Diverging Safety Signals: A Trend Analysis of Suspected Adverse Drug Reactions Reporting for Spinal Muscular Atrophy Therapies in the European Union
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darras, B.T. Spinal muscular atrophies. Pediatr. Clin. N. Am. 2015, 62, 743–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H. New and Developing Therapies in Spinal Muscular Atrophy: From Genotype to Phenotype to Treatment and Where Do We Stand? Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef] [PubMed]
- Talbot, K.; Tizzano, E.F. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017, 24, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef] [PubMed]
- European Public Assessment Report (EPAR) for Spinraza. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spinraza (accessed on 25 April 2025).
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Peña, L.D.M.; Shieh, P.B.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Muntoni, F.; Baranello, G.; Masson, R.; Boespflug-Tanguy, O.; Bruno, C.; Corti, S.; Daron, A.; Deconinck, N.; Servais, L.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 832–841. [Google Scholar] [CrossRef] [PubMed]
- European Public Assessment Report (EPAR) for Zolgensma. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma (accessed on 25 April 2025).
- Darras, B.T.; Masson, R.; Mazurkiewicz-Bełdzińska, M.; Rose, K.; Xiong, H.; Zanoteli, E.; Baranello, G.; Bruno, C.; Vlodavets, D.; Wang, Y.; et al. Risdiplam-Treated Infants with Type 1 Spinal Muscular Atrophy versus Historical Controls. N. Engl. J. Med. 2021, 385, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022, 21, 42–52. [Google Scholar] [CrossRef] [PubMed]
- European Public Assessment Report (EPAR) for Evrysdi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evrysdi (accessed on 25 April 2025).
- Post-Authorisation Efficacy Studies. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/post-authorisation-efficacy-studies-questions-answers (accessed on 25 April 2025).
- Risk Management Plans. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/pharmacovigilance-marketing-authorisation/risk-management/risk-management-plans (accessed on 25 April 2025).
- EudraVigilance System Overview. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance/eudravigilance-system-overview (accessed on 25 April 2025).
- European Medicines Agency Policy on Access to EudraVigilance Data for Medicinal Products for Human Use. Available online: https://www.ema.europa.eu/en/documents/other/european-medicines-agency-policy-access-eudravigilance-data-medicinal-products-human-use-revision-4_en.pdf (accessed on 25 April 2025).
- Rieder, M.; Belančić, A. Past, present and future of drug safety: Editorial. Br. J. Clin. Pharmacol. 2024, 90, 1760–1762. [Google Scholar] [CrossRef] [PubMed]
- European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/en/index.html (accessed on 25 April 2025).
- Belančić, A.; Mas, P.; Miletić, L.; Kovačić Bytyqi, B.; Vitezić, D. Post-Marketing Safety of Spinal Muscular Atrophy Therapies: Analysis of Spontaneous Adverse Drug Reactions from EudraVigilance. J. Clin. Med. 2025, 14, 3173. [Google Scholar] [CrossRef] [PubMed]
- Joinpoint Regression Program. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 25 April 2025).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000, 19, 335–351, Erratum in Stat. Med. 2001, 20, 655. [Google Scholar] [CrossRef]
- Moulis, G.; Sommet, A.; Durrieu, G.; Bagheri, H.; Lapeyre-Mestre, M.; Montastruc, J.L.; French Association of PharmacoVigilance Centres. Trends of reporting of ‘serious’ vs. ‘non-serious’ adverse drug reactions over time: A study in the French PharmacoVigilance Database. Br. J. Clin. Pharmacol. 2012, 74, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Weber, J. Epidemiology of adverse reactions to nonsteroidal antiinflammatory drugs. In Advances in Inflammatory Research; Rainsford, K.D., Velo, G.P., Eds.; Raven Press: New York, NY, USA, 1984; pp. 1–7. [Google Scholar]
- Hoffman, K.B.; Dimbil, M.; Erdman, C.B.; Tatonetti, N.P.; Overstreet, B.M. The Weber effect and the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS): Analysis of sixty-two drugs approved from 2006 to 2010. Drug Saf. 2014, 37, 283–294, Erratum in Drug Saf. 2014, 37, 381. [Google Scholar] [CrossRef] [PubMed]
- McAdams, M.A.; Governale, L.A.; Swartz, L.; Hammad, T.A.; Dal Pan, G.J. Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: Revisiting the Weber effect. Pharmacoepidemiol. Drug Saf. 2008, 17, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Fan, M.; Gao, W.; Chen, C.; Dai, G. Adverse events in different administration routes of semaglutide: A pharmacovigilance study based on the FDA adverse event reporting system. Front. Pharmacol. 2024, 15, 1414268. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Product Information for Evrysdi. Available online: https://www.ema.europa.eu/en/documents/product-information/evrysdi-epar-product-information_en.pdf (accessed on 25 May 2025).
- Day, J.W.; Mendell, J.R.; Mercuri, E.; Finkel, R.S.; Strauss, K.A.; Kleyn, A.; Tauscher-Wisniewski, S.; Tukov, F.F.; Reyna, S.P.; Chand, D.H. Clinical Trial and Postmarketing Safety of Onasemnogene Abeparvovec Therapy. Drug Saf. 2021, 44, 1109–1119, Erratum in Drug Saf. 2022, 45, 191–192. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Direct Healthcare Professional Communication—Zolgensma (onasemnogene abeparvovec): Risk for thrombotic microangiopathy. Available online: https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-zolgensma-onasemnogene-abeparvovec-risk-thrombotic-microangiopathy_en.pdf (accessed on 25 May 2025).
- European Medicines Agency. Direct Healthcare Professional Communication ZOLGENSMA (onasemnogene abeparvovec)—Fatal cases of acute liver failure. Available online: https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-zolgensma-onasemnogene-abeparvovec-fatal-cases-acute-liver-failure_en.pdf (accessed on 25 May 2025).
- Hazell, L.; Shakir, S.A. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
- Shalviri, G.; Mohebbi, N.; Mirbaha, F.; Majdzadeh, R.; Yazdizadeh, B.; Gholami, K.; Grobler, L.; Rose, C.J.; Chin, W.Y. Improving adverse drug event reporting by healthcare professionals. Cochrane Database Syst. Rev. 2024, 10, CD012594. [Google Scholar] [CrossRef] [PubMed]
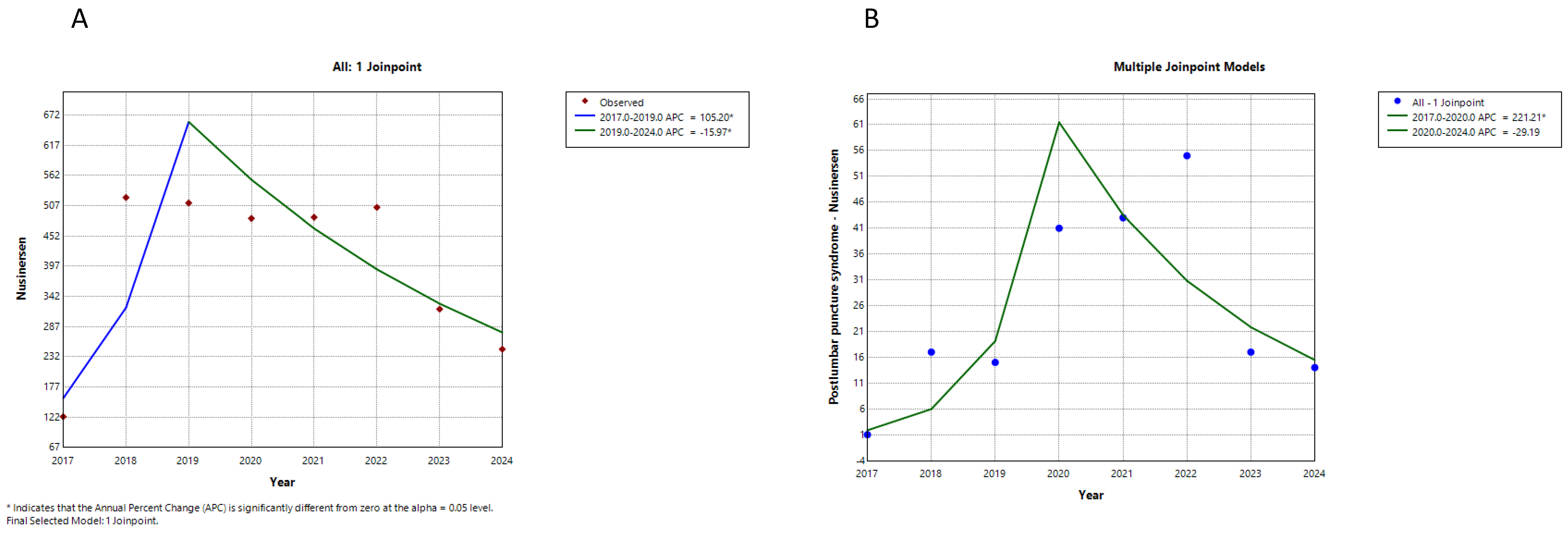

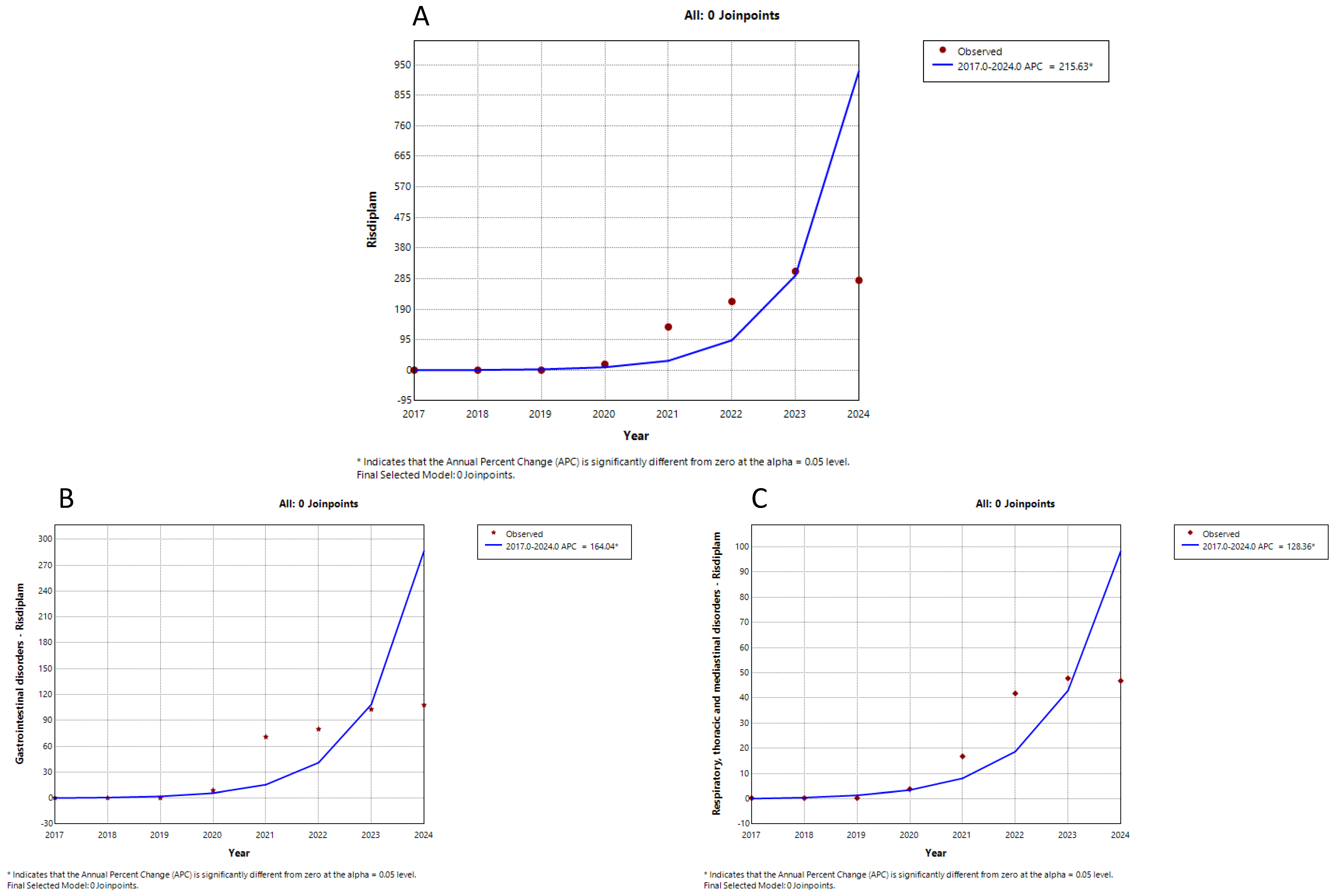
| Medicine | Trend Segment | Annual Percent Change (%) | p-Value |
|---|---|---|---|
| Nusinersen all suspected ADRs | 1 | 105.2 | 0.004 |
| 2 | −16.0 | 0.016 | |
| Nusinersen—postlumbar puncture syndrome | 1 | 221.2 | 0.000 |
| 2 | −29.2 | 0.083 | |
| Onasemnogene abeparvovec all suspected ADRs | 1 | 657.4 | 0.000 |
| 2 | 18.1 | 0.567 | |
| Onasemnogene abeparvovec—suspected ADRs affecting liver | 1 | 394.3 | 0.000 |
| 2 | −14.0 | 0.669 | |
| Onasemnogene abeparvovec—troponin increase | 1 | 174.7 | 0.033 |
| 2 | 9.6 | 0.775 | |
| Risdiplam all suspected ADRs | 1 | 215.6 | 0.000 |
| Risdiplam gastrointestinal suspected ADRs | 1 | 164.0 | 0.000 |
| Risdiplam respiratory suspected ADRs | 1 | 128.3 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belančić, A.; Mas, P.; Stević, I.; Vitezić, D.; Janković, S. Diverging Safety Signals: A Trend Analysis of Suspected Adverse Drug Reactions Reporting for Spinal Muscular Atrophy Therapies in the European Union. Neurol. Int. 2025, 17, 165. https://doi.org/10.3390/neurolint17100165
Belančić A, Mas P, Stević I, Vitezić D, Janković S. Diverging Safety Signals: A Trend Analysis of Suspected Adverse Drug Reactions Reporting for Spinal Muscular Atrophy Therapies in the European Union. Neurology International. 2025; 17(10):165. https://doi.org/10.3390/neurolint17100165
Chicago/Turabian StyleBelančić, Andrej, Petar Mas, Ivana Stević, Dinko Vitezić, and Slobodan Janković. 2025. "Diverging Safety Signals: A Trend Analysis of Suspected Adverse Drug Reactions Reporting for Spinal Muscular Atrophy Therapies in the European Union" Neurology International 17, no. 10: 165. https://doi.org/10.3390/neurolint17100165
APA StyleBelančić, A., Mas, P., Stević, I., Vitezić, D., & Janković, S. (2025). Diverging Safety Signals: A Trend Analysis of Suspected Adverse Drug Reactions Reporting for Spinal Muscular Atrophy Therapies in the European Union. Neurology International, 17(10), 165. https://doi.org/10.3390/neurolint17100165








