.; Cai, G.; Chaste, P.; Nygren, G.; Goldsmith, J.; Reichert, J.; Anckarsäter, H.; Rastam, M.; Smith, C.J.; Silverman, J.M.; et al. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 484–491. [Google Scholar] [CrossRef]
- Rademacher, S.; Eickholt, B.J. PTEN in Autism and Neurodevelopmental Disorders. Cold Spring Harb. Perspect. Med. 2019, 9, a036780. [Google Scholar] [CrossRef]
- Greer, J.M.; Wynshaw-Boris, A. Pten and the brain: Sizing up social interaction. Neuron 2006, 50, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.-H.; Luikart, B.W.; Powell, C.M.; Zhou, J.; Matheny, S.A.; Zhang, W.; Li, Y.; Baker, S.J.; Parada, L.F. Pten regulates neuronal arborization and social interaction in mice. Neuron 2006, 50, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Luikart, B.W.; Schnell, E.; Washburn, E.K.; Bensen, A.L.; Tovar, K.R.; Westbrook, G.L. Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J. Neurosci. 2011, 31, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Haws, M.E.; Jaramillo, T.C.; Espinosa, F.; Widman, A.J.; Stuber, G.D.; Sparta, D.R.; Tye, K.M.; Russo, S.J.; Parada, L.F.; Stavarache, M.; et al. PTEN knockdown alters dendritic spine/protrusion morphology, not density. J. Comp. Neurol. 2014, 522, 1171–1190. [Google Scholar] [CrossRef]
- Williams, M.R.; DeSpenza, T.; Li, M.; Gulledge, A.T.; Luikart, B.W. Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive. J. Neurosci. 2015, 35, 943–959. [Google Scholar] [CrossRef]
- Getz, S.A.; DeSpenza, T.; Li, M.; Luikart, B.W. Rapamycin prevents, but does not reverse, aberrant migration in Pten knockout neurons. Neurobiol. Dis. 2016, 93, 12–20. [Google Scholar] [CrossRef]
- Getz, S.A.; Tariq, K.; Marchand, D.H.; Dickson, C.R.; Howe Vi, J.R.; Skelton, P.D.; Wang, W.; Li, M.; Barry, J.M.; Hong, J.; et al. PTEN Regulates Dendritic Arborization by Decreasing Microtubule Polymerization Rate. J. Neurosci. 2022, 42, 1945–1957. [Google Scholar] [CrossRef]
- Skelton, P.D.; Poquerusse, J.; Salinaro, J.R.; Li, M.; Luikart, B.W. Activity-dependent dendritic elaboration requires Pten. Neurobiol. Dis. 2020, 134, 104703. [Google Scholar] [CrossRef]
- Kwon, C.H.; Zhu, X.; Zhang, J.; Knoop, L.L.; Tharp, R.; Smeyne, R.J.; Eberhart, C.G.; Burger, P.C.; Baker, S.J. Pten regulates neuronal soma size: A mouse model of Lhermitte-Duclos disease. Nat. Genet. 2001, 29, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Cupolillo, D.; Hoxha, E.; Faralli, A.; De Luca, A.; Rossi, F.; Tempia, F.; Carulli, D. Autistic-Like Traits and Cerebellar Dysfunction in Purkinje Cell PTEN Knock-Out Mice. Neuropsychopharmacology 2016, 41, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Blundell, J.; Ogawa, S.; Kwon, C.-H.; Zhang, W.; Sinton, C.; Powell, C.M.; Parada, L.F. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 2009, 29, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Itoh, K.; Yamada, T.; Adachi, Y.; Kato, T.; Murata, D.; Sesaki, H.; Iijima, M. Nuclear PTEN deficiency causes microcephaly with decreased neuronal soma size and increased seizure susceptibility. J. Biol. Chem. 2018, 293, 9292–9300. [Google Scholar] [CrossRef]
- Johnson, K.P.; Zarrinnegar, P. Autism Spectrum Disorder and Sleep. Psychiatr. Clin. N. Am. 2024, 47, 199–212. [Google Scholar] [CrossRef]
- Sidhu, N.; Wong, Z.; Bennett, A.E.; Souders, M.C. Sleep Problems in Autism Spectrum Disorder. Pediatr. Clin. N. Am. 2024, 71, 253–268. [Google Scholar] [CrossRef]
- Pinato, L.; Galina Spilla, C.S.; Markus, R.P.; da Silveira Cruz-Machado, S. Dysregulation of Circadian Rhythms in Autism Spectrum Disorders. Curr. Pharm. Des. 2019, 25, 4379–4393. [Google Scholar] [CrossRef]
- Ferraro, S.; de Zavalia, N.; Belforte, N.; Amir, S. In utero Exposure to Valproic-Acid Alters Circadian Organisation and Clock-Gene Expression: Implications for Autism Spectrum Disorders. Front. Behav. Neurosci. 2021, 15, 711549. [Google Scholar] [CrossRef]
- Takahashi, J.S.; Hong, H.-K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Liu, D.; Nanclares, C.; Simbriger, K.; Fang, K.; Lorsung, E.; Le, N.; Amorim, I.S.; Chalkiadaki, K.; Pathak, S.S.; Li, J.; et al. Autistic-like behavior and cerebellar dysfunction in Bmal1 mutant mice ameliorated by mTORC1 inhibition. Mol. Psychiatry 2023, 28, 3727–3738. [Google Scholar] [CrossRef]
- Singla, R.; Mishra, A.; Lin, H.; Lorsung, E.; Le, N.; Tin, S.; Jin, V.X.; Cao, R. Haploinsufficiency of a Circadian Clock Gene Bmal1 (Arntl or Mop3) Causes Brain-Wide mTOR Hyperactivation and Autism-like Behavioral Phenotypes in Mice. Int. J. Mol. Sci. 2022, 23, 6317. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Morín, J.G.; Santillán, M. Crosstalk dynamics between the circadian clock and the mTORC1 pathway. J. Theor. Biol. 2020, 501, 110360. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.O.; Boyle, L.M.; Yuan, E.D.; Hochstrasser, K.J.; Chifamba, F.F.; Nathan, A.; Tsai, P.T.; Davis, F.; Sahin, M. Aberrant Proteostasis of BMAL1 Underlies Circadian Abnormalities in a Paradigmatic mTOR-opathy. Cell Rep. 2017, 20, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Li, X.; Huang, J.; Xu, C.; Liang, Q.; Ren, K.; Bai, A.; Lu, C.; Qian, R.; Sun, N. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell 2020, 11, 661–679. [Google Scholar] [CrossRef]
- McKee, C.A.; Polino, A.J.; King, M.W.; Musiek, E.S. Circadian clock protein BMAL1 broadly influences autophagy and endolysosomal function in astrocytes. Proc. Natl. Acad. Sci. USA 2023, 120, e2220551120. [Google Scholar] [CrossRef]
- Liu, W.-W.; Wei, S.-Z.; Huang, G.-D.; Liu, L.-B.; Gu, C.; Shen, Y.; Wang, X.-H.; Xia, S.-T.; Xie, A.-M.; Hu, L.-F.; et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson’s disease mouse model. FASEB J. 2020, 34, 6570–6581. [Google Scholar] [CrossRef]
- Iweka, C.A.; Seigneur, E.; Hernandez, A.L.; Paredes, S.H.; Cabrera, M.; Blacher, E.; Pasternak, C.T.; Longo, F.M.; de Lecea, L.; Andreasson, K.I. Myeloid deficiency of the intrinsic clock protein BMAL1 accelerates cognitive aging by disrupting microglial synaptic pruning. J. Neuroinflammation 2023, 20, 48. [Google Scholar] [CrossRef]
- Rojo, D.; Dal Cengio, L.; Badner, A.; Kim, S.; Sakai, N.; Greene, J.; Dierckx, T.; Mehl, L.C.; Eisinger, E.; Ransom, J.; et al. BMAL1 loss in oligodendroglia contributes to abnormal myelination and sleep. Neuron 2023, 111, 3604–3618.e11. [Google Scholar] [CrossRef]
- Sayad, A.; Noroozi, R.; Omrani, M.D.; Taheri, M.; Ghafouri-Fard, S. Retinoic acid-related orphan receptor alpha (RORA) variants are associated with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 1595–1601. [Google Scholar] [CrossRef]
- Ribeiro, S.; Sherrard, R.M. Cerebellum and neurodevelopmental disorders: RORα is a unifying force. Front. Cell. Neurosci. 2023, 17, 1108339. [Google Scholar] [CrossRef]
- Maden, M.; Holder, N. Retinoic acid and development of the central nervous system. Bioessays 1992, 14, 431–438. [Google Scholar] [CrossRef]
- Maden, M.; Holder, N. The involvement of retinoic acid in the development of the vertebrate central nervous system. Dev. Suppl. 1991, 113 (Suppl. S2), 87–94. [Google Scholar] [CrossRef]
- Moramarco, F.; McCaffery, P. Retinoic acid regulation of homoeostatic synaptic plasticity and its relationship to cognitive disorders. J. Mol. Endocrinol. 2024, 72, e220177. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Hu, V.W.; Sarachana, T.; Kim, K.S.; Nguyen, A.; Kulkarni, S.; Steinberg, M.E.; Luu, T.; Lai, Y.; Lee, N.H. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: Evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009, 2, 78–97. [Google Scholar] [CrossRef]
- Hu, V.W.; Sarachana, T.; Sherrard, R.M.; Kocher, K.M. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol. Autism 2015, 6, 7. [Google Scholar] [CrossRef]
- Xu, L.-M.; Li, J.-R.; Huang, Y.; Zhao, M.; Tang, X.; Wei, L. AutismKB: An evidence-based knowledgebase of autism genetics. Nucleic Acids Res. 2012, 40, D1016–D1022. [Google Scholar] [CrossRef]
- Guissart, C.; Latypova, X.; Rollier, P.; Khan, T.N.; Stamberger, H.; McWalter, K.; Cho, M.T.; Kjaergaard, S.; Weckhuysen, S.; Lesca, G.; et al. Dual Molecular Effects of Dominant RORA Mutations Cause Two Variants of Syndromic Intellectual Disability with Either Autism or Cerebellar Ataxia. Am. J. Hum. Genet. 2018, 102, 744–759. [Google Scholar] [CrossRef]
- Boukhtouche, F.; Janmaat, S.; Vodjdani, G.; Gautheron, V.; Mallet, J.; Dusart, I.; Mariani, J. Retinoid-related orphan receptor alpha controls the early steps of Purkinje cell dendritic differentiation. J. Neurosci. 2006, 26, 1531–1538. [Google Scholar] [CrossRef]
- Chen, X.R.; Heck, N.; Lohof, A.M.; Rochefort, C.; Morel, M.-P.; Wehrlé, R.; Doulazmi, M.; Marty, S.; Cannaya, V.; Avci, H.X.; et al. Mature Purkinje cells require the retinoic acid-related orphan receptor-α (RORα) to maintain climbing fiber mono-innervation and other adult characteristics. J. Neurosci. 2013, 33, 9546–9562. [Google Scholar] [CrossRef]
- Takeo, Y.H.; Kakegawa, W.; Miura, E.; Yuzaki, M. RORα Regulates Multiple Aspects of Dendrite Development in Cerebellar Purkinje Cells In Vivo. J. Neurosci. 2015, 35, 12518–12534. [Google Scholar] [CrossRef] [PubMed]
- Boukhtouche, F.; Doulazmi, M.; Frederic, F.; Dusart, I.; Brugg, B.; Mariani, J. RORalpha, a pivotal nuclear receptor for Purkinje neuron survival and differentiation: From development to ageing. Cerebellum 2006, 5, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Doulazmi, M.; Frédéric, F.; Capone, F.; Becker-André, M.; Delhaye-Bouchaud, N.; Mariani, J. A comparative study of Purkinje cells in two RORalpha gene mutant mice: Staggerer and RORalpha(-/-). Brain Res. Dev. Brain Res. 2001, 127, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, C.I.; Staels, B.; Brugg, B.; Lemaigre-Dubreuil, Y.; Tedgui, A.; Mariani, J. Age-related phenotypes in the staggerer mouse expand the RORα nuclear receptor’s role beyond the cerebellum. Mol. Cell. Endocrinol. 2002, 186, 1–5. [Google Scholar] [CrossRef]
- Yuan, B.; Luo, L.; Hu, C.; Lin, F.; Yang, T.; Chen, J.; Li, T. Retinoic acid supplementation ameliorates motor incoordination via RARα-CBLN2 in the cerebellum of a prenatal valproic acid-exposed rat autism model. Neurosci. Lett. 2023, 809, 137316. [Google Scholar] [CrossRef]
- Bi, W.; Yan, J.; Shi, X.; Yuva-Paylor, L.A.; Antalffy, B.A.; Goldman, A.; Yoo, J.W.; Noebels, J.L.; Armstrong, D.L.; Paylor, R.; et al. Rai1 deficiency in mice causes learning impairment and motor dysfunction, whereas Rai1 heterozygous mice display minimal behavioral phenotypes. Hum. Mol. Genet. 2007, 16, 1802–1813. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Stoney, P.N.; Shearer, K.D.; Sementilli, A.; Nanescu, S.E.; Sementilli, P.; McCaffery, P. Expression in the human brain of retinoic acid induced 1, a protein associated with neurobehavioural disorders. Brain Struct. Funct. 2015, 220, 1195–1203. [Google Scholar] [CrossRef]
- Cao, L.; Molina, J.; Abad, C.; Carmona-Mora, P.; Cárdenas Oyarzo, A.; Young, J.I.; Walz, K. Correct developmental expression level of Rai1 in forebrain neurons is required for control of body weight, activity levels and learning and memory. Hum. Mol. Genet. 2014, 23, 1771–1782. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Kowalczyk, M.; Fogerson, P.M.; Lee, Y.-J.; Haque, M.; Adams, E.L.; Wang, D.C.; DeNardo, L.A.; Tessier-Lavigne, M.; Huguenard, J.R.; et al. Loss of Rai1 enhances hippocampal excitability and epileptogenesis in mouse models of Smith-Magenis syndrome. Proc. Natl. Acad. Sci. USA 2022, 119, e2210122119. [Google Scholar] [CrossRef]
- Journiac, N.; Jolly, S.; Jarvis, C.; Gautheron, V.; Rogard, M.; Trembleau, A.; Blondeau, J.-P.; Mariani, J.; Vernet-der Garabedian, B. The nuclear receptor ROR(alpha) exerts a bi-directional regulation of IL-6 in resting and reactive astrocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 21365–21370. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ni, J.; Nonaka, S.; Hayashi, Y. Microglial circadian clock regulation of microglial structural complexity, dendritic spine density and inflammatory response. Neurochem. Int. 2021, 142, 104905. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.; Sutcliffe, J.S.; Cattanach, B.M.; Beechey, C.V.; Armstrong, D.; Eichele, G.; Beaudet, A.L. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat. Genet. 1997, 17, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Gustin, R.M.; Bichell, T.J.; Bubser, M.; Daily, J.; Filonova, I.; Mrelashvili, D.; Deutch, A.Y.; Colbran, R.J.; Weeber, E.J.; Haas, K.F. Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiol. Dis. 2010, 39, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, C.F.; Schonewille, M.; Gao, Z.; Aronica, E.M.A.; Judson, M.C.; Philpot, B.D.; Hoebeek, F.E.; van Woerden, G.M.; De Zeeuw, C.I.; Elgersma, Y. Dissociation of locomotor and cerebellar deficits in a murine Angelman syndrome model. J. Clin. Investig. 2015, 125, 4305–4315. [Google Scholar] [CrossRef]
- Vatsa, N.; Jana, N.R. UBE3A and Its Link With Autism. Front. Mol. Neurosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Roy, B.; Amemasor, E.; Hussain, S.; Castro, K. UBE3A: The Role in Autism Spectrum Disorders (ASDs) and a Potential Candidate for Biomarker Studies and Designing Therapeutic Strategies. Diseases 2023, 12, 7. [Google Scholar] [CrossRef]
- Fang, P.; Lev-Lehman, E.; Tsai, T.F.; Matsuura, T.; Benton, C.S.; Sutcliffe, J.S.; Christian, S.L.; Kubota, T.; Halley, D.J.; Meijers-Heijboer, H.; et al. The spectrum of mutations in UBE3A causing Angelman syndrome. Hum. Mol. Genet. 1999, 8, 129–135. [Google Scholar] [CrossRef]
- Dindot, S.V.; Antalffy, B.A.; Bhattacharjee, M.B.; Beaudet, A.L. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum. Mol. Genet. 2008, 17, 111–118. [Google Scholar] [CrossRef]
- Gardner, Z.; Holbrook, O.; Tian, Y.; Odamah, K.; Man, H.-Y. The role of glia in the dysregulation of neuronal spinogenesis in Ube3a-dependent ASD. Exp. Neurol. 2024, 376, 114756. [Google Scholar] [CrossRef]
- Cheron, G.; Márquez-Ruiz, J.; Kishino, T.; Dan, B. Disruption of the LTD dialogue between the cerebellum and the cortex in Angelman syndrome model: A timing hypothesis. Front. Syst. Neurosci. 2014, 8, 221. [Google Scholar] [CrossRef]
- Hao, X.; Sun, J.; Zhong, L.; Baudry, M.; Bi, X. UBE3A deficiency-induced autophagy is associated with activation of AMPK-ULK1 and p53 pathways. Exp. Neurol. 2023, 363, 114358. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, M.-H.; Shim, W.H.; Kim, J.K.; Jeon, E.-Y.; Kim, D.-H.; Yoon, S.-Y. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 2017, 22, 1576–1584. [Google Scholar] [CrossRef]
- Angrand, L.; Masson, J.-D.; Rubio-Casillas, A.; Nosten-Bertrand, M.; Crépeaux, G. Inflammation and Autophagy: A Convergent Point between Autism Spectrum Disorder (ASD)-Related Genetic and Environmental Factors: Focus on Aluminum Adjuvants. Toxics 2022, 10, 518. [Google Scholar] [CrossRef]
- Jones, C.; Barrera, I.; Brothers, S.; Ring, R.; Wahlestedt, C. Oxytocin and social functioning. Dialogues Clin. Neurosci. 2017, 19, 193–201. [Google Scholar] [CrossRef]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef] [PubMed]
- Froemke, R.C.; Young, L.J. Oxytocin, Neural Plasticity, and Social Behavior. Annu. Rev. Neurosci. 2021, 44, 359–381. [Google Scholar] [CrossRef] [PubMed]
- LoParo, D.; Waldman, I.D. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: A meta-analysis. Mol. Psychiatry 2015, 20, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Otowa, T.; Abe, O.; Kuwabara, H.; Aoki, Y.; Natsubori, T.; Takao, H.; Kakiuchi, C.; Kondo, K.; Ikeda, M.; et al. Oxytocin receptor gene variations predict neural and behavioral response to oxytocin in autism. Soc. Cogn. Affect. Neurosci. 2017, 12, 496–506. [Google Scholar] [CrossRef]
- Al-Ali, Z.; Yasseen, A.A.; Al-Dujailli, A.; Al-Karaqully, A.J.; McAllister, K.A.; Jumaah, A.S. The oxytocin receptor gene polymorphism rs2268491 and serum oxytocin alterations are indicative of autism spectrum disorder: A case-control paediatric study in Iraq with personalized medicine implications. PLoS ONE 2022, 17, e0265217. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [CrossRef]
- Winslow, J.T.; Insel, T.R. The social deficits of the oxytocin knockout mouse. Neuropeptides 2002, 36, 221–229. [Google Scholar] [CrossRef]
- Choe, K.Y.; Bethlehem, R.A.I.; Safrin, M.; Dong, H.; Salman, E.; Li, Y.; Grinevich, V.; Golshani, P.; DeNardo, L.A.; Peñagarikano, O.; et al. Oxytocin normalizes altered circuit connectivity for social rescue of the Cntnap2 knockout mouse. Neuron 2022, 110, 795–808.e6. [Google Scholar] [CrossRef]
- Hörnberg, H.; Pérez-Garci, E.; Schreiner, D.; Hatstatt-Burklé, L.; Magara, F.; Baudouin, S.; Matter, A.; Nacro, K.; Pecho-Vrieseling, E.; Scheiffele, P. Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature 2020, 584, 252–256. [Google Scholar] [CrossRef]
- Reichova, A.; Bacova, Z.; Bukatova, S.; Kokavcova, M.; Meliskova, V.; Frimmel, K.; Ostatnikova, D.; Bakos, J. Abnormal neuronal morphology and altered synaptic proteins are restored by oxytocin in autism-related SHANK3 deficient model. Mol. Cell. Endocrinol. 2020, 518, 110924. [Google Scholar] [CrossRef] [PubMed]
- Peñagarikano, O.; Lázaro, M.T.; Lu, X.-H.; Gordon, A.; Dong, H.; Lam, H.A.; Peles, E.; Maidment, N.T.; Murphy, N.P.; Yang, X.W.; et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med. 2015, 7, 271ra8. [Google Scholar] [CrossRef] [PubMed]
- Szabó, J.; Mlynár, M.; Feješ, A.; Renczés, E.; Borbélyová, V.; Ostatníková, D.; Celec, P. Intranasal oxytocin in a genetic animal model of autism. Mol. Psychiatry 2024, 29, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, X.; Geng, Y.; Zhao, W.; Zhou, F.; Wang, J.; Markett, S.; Biswal, B.B.; Ma, Y.; Kendrick, K.M.; et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 2019, 184, 781–789. [Google Scholar] [CrossRef]
- Shen, L.-P.; Li, W.; Pei, L.-Z.; Yin, J.; Xie, S.-T.; Li, H.-Z.; Yan, C.; Wang, J.-J.; Zhang, Q.; Zhang, X.-Y.; et al. Oxytocin Receptor in Cerebellar Purkinje Cells Does Not Engage in Autism-Related Behaviors. Cerebellum 2023, 22, 888–904. [Google Scholar] [CrossRef]
- Inutsuka, A.; Hattori, A.; Yoshida, M.; Takayanagi, Y.; Onaka, T. Cerebellar damage with inflammation upregulates oxytocin receptor expression in Bergmann Glia. Mol. Brain 2024, 17, 41. [Google Scholar] [CrossRef]
- Muhammad, T.; Pastore, S.F.; Good, K.; Ausió, J.; Vincent, J.B. Chromatin gatekeeper and modifier CHD proteins in development, and in autism and other neurological disorders. Psychiatr. Genet. 2023, 33, 213–232. [Google Scholar] [CrossRef]
- Weissberg, O.; Elliott, E. The Mechanisms of CHD8 in Neurodevelopment and Autism Spectrum Disorders. Genes 2021, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Kasah, S.; Oddy, C.; Basson, M.A. Autism-linked CHD gene expression patterns during development predict multi-organ disease phenotypes. J. Anat. 2018, 233, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Katayama, Y.; Kakegawa, W.; Ino, D.; Nishiyama, M.; Yuzaki, M.; Nakayama, K.I. The autism-associated protein CHD8 is required for cerebellar development and motor function. Cell Rep. 2021, 35, 108932. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Dong, C.; Chen, H.; Dong, X.; Yang, L.; Hu, L.; Wang, H.; Wu, B.; Yao, Y.; et al. Deletion of CHD8 in cerebellar granule neuron progenitors leads to severe cerebellar hypoplasia, ataxia, and psychiatric behavior in mice. J. Genet. Genom. 2022, 49, 859–869. [Google Scholar] [CrossRef]
- Behesti, H.; Fore, T.R.; Wu, P.; Horn, Z.; Leppert, M.; Hull, C.; Hatten, M.E. ASTN2 modulates synaptic strength by trafficking and degradation of surface proteins. Proc. Natl. Acad. Sci. USA 2018, 115, E9717–E9726. [Google Scholar] [CrossRef]
- Wilson, P.M.; Fryer, R.H.; Fang, Y.; Hatten, M.E. Astn2, a novel member of the astrotactin gene family, regulates the trafficking of ASTN1 during glial-guided neuronal migration. J. Neurosci. 2010, 30, 8529–8540. [Google Scholar] [CrossRef]
- Lionel, A.C.; Tammimies, K.; Vaags, A.K.; Rosenfeld, J.A.; Ahn, J.W.; Merico, D.; Noor, A.; Runke, C.K.; Pillalamarri, V.K.; Carter, M.T.; et al. Disruption of the ASTN2/TRIM32 locus at 9q33.1 is a risk factor in males for autism spectrum disorders, ADHD and other neurodevelopmental phenotypes. Hum. Mol. Genet. 2014, 23, 2752–2768. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature 2017, 542, 433–438. [Google Scholar] [CrossRef]
- Kaplanis, J.; Samocha, K.E.; Wiel, L.; Zhang, Z.; Arvai, K.J.; Eberhardt, R.Y.; Gallone, G.; Lelieveld, S.H.; Martin, H.C.; McRae, J.F.; et al. Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature 2020, 586, 757–762. [Google Scholar] [CrossRef]
- Nóbrega, I.d.S.; e Silva, A.L.T.; Yokota-Moreno, B.Y.; Sertié, A.L. The Importance of Large-Scale Genomic Studies to Unravel Genetic Risk Factors for Autism. Int. J. Mol. Sci. 2024, 25, 5816. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pramparo, T.; Gazestani, V.H.; Lombardo, M.V.; Pierce, K.; Lewis, N.E. The ASD Living Biology: From cell proliferation to clinical phenotype. Mol. Psychiatry 2019, 24, 88–107. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.; Volkmar, F.R. Autism and related disorders. Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 106, pp. 407–418. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes 2023, 14, 677. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.S.; Breunig, S.; Lawrence, J.M.; Foote, I.F.; Grotzinger, A.D. Characterizing Genetic Pathways Unique to Autism Spectrum Disorder at Multiple Levels of Biological Analysis. medRxiv 2024, 2024.06.07.24308616. [Google Scholar] [CrossRef]
- Zerbo, O.; Leong, A.; Barcellos, L.; Bernal, P.; Fireman, B.; Croen, L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015, 46, 232–236. [Google Scholar] [CrossRef]
- Muratori, F.; Tonacci, A.; Billeci, L.; Catalucci, T.; Igliozzi, R.; Calderoni, S.; Narzisi, A. Olfactory Processing in Male Children with Autism: Atypical Odor Threshold and Identification. J. Autism Dev. Disord. 2017, 47, 3243–3251. [Google Scholar] [CrossRef]
- Jameson, C.; Boulton, K.A.; Silove, N.; Guastella, A.J. Eczema and related atopic diseases are associated with increased symptom severity in children with autism spectrum disorder. Transl. Psychiatry 2022, 12, 415. [Google Scholar] [CrossRef]
- Man, M.-Q.; Yang, S.; Mauro, T.M.; Zhang, G.; Zhu, T. Link between the skin and autism spectrum disorder. Front. Psychiatry 2023, 14, 1265472. [Google Scholar] [CrossRef]
- Long, J.; Dang, H.; Su, W.; Moneruzzaman, M.; Zhang, H. Interactions between circulating inflammatory factors and autism spectrum disorder: A bidirectional Mendelian randomization study in European population. Front. Immunol. 2024, 15, 1370276. [Google Scholar] [CrossRef]
- Salman, E.D.; Kadlubar, S.A.; Falany, C.N. Expression and localization of cytosolic sulfotransferase (SULT) 1A1 and SULT1A3 in normal human brain. Drug Metab. Dispos. 2009, 37, 706–709. [Google Scholar] [CrossRef]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.K.; Won, H.; Pallesen, J.; Agerbo, E.; Andreassen, O.A.; Anney, R.; et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 2019, 51, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Sadakata, T.; Shinoda, Y.; Sato, A.; Iguchi, H.; Ishii, C.; Matsuo, M.; Yamaga, R.; Furuichi, T. Mouse models of mutations and variations in autism spectrum disorder-associated genes: Mice expressing Caps2/Cadps2 copy number and alternative splicing variants. Int. J. Environ. Res. Public Health 2013, 10, 6335–6353. [Google Scholar] [CrossRef] [PubMed]
- Sadakata, T.; Shinoda, Y.; Oka, M.; Sekine, Y.; Furuichi, T. Autistic-like behavioral phenotypes in a mouse model with copy number variation of the CAPS2/CADPS2 gene. FEBS Lett. 2013, 587, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Graziano, C.; Minopoli, F.; Bacchelli, E.; Magini, P.; Diquigiovanni, C.; Lomartire, S.; Bianco, F.; Vargiolu, M.; Parchi, P.; et al. Maternally inherited genetic variants of CADPS2 are present in autism spectrum disorders and intellectual disability patients. EMBO Mol. Med. 2014, 6, 795–809. [Google Scholar] [CrossRef]
- Okamoto, N.; Hatsukawa, Y.; Shimojima, K.; Yamamoto, T. Submicroscopic deletion in 7q31 encompassing CADPS2 and TSPAN12 in a child with autism spectrum disorder and PHPV. Am. J. Med. Genet. A 2011, 155A, 1568–1573. [Google Scholar] [CrossRef]
- Aristidou, C.; Koufaris, C.; Theodosiou, A.; Bak, M.; Mehrjouy, M.M.; Behjati, F.; Tanteles, G.; Christophidou-Anastasiadou, V.; Tommerup, N.; Sismani, C. Accurate Breakpoint Mapping in Apparently Balanced Translocation Families with Discordant Phenotypes Using Whole Genome Mate-Pair Sequencing. PLoS ONE 2017, 12, e0169935. [Google Scholar] [CrossRef]
- Pavone, P.; Corsello, G.; Marino, S.D.; Ruggieri, M.; Falsaperla, R. 7q31.32 partial duplication: First report of a child with dysmorphism, autistic spectrum disorder, moderate intellectual disability and, epilepsy. Literature review. Epilepsy Res. 2019, 158, 106223. [Google Scholar] [CrossRef]
- Sadakata, T.; Furuichi, T. Developmentally regulated Ca2+-dependent activator protein for secretion 2 (CAPS2) is involved in BDNF secretion and is associated with autism susceptibility. Cerebellum 2009, 8, 312–322. [Google Scholar] [CrossRef]
- Fujima, S.; Yamaga, R.; Minami, H.; Mizuno, S.; Shinoda, Y.; Sadakata, T.; Abe, M.; Sakimura, K.; Sano, Y.; Furuichi, T. CAPS2 Deficiency Impairs the Release of the Social Peptide Oxytocin, as Well as Oxytocin-Associated Social Behavior. J. Neurosci. 2021, 41, 4524–4535. [Google Scholar] [CrossRef]
- Rahman, M.A.; Orfali, R.; Dave, N.; Lam, E.; Naguib, N.; Nam, Y.-W.; Zhang, M. KCa 2.2 (KCNN2): A physiologically and therapeutically important potassium channel. J. Neurosci. Res. 2023, 101, 1699–1710. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, G.; Liu, Y.; Standley, S.; Ji, A.; Tunuguntla, R.; Wang, Y.; Claus, C.; Luo, Y.; Baudry, M.; et al. UBE3A Regulates Synaptic Plasticity and Learning and Memory by Controlling SK2 Channel Endocytosis. Cell Rep. 2015, 12, 449–461. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Zhu, G.; Cato, C.; Hao, X.; Qian, L.; Lin, W.; Adhikari, R.; Luo, Y.; Baudry, M.; et al. PKA and Ube3a regulate SK2 channel trafficking to promote synaptic plasticity in hippocampus: Implications for Angelman Syndrome. Sci. Rep. 2020, 10, 9824. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Baudry, M.; Bi, X. SK2 channel regulation of neuronal excitability, synaptic transmission, and brain rhythmic activity in health and diseases. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118834. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Hao, X.; Baudry, M.; Bi, X. Lack of UBE3A-Mediated Regulation of Synaptic SK2 Channels Contributes to Learning and Memory Impairment in the Female Mouse Model of Angelman Syndrome. Neural Plast. 2022, 2022, 3923384. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, G.; Piochon, C.; Adelman, J.P.; Hansel, C. SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron 2012, 75, 108–120. [Google Scholar] [CrossRef]
- Grasselli, G.; He, Q.; Wan, V.; Adelman, J.P.; Ohtsuki, G.; Hansel, C. Activity-Dependent Plasticity of Spike Pauses in Cerebellar Purkinje Cells. Cell Rep. 2016, 14, 2546–2553. [Google Scholar] [CrossRef]
- Grasselli, G.; Boele, H.-J.; Titley, H.K.; Bradford, N.; van Beers, L.; Jay, L.; Beekhof, G.C.; Busch, S.E.; De Zeeuw, C.I.; Schonewille, M.; et al. SK2 channels in cerebellar Purkinje cells contribute to excitability modulation in motor-learning-specific memory traces. PLoS Biol. 2020, 18, e3000596. [Google Scholar] [CrossRef]
- Zhang, X.; Novera, W.; Zhang, Y.; Deng, L.-W. MLL5 (KMT2E): Structure, function, and clinical relevance. Cell. Mol. Life Sci. 2017, 74, 2333–2344. [Google Scholar] [CrossRef]
- Pais, L.; Rodan, L.; O’Donnell-Luria, A. KMT2E-Related Neurodevelopmental Disorder. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- O’Donnell-Luria, A.H.; Pais, L.S.; Faundes, V.; Wood, J.C.; Sveden, A.; Luria, V.; Abou Jamra, R.; Accogli, A.; Amburgey, K.; Anderlid, B.M.; et al. Heterozygous Variants in KMT2E Cause a Spectrum of Neurodevelopmental Disorders and Epilepsy. Am. J. Hum. Genet. 2019, 104, 1210–1222. [Google Scholar] [CrossRef]
- Li, Y.-J.; Li, C.-Y.; Li, C.-Y.; Hu, D.-X.; Xv, Z.-B.; Zhang, S.-H.; Li, Q.; Zhang, P.; Tian, B.; Lan, X.-L.; et al. KMT2E Haploinsufficiency Manifests Autism-Like Behaviors and Amygdala Neuronal Development Dysfunction in Mice. Mol. Neurobiol. 2023, 60, 1609–1625. [Google Scholar] [CrossRef]
- Li, Y.; Fan, L.; Luo, R.; Yang, Z.; Yuan, M.; Zhang, J.; Gan, J. Case Report: De novo Variants of KMT2E Cause O’Donnell-Luria-Rodan Syndrome: Additional Cases and Literature Review. Front. Pediatr. 2021, 9, 641841. [Google Scholar] [CrossRef]
- Abreu, N.J.; Siemon, A.E.; Baylis, A.L.; Kirschner, R.E.; Pfau, R.B.; Ho, M.-L.; Hickey, S.E.; Truxal, K.V. Novel truncating variant in KMT2E associated with cerebellar hypoplasia and velopharyngeal dysfunction. Clin. Case Rep. 2022, 10, e05277. [Google Scholar] [CrossRef]
- Schaffner, S.L.; Lussier, A.A.; Baker, J.A.; Goldowitz, D.; Hamre, K.M.; Kobor, M.S. Neonatal Alcohol Exposure in Mice Induces Select Differentiation- and Apoptosis-Related Chromatin Changes Both Independent of and Dependent on Sex. Front. Genet. 2020, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Licatalosi, D.D.; Yano, M.; Fak, J.J.; Mele, A.; Grabinski, S.E.; Zhang, C.; Darnell, R.B. Ptbp2 represses adult-specific splicing to regulate the generation of neuronal precursors in the embryonic brain. Genes Dev. 2012, 26, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Fisher, E.; Li, L.; Zhong, P.; Yan, Z.; Feng, J. PTBP2 attenuation facilitates fibroblast to neuron conversion by promoting alternative splicing of neuronal genes. Stem Cell Rep. 2023, 18, 2268–2282. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, A.; Calaza, M.; Rodriguez-Fontenla, C.; Carracedo, A. Novel Gene-Based Analysis of ASD GWAS: Insight Into the Biological Role of Associated Genes. Front. Genet. 2019, 10, 733. [Google Scholar] [CrossRef]
- Jones, R.M.; Cadby, G.; Blangero, J.; Abraham, L.J.; Whitehouse, A.J.O.; Moses, E.K. MACROD2 gene associated with autistic-like traits in a general population sample. Psychiatr. Genet. 2014, 24, 241–248. [Google Scholar] [CrossRef]
- Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism 2017, 8, 21. [Google Scholar] [CrossRef]
- Bacchelli, E.; Cameli, C.; Viggiano, M.; Igliozzi, R.; Mancini, A.; Tancredi, R.; Battaglia, A.; Maestrini, E. An integrated analysis of rare CNV and exome variation in Autism Spectrum Disorder using the Infinium PsychArray. Sci. Rep. 2020, 10, 3198. [Google Scholar] [CrossRef]
- Alnak, A.; Kuşcu Özücer, İ.; Okay Çağlayan, A.; Coşkun, M. Peripheral Expression of MACROD2 Gene Is Reduced Among a Sample of Turkish Children with Autism Spectrum Disorder. Psychiatry Clin. Psychopharmacol. 2021, 31, 261–268. [Google Scholar] [CrossRef]
- Jin, N.; Burkard, M.E. MACROD2, an Original Cause of CIN? Cancer Discov. 2018, 8, 921–923. [Google Scholar] [CrossRef]
- DeWitt, J.J.; Hecht, P.M.; Grepo, N.; Wilkinson, B.; Evgrafov, O.V.; Morris, K.V.; Knowles, J.A.; Campbell, D.B. Transcriptional Gene Silencing of the Autism-Associated Long Noncoding RNA MSNP1AS in Human Neural Progenitor Cells. Dev. Neurosci. 2016, 38, 375–383. [Google Scholar] [CrossRef]
- DeWitt, J.J.; Grepo, N.; Wilkinson, B.; Evgrafov, O.V.; Knowles, J.A.; Campbell, D.B. Impact of the Autism-Associated Long Noncoding RNA MSNP1AS on Neuronal Architecture and Gene Expression in Human Neural Progenitor Cells. Genes 2016, 7, 76. [Google Scholar] [CrossRef]
- Bilinovich, S.M.; Lewis, K.; Grepo, N.; Campbell, D.B. The Long Noncoding RNA RPS10P2-AS1 Is Implicated in Autism Spectrum Disorder Risk and Modulates Gene Expression in Human Neuronal Progenitor Cells. Front. Genet. 2019, 10, 970. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Sands, T.T.; Miceli, F.; Lesca, G.; Beck, A.E.; Sadleir, L.G.; Arrington, D.K.; Schönewolf-Greulich, B.; Moutton, S.; Lauritano, A.; Nappi, P.; et al. Autism and developmental disability caused by KCNQ3 gain-of-function variants. Ann. Neurol. 2019, 86, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.P.; Doddato, G.; Valentino, F.; Baldassarri, M.; Tita, R.; Fallerini, C.; Bruttini, M.; Lo Rizzo, C.; Mencarelli, M.A.; Mari, F.; et al. New Candidates for Autism/Intellectual Disability Identified by Whole-Exome Sequencing. Int. J. Mol. Sci. 2021, 22, 13439. [Google Scholar] [CrossRef]
- Wright, D.; Kenny, A.; Eley, S.; McKechanie, A.G.; Stanfield, A.C. Visual social attention in SYNGAP1-related intellectual disability. Autism Res. 2024, 17, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Kenny, A.; Mizen, L.A.M.; McKechanie, A.G.; Stanfield, A.C. The Behavioral Profile of SYNGAP1-Related Intellectual Disability. Am. J. Intellect. Dev. Disabil. 2024, 129, 199–214. [Google Scholar] [CrossRef]
- Agarwal, M.; Johnston, M.V.; Stafstrom, C.E. SYNGAP1 mutations: Clinical, genetic, and pathophysiological features. Int. J. Dev. Neurosci. 2019, 78, 65–76. [Google Scholar] [CrossRef]
- Chen, H.J.; Rojas-Soto, M.; Oguni, A.; Kennedy, M.B. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 1998, 20, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Liao, D.; Lau, L.F.; Huganir, R.L. SynGAP: A synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron 1998, 20, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Birtele, M.; Del Dosso, A.; Xu, T.; Nguyen, T.; Wilkinson, B.; Hosseini, N.; Nguyen, S.; Urenda, J.-P.; Knight, G.; Rojas, C.; et al. Non-synaptic function of the autism spectrum disorder-associated gene SYNGAP1 in cortical neurogenesis. Nat. Neurosci. 2023, 26, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sanders, S.J.; Liu, L.; De Rubeis, S.; Lim, E.T.; Sutcliffe, J.S.; Schellenberg, G.D.; Gibbs, R.A.; Daly, M.J.; Buxbaum, J.D.; et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS Genet. 2013, 9, e1003671. [Google Scholar] [CrossRef]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Zhou, X.; Feliciano, P.; Shu, C.; Wang, T.; Astrovskaya, I.; Hall, J.B.; Obiajulu, J.U.; Wright, J.R.; Murali, S.C.; Xu, S.X.; et al. Integrating de novo and inherited variants in 42,607 autism cases identifies mutations in new moderate-risk genes. Nat. Genet. 2022, 54, 1305–1319. [Google Scholar] [CrossRef]
- Gozes, I.; Bassan, M.; Zamostiano, R.; Pinhasov, A.; Davidson, A.; Giladi, E.; Perl, O.; Glazner, G.W.; Brenneman, D.E. A novel signaling molecule for neuropeptide action: Activity-dependent neuroprotective protein. Ann. N. Y. Acad. Sci. 1999, 897, 125–135. [Google Scholar] [CrossRef]
- D’Incal, C.; Van Dijck, A.; Ibrahim, J.; De Man, K.; Bastini, L.; Konings, A.; Elinck, E.; Gozes, L.; Marusic, Z.; Anicic, M.; et al. ADNP dysregulates methylation and mitochondrial gene expression in the cerebellum of a Helsmoortel-Van der Aa syndrome autopsy case. Acta Neuropathol. Commun. 2024, 12, 62. [Google Scholar] [CrossRef]
- Gould, E.; Kim, J.H. SCN2A contributes to oligodendroglia excitability and development in the mammalian brain. Cell Rep. 2021, 36, 109653. [Google Scholar] [CrossRef]
- Murray, M.; Martindale, J.M.; Otallah, S.I. SCN2A- Associated Episodic and Persistent Ataxia with Cerebellar Atrophy: A Case Report. Child Neurol. Open 2023, 10, 2329048X231163944. [Google Scholar] [CrossRef]
- Fu, J.M.; Satterstrom, F.K.; Peng, M.; Brand, H.; Collins, R.L.; Dong, S.; Wamsley, B.; Klei, L.; Wang, L.; Hao, S.P.; et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat. Genet. 2022, 54, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Derderian, K.D.; Hamada, E.; Zhou, X.; Nelson, A.D.; Kyoung, H.; Ahituv, N.; Bouvier, G.; Bender, K.J. Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-associated ASD. Neuron 2024, 112, 1444–1455.e5. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.; Satterstrom, F.K.; Buxbaum, J.D.; Mahjani, B. Identification of moderate effect size genes in autism spectrum disorder through a novel gene pairing approach. medRxiv 2024, 2024.04.03.24305278. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, P.; Jin, X.; Xu, X.; Li, J.; Li, Z.; Wang, M.; Wang, T.; Wu, X.; Jiang, Y.; et al. Genomic landscapes of Chinese sporadic autism spectrum disorders revealed by whole-genome sequencing. J. Genet. Genom. 2018, 45, 527–538. [Google Scholar] [CrossRef]
- Chernikova, M.A.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-Zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef]
- Wang, L.W.; Tancredi, D.J.; Thomas, D.W. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J. Dev. Behav. Pediatr. 2011, 32, 351–360. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wei, H.; Chen, J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems 2021, 6, e01343-20. [Google Scholar] [CrossRef]
- Avolio, E.; Olivito, I.; Rosina, E.; Romano, L.; Angelone, T.; De Bartolo, A.; Scimeca, M.; Bellizzi, D.; D’Aquila, P.; Passarino, G.; et al. Modifications of Behavior and Inflammation in Mice Following Transplant with Fecal Microbiota from Children with Autism. Neuroscience 2022, 498, 174–189. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Fang, J.; Guo, J.; Lao, Y.; Kang, S.-G.; Huang, K.; Tong, T. L-tyrosine alleviates autism-like behavior in mice by remodeling the gut microbiota. Brain Behav. Immun. 2025, 127, 358–374. [Google Scholar] [CrossRef]
- Borbélyová, V.; Szabó, J.; Sušienková, P.; Potvin, J.; Belvončíková, P.; Groß, T.; Jančovičová, A.; Bačová, Z.; Rašková, B.; Szadvári, I.; et al. The Effect of Parental Faecal Microbiome Transplantation from Children with Autism Spectrum Disorder on Behavior and Gastrointestinal Manifestations in the Male Offspring of Shank3 Mice. Int. J. Mol. Sci. 2025, 26, 5927. [Google Scholar] [CrossRef] [PubMed]
- Prince, N.; Peralta Marzal, L.N.; Markidi, A.; Ahmed, S.; Adolfs, Y.; Pasterkamp, R.J.; Kumar, H.; Roeselers, G.; Garssen, J.; Kraneveld, A.D.; et al. Prebiotic diet normalizes aberrant immune and behavioral phenotypes in a mouse model of autism spectrum disorder. Acta Pharmacol. Sin. 2024, 45, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiong, Y.; Li, Y. The Impact of Valproic Acid on Microbiota in a Mouse Model of Autism Spectrum Disorder. Psychiatry Clin. Psychopharmacol. 2025, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Hou, W.; Bi, D.; Yin, F.; Gao, Y.; Huang, D.; Li, Y.; Cao, Z.; Yan, Y.; et al. Fecal microbiota transplantation improves VPA-induced ASD mice by modulating the serotonergic and glutamatergic synapse signaling pathways. Transl. Psychiatry 2023, 13, 17. [Google Scholar] [CrossRef]
- Cristiano, C.; Hoxha, E.; Lippiello, P.; Balbo, I.; Russo, R.; Tempia, F.; Miniaci, M.C. Maternal treatment with sodium butyrate reduces the development of autism-like traits in mice offspring. Biomed. Pharmacother. 2022, 156, 113870. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Blanchard, D.C. The BTBR mouse model of idiopathic autism—Current view on mechanisms. Neurosci. Biobehav. Rev. 2017, 76, 99–110. [Google Scholar] [CrossRef]
- Xiao, R.; Zhong, H.; Li, X.; Ma, Y.; Zhang, R.; Wang, L.; Zang, Z.; Fan, X. Abnormal Cerebellar Development Is Involved in Dystonia-Like Behaviors and Motor Dysfunction of Autistic BTBR Mice. Front. Cell. Dev. Biol. 2020, 8, 231. [Google Scholar] [CrossRef]
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav. Immun. 2018, 73, 310–319. [Google Scholar] [CrossRef]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered Intestinal Morphology and Microbiota Composition in the Autism Spectrum Disorders Associated SHANK3 Mouse Model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef]
- Robinson-Agramonte, M.d.L.A.; Noris García, E.; Fraga Guerra, J.; Vega Hurtado, Y.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- He, Y.; He, Y.; Cheng, B. Identification of Bacterial Lipopolysaccharide-Associated Genes and Molecular Subtypes in Autism Spectrum Disorder. Pharmacogenomics Pers. Med. 2025, 18, 1–18. [Google Scholar] [CrossRef]
- Cerilli, E.; Dall’O, G.M.; Chelini, G.; Catena, B.; Weinberger, B.; Bozzi, Y.; Pangrazzi, L. Immune system dysfunction and inflammation in aging Shank3b mutant mice, a model of autism spectrum disorder. Front. Immunol. 2024, 15, 1447385. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Cerilli, E.; Balasco, L.; Dall’O’, G.M.; Chelini, G.; Pastore, A.; Weinberger, B.; Bozzi, Y. N-acetylcysteine counteracts immune dysfunction and autistic-related behaviors in the Shank3b mouse model of Autism Spectrum Disorders. bioRxiv 2024, 2024.03.13.584809. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-K.; Choi, G.B.; Huh, J.R. Maternal inflammation and its ramifications on fetal neurodevelopment. Trends Immunol. 2022, 43, 230–244. [Google Scholar] [CrossRef]
- Haida, O.; Al Sagheer, T.; Balbous, A.; Francheteau, M.; Matas, E.; Soria, F.; Fernagut, P.O.; Jaber, M. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl. Psychiatry 2019, 9, 124. [Google Scholar] [CrossRef]
- Patel, S.; Dale, R.C.; Rose, D.; Heath, B.; Nordahl, C.W.; Rogers, S.; Guastella, A.J.; Ashwood, P. Maternal immune conditions are increased in males with autism spectrum disorders and are associated with behavioural and emotional but not cognitive co-morbidity. Transl. Psychiatry 2020, 10, 286. [Google Scholar] [CrossRef]
- Aavani, T.; Rana, S.A.; Hawkes, R.; Pittman, Q.J. Maternal immune activation produces cerebellar hyperplasia and alterations in motor and social behaviors in male and female mice. Cerebellum 2015, 14, 491–505. [Google Scholar] [CrossRef]
- Pendyala, G.; Chou, S.; Jung, Y.; Coiro, P.; Spartz, E.; Padmashri, R.; Li, M.; Dunaevsky, A. Maternal Immune Activation Causes Behavioral Impairments and Altered Cerebellar Cytokine and Synaptic Protein Expression. Neuropsychopharmacology 2017, 42, 1435–1446. [Google Scholar] [CrossRef]
- Zhang, X.; Ibi, M.; Haga, R.; Iwata, K.; Matsumoto, M.; Asaoka, N.; Liu, J.; Katsuyama, M.; Yabe-Nishimura, C. NOX1/NADPH oxidase affects the development of autism-like behaviors in a maternal immune activation model. Biochem. Biophys. Res. Commun. 2021, 534, 59–66. [Google Scholar] [CrossRef]
- Chauhan, A.; Audhya, T.; Chauhan, V. Brain region-specific glutathione redox imbalance in autism. Neurochem. Res. 2012, 37, 1681–1689. [Google Scholar] [CrossRef]
- Shi, L.; Smith, S.E.P.; Malkova, N.; Tse, D.; Su, Y.; Patterson, P.H. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lucchina, L.; Depino, A.M. Altered peripheral and central inflammatory responses in a mouse model of autism. Autism Res. 2014, 7, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Kanner, L.; Lesser, L.I. Early infantile autism. Pediatr. Clin. N. Am. 1958, 5, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Tonge, B.J.; Dissanayake, C.; Brereton, A.V. Autism: Fifty years on from Kanner. J. Paediatr. Child Health 1994, 30, 102–107. [Google Scholar] [CrossRef]
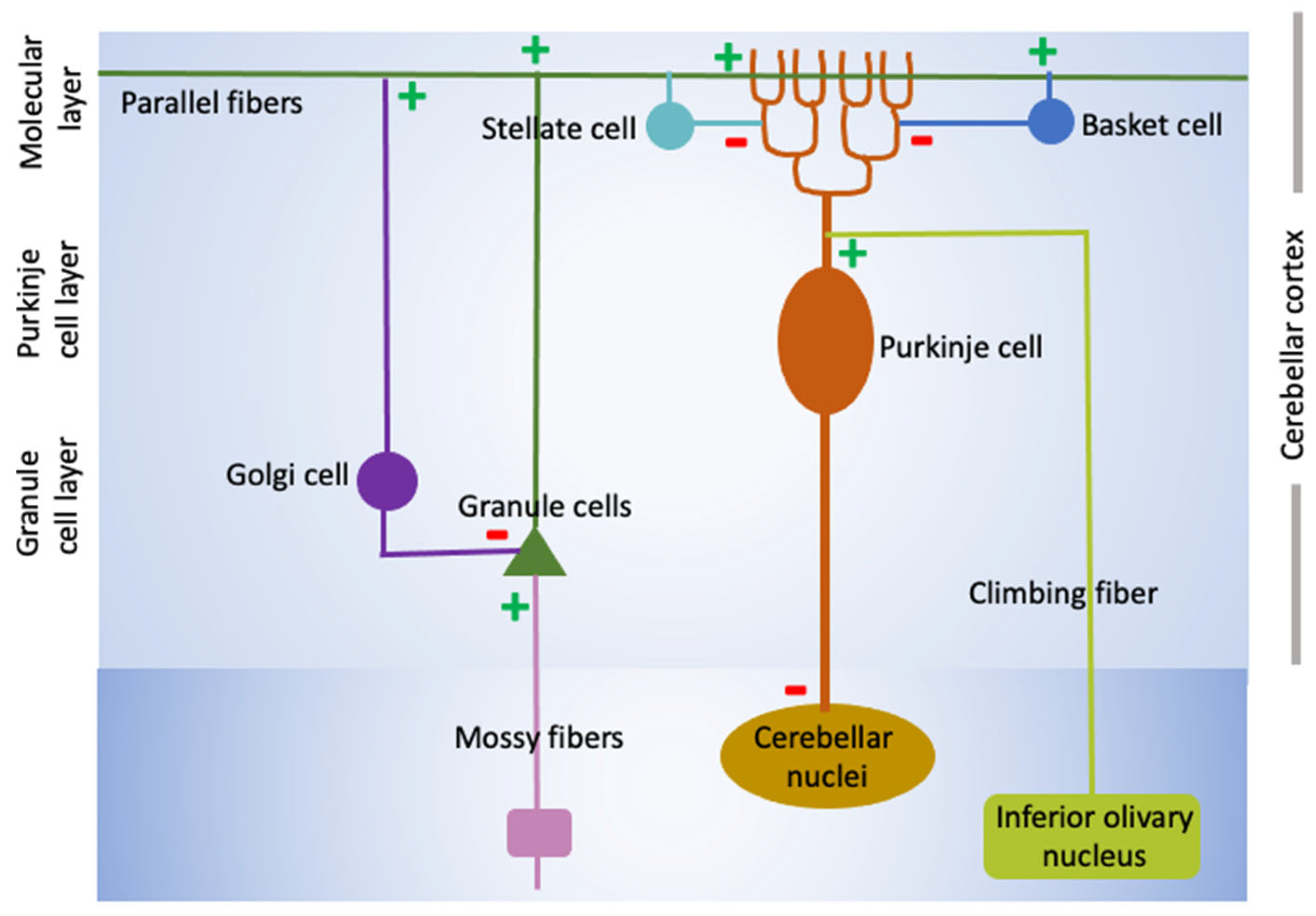
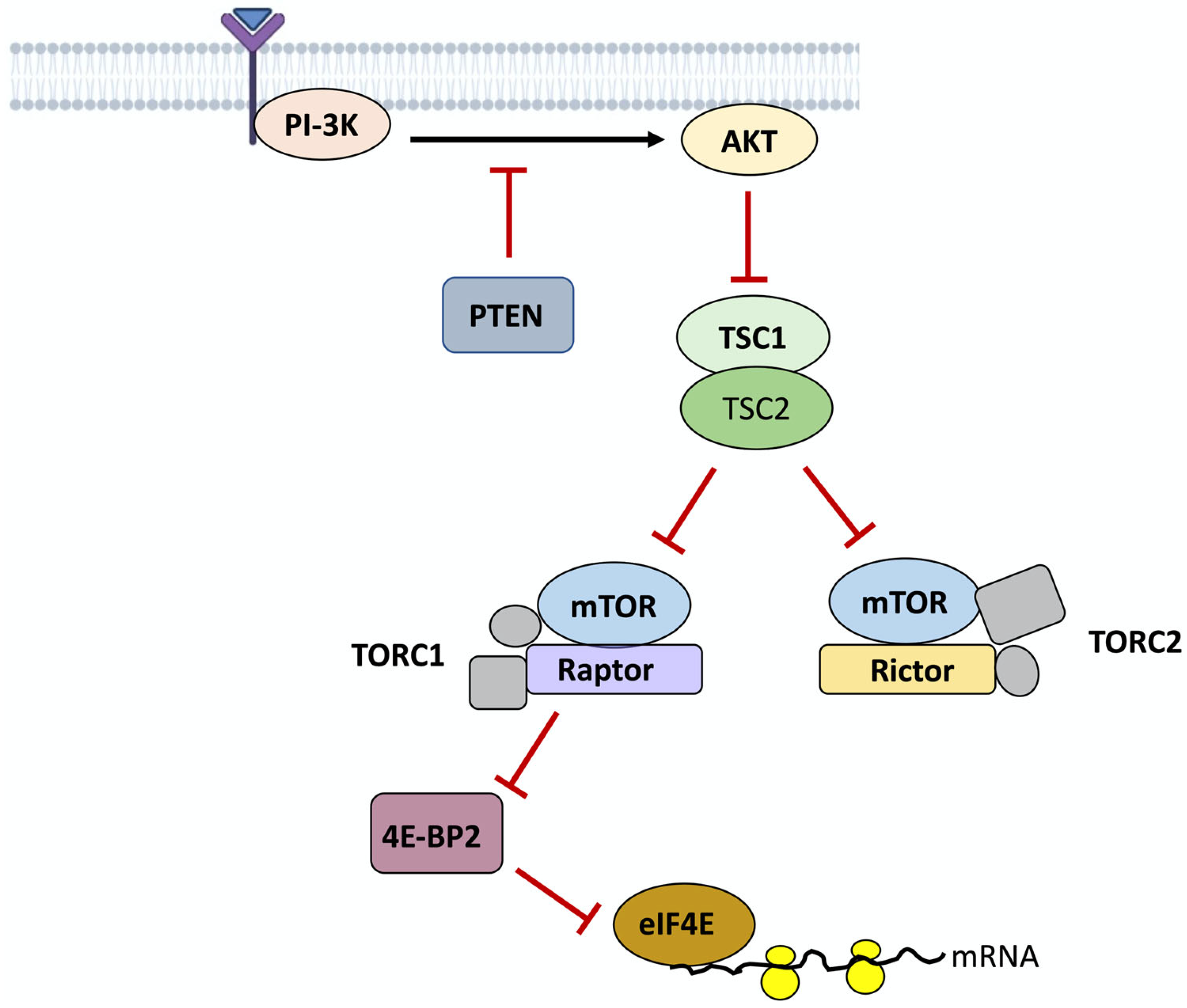
| Protein | Functional Class | Primary Function | Association with ASD and Potential Mechanisms |
|---|---|---|---|
| SHANKs | Synaptic | Postsynaptic scaffolding proteins at glutaminergic synapses. |
|
| Neuroligins (NLGNs) | Synaptic | Postsynaptic transmembrane proteins regulating synapse formation, organization, and function. |
|
| Neurexins (NRXNs) | Synaptic | Presynaptic transmembrane cell adhesion proteins that regulate synapse formation, organization of release machinery, and E/I balance. |
|
| Contactin-associated protein-2 (CNTNAP2) | Synaptic | Cell adhesion molecule involved in synapse organization. Also involved in the clustering voltage-gated K+ channels myelinated axons at Nodes of Ranvier. |
|
| Cadherins (CDHs) | Cell adhesion | Involved in the formation of neural circuitry and regulation of synaptic plasticity. |
|
| CUB and sushi multiple domains-3 (CSDM3) | Synaptic Development | Regulates dendrite development and synaptogenesis. |
|
| Engrailed-2 (EN-2) | Development | Transcription factor regulating pattern formation that is expressed highly in the cerebellum and hindbrain. |
|
| Tuberous sclerosis complex protein-1 and 2 (TSC1/TSC2) | Tissue homeostasis | Act as part of a complex to inhibit mTOR signaling. TSC1/TSC2 mutations cause tuberous sclerosis complex. |
|
| PTEN | Tumor suppressor | Lipid phosphatase that negatively regulates cell division and survival. |
|
| BMAL1 | Transcription factor | Critical component of the circadian rhythm regulatory machinery. Also regulates mRNA translation. |
|
| Retinoic acid receptor-α (RORα) | Nuclear receptor | Multiple roles. In the CNS, RORα regulates stem cell production, neuronal differentiation, and morphogenesis of brain structures. |
|
| UBE3A | E3 ubiquitin ligase and transcriptional regulator for steroid hormone receptors | Regulates synaptic function and plasticity |
|
| Oxytocin (OXT) | Neuropeptide | Uterine contractions. |
|
| Chromodomain helicase DNA-binding protein-2 and 8 (CHD2/CHD8) | Chromatin remodeling protein. | Regulates transcription, DNA replication, and DNA repair by controlling chromatin structure and accessibility. |
|
| Astrotactin-2 (ASTN2) | Transmembrane glycoprotein | Regulates trafficking and degradation of cell surface proteins. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Mello, S.R. Autism Spectrum Disorder: The Cerebellum, Genes, and Pathways. Neurol. Int. 2025, 17, 173. https://doi.org/10.3390/neurolint17100173
D’Mello SR. Autism Spectrum Disorder: The Cerebellum, Genes, and Pathways. Neurology International. 2025; 17(10):173. https://doi.org/10.3390/neurolint17100173
Chicago/Turabian StyleD’Mello, Santosh R. 2025. "Autism Spectrum Disorder: The Cerebellum, Genes, and Pathways" Neurology International 17, no. 10: 173. https://doi.org/10.3390/neurolint17100173
APA StyleD’Mello, S. R. (2025). Autism Spectrum Disorder: The Cerebellum, Genes, and Pathways. Neurology International, 17(10), 173. https://doi.org/10.3390/neurolint17100173






