Abstract
Background and objectives: Frontotemporal dementia (FTD) is a heterogeneous neurodegenerative disorder with autosomal dominant forms most often linked to MAPT, GRN, and C9orf72. We aimed to evaluate the prevalence of pathogenic variants in these genes in a hospital-based cohort of FTD patients assessed at a tertiary referral center in southeastern Serbia. Methods: We studied 58 consecutive patients with FTD spectrum syndromes evaluated at a tertiary referral center. All underwent standardized neurological, neuropsychological, and imaging assessments, and family history was recorded. Genetic testing included validated assays for C9orf72 repeat expansions and next-generation sequencing of MAPT and GRN. Results: Women comprised 53.45% of the cohort. The mean age was 67.88 years, with mean onset at 61.70 years. Behavioral variant FTD predominated (75.87%), while language forms were less frequent. Positive family history was present in 16 patients (27.59%). Pathogenic variants were identified in three individuals (5.17%): two unrelated carriers of the intronic MAPT mutation c.1920+16C>T and one patient with a C9orf72 expansion. No GRN variants were detected. Mutation frequency was 18.75% in familial cases, while none were found among sporadic patients (p = 0.018). Four of nine relatives were asymptomatic MAPT mutation carriers. Conclusions: This first genetic study of FTD in southeastern Serbia revealed a lower mutation frequency than in Northern and Western Europe, but similar to cohorts from Southeastern Europe. The detection of MAPT c.1920+16C>T in two unrelated families extends the geographic range of this splice-site variant and underscores the importance of systematic genetic testing and larger collaborative studies in the Balkans.
1. Introduction
Frontotemporal dementia (FTD) represents a spectrum of clinical syndromes caused by neurodegenerative diseases collectively referred to as frontotemporal lobar degeneration (FTLD). These entities are clinically, pathologically, and genetically highly heterogeneous. In a narrower sense, the clinical spectrum of frontotemporal dementias includes the behavioral variant of FTD (bvFTD), characterized by progressive changes in behavior and personality, as well as the primary progressive aphasias, most commonly the non-fluent/agrammatic variant (nfvPPA) and the semantic variant (svPPA), which predominantly affect language functions. In addition, although less frequent, either at disease onset or later in the course of progression, overlap with extrapyramidal disorders such as corticobasal syndrome (CBS) and progressive supranuclear palsy (PSP), or with motor neuron disease, may occur [1].
From a genetic perspective, most cases are sporadic; however, autosomal dominant mutations are registered in approximately 30–40% of cases [2]. The most common autosomal dominant familial forms of FTD are associated with mutations in three genes: MAPT (microtubule-associated protein tau), GRN (progranulin), and C9orf72 (chromosome 9 open reading frame 72). These mutations result in distinct pathological changes and neurodegeneration, but ultimately lead to clinical syndromes with considerable phenotypic overlap [3].
The prevalence of different mutations varies across geographic regions and populations. For example, C9orf72 repeat expansions show a markedly higher prevalence in Northern Europe, particularly in Scandinavia [4]. Likewise, GRN and MAPT mutations exhibit regional founder effects in certain European populations [5]. For southeastern Europe and the Balkan region, only a limited number of studies have examined the prevalence of these mutations. Available evidence suggests a potentially lower frequency of autosomal dominant mutations associated with the FTD spectrum compared to Northern and Western Europe [6,7]. The reasons for these regional differences may lie in population-specific genetic backgrounds, underdiagnosis, or limited access to genetic testing. Therefore, further research is necessary to provide a clearer picture of FTD genetics in southeastern Europe.
The aim of this study was to investigate the frequency of MAPT, GRN, and C9orf72 mutations in a hospital-based cohort of patients evaluated at a tertiary university hospital serving the population of southeastern Serbia.
2. Materials and Methods
2.1. Study Population
This study included 58 consecutive patients with a diagnosis of either bvFTD, nfvPPA, or svPPA who were evaluated at the Clinic of Neurology, University Clinical Center Niš, Serbia, between 2023 and 2025. This university hospital serves as a tertiary referral center for the southern and eastern regions of Serbia, covering a population of approximately 1.4 million people. The diagnoses of bvFTD, nfvPPA and svPPA were established according to internationally accepted criteria [8,9]. Patients with an initial presentation of bvFTD who subsequently developed extrapyramidal features consistent with CBS and PSP, or clinical features of amyotrophic lateral sclerosis (FTD-ALS), were also included [10,11,12]. This is a hospital-based, consecutive cohort from a tertiary referral center and does not represent a defined percentage of all FTD cases in the region served by the center. A flow chart of the study population and genetic testing is shown in Figure 1.
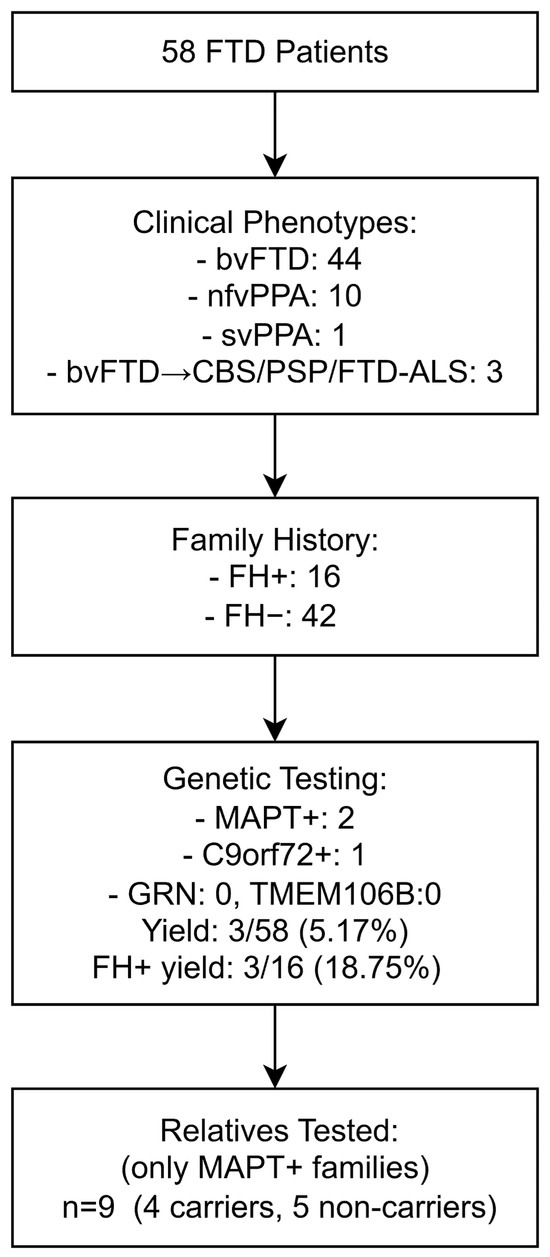
Figure 1.
Flow chart of the study population, clinical classification, results of genetic testing of patients, and cascade testing of relatives. Note: MAPT+, patients positive for MAPT mutation; C9orf72+, patients positive for C9orf72 expansion; FH+, positive family history; FH−, negative family history. Other abbreviations are listed at the end of the manuscript.
2.2. Clinical Assessment
Clinical data were obtained as part of the routine diagnostic work-up for patients with cognitive disorders. All patients underwent a standardized diagnostic protocol including neurological examination, cognitive screening, and behavioral evaluation, followed by laboratory investigations, brain computed tomography (CT) or magnetic resonance imaging (MRI), and detailed neuropsychological testing. For the purposes of this study, results from the Mini-Mental State Examination (MMSE) [13] and the Frontal Assessment Battery (FAB) [14] were extracted from the neuropsychological evaluation. In patients who provided informed consent, lumbar puncture with cerebrospinal fluid (CSF) biomarker analysis was performed to exclude Alzheimer’s disease (AD).
Family history was considered positive (FH+) if, based on information obtained from the patient, caregivers, family members, or available medical records, one or more relatives up to the third degree of kinship were identified with clinical syndromes consistent with FTLD spectrum disorders (bvFTD, PPA, FTD-ALS, ALS, CBS, PSP) or with undefined early-onset dementia, in accordance with Goldman categories 1–3 [15]; otherwise, patients were classified as FH−.
Genetic testing was performed for the three genes most commonly implicated in autosomal dominant forms of FTD: MAPT, GRN, and C9orf72. In addition, the TMEM106B gene, a known genetic risk modifier, was analyzed. Genetic counseling was offered to all patients, and genetic testing for the same panel of genes was made available to family members of identified mutation carriers.
2.3. Genetic Analysis
Genomic DNA was extracted from peripheral blood and analyzed at PreventionGenetics (Marshfield, WI, USA), a CLIA-certified (CLIA #52D2065132) and CAP-accredited (CAP #7185561) laboratory. The C9orf72 hexanucleotide repeat expansion was evaluated with four complementary assays [16,17]. Two repeat-primed PCR assays targeted the 3′ and 5′ ends of the repeat, and two additional fragment-length assays provided size estimates for non-expanded alleles. This combination allows both detection of expanded alleles and sizing of shorter ones, up to ~40 repeats. Alleles with fewer than 25 repeats are considered normal, those with more than 30 as pathogenic, while the intermediate range (25–30 repeats) is regarded as uncertain. The method is reliable but not without its limitations: very large expansions cannot be sized, allelic dropout may occur in heterozygous cases, and variants outside the repeat region remain undetected.
For GRN, MAPT, and TMEM106B, sequencing was performed with a hybrid-capture next-generation sequencing (NGS) panel on a NovaSeq 6000 (Illumina, Inc., San Diego, CA, USA) platform using 150 bp paired-end reads. The assay covered all coding exons and plus 10 base pairs of flanking intronic sequences, as well as selected non-coding regions known to harbor pathogenic variants. Data processing relied on validated pipelines for alignment, variant calling, and quality checks. Average coverage was close to 100×, and more than 98% of bases were sequenced at a depth of at least 20×. Regions that fell below this threshold were resequenced by Sanger to ensure completeness.
Copy number variants (CNVs) for GRN, MAPT, and TMEM106B were evaluated from NGS data at PreventionGenetics using an internally developed read-depth algorithm that compares mean read depth and distribution for each target against multiple matched controls; neighboring target read depth and the zygosity of variants within each target were used to reinforce calls. The pipeline shows sensitivity approaching 100% for CNVs affecting ≥4 exons, whereas sensitivity for smaller events is lower (~75%). Where technically indicated, smaller CNVs were not reported if orthogonal confirmation was not feasible.
Primary processing (alignment, variant calling, and CNV calling) was performed at the sequencing laboratory (PreventionGenetics). The authors interpreted finalized clinical reports, which also included methodological details, and did not re-analyze raw data.
Variants were classified according to American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) guidelines [18] into the standard categories: pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, benign, risk allele, or pseudodeficiency. Synonymous variants without predicted impact on splicing or protein function were generally considered likely benign and not reported. Similarly, benign, likely benign, risk, and pseudodeficiency variants were not included. Interpretation drew on both public and commercial databases, and nomenclature followed the recommendations of the Human Genome Variation Society (HGVS) (http://www.hgvs.org (accessed on 30 August 2025)).
2.4. Statistical Analysis
Statistical analyses were performed using SPSS Statistics (IBM Corp., version 31) and R (version 4.0.5). Categorical variables were summarized as absolute and relative frequencies with exact 95% confidence intervals calculated by the Clopper–Pearson method. Proportions were compared using Fisher’s exact test (two-sided). Risk differences were reported with 95% confidence intervals estimated by the Newcombe–Wilson score method (without continuity correction). Continuous variables were summarized as means ± standard deviations (SD) with ranges, and group comparisons (FH+ vs. FH−) were performed using the Mann–Whitney U test and the chi-square test, where appropriate. A p-value < 0.05 was considered statistically significant. Group-level statistical testing was performed only for FH+ vs. FH− patients. Findings from the mutation-carrier subgroup are shown descriptively.
2.5. Reporting Guidelines
This observational cohort study was conducted and is reported in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [19].
3. Results
A total of 67 individuals were included in the study, comprising 58 patients with FTD and 9 unaffected relatives from MAPT-positive families. Among the patients, 31 were female (53.45%) and 27 male (46.55%). The mean age was 67.88 ± 9.09 years (range 46–88), with a mean age at onset of 61.70 ± 8.71 years (range 42–75). The mean disease duration was 6.17 ± 3.31 years (range 2–16). Patients had on average 12.47 ± 2.98 years of education (range 4–21). Neuropsychological assessment showed moderate impairment, with a mean MMSE of 21.05 ± 6.95 (range 3–30) and a mean FAB of 8.58 ± 3.90 (range 2–17). The most common clinical phenotype was bvFTD, observed in 44 patients (75.87%), followed by nfvPPA in 10 (17.25%) and svPPA in 1 (1.72%). Three additional patients (1.72% each) later developed features of CBS, PSP, or FTD-ALS, although their initial presentation was characterized by behavioral changes consistent with bvFTD. This distribution reflects the expected phenotypic heterogeneity in tertiary-care FTD cohorts, with standardized diagnostic criteria applied across all cases. A positive family history (FH+) was documented in 16 patients (27.59%), while the remaining 42 patients were classified as FH− (Table 1).

Table 1.
Demographic and clinical characteristics of patients with frontotemporal dementia (n = 58).
Genetic analysis identified pathogenic variants in a minority of cases (Table 2). Two unrelated patients carried the heterozygous intronic MAPT pathogenic variant c.1920+16C>T, which affects splicing and is associated with autosomal dominant familial FTD. Both index cases presented with a bvFTD phenotype and had a positive family history. Genetic testing was extended to nine unaffected relatives from these two families. In Family A, two of four children were mutation carriers, whereas the proband’s mother and brother tested negative. In Family B, both children carried the MAPT variant, while the patient’s sister was non-carrier. Altogether, MAPT mutations were identified in six individuals (two affected patients and four unaffected relatives), corresponding to a carrier rate of 44.44% (4/9) among at-risk relatives. One additional patient with a bvFTD phenotype tested positive for a pathogenic C9orf72 expansion (>30 repeats), while all other tested individuals carried normal alleles (<25 repeats). No intermediate alleles (25–30 repeats) were detected. No pathogenic variants were detected in GRN or TMEM106B, and no variants of uncertain significance (VUS) were identified. No exon-level CNVs were detected across GRN, MAPT, or TMEM106B. Analyses concerning the three mutation carriers are descriptive only (Table 3).

Table 2.
Genetic findings in patients with frontotemporal dementia (n = 58).

Table 3.
Clinical and genetic characteristics of mutation carriers.
The overall diagnostic yield among clinically diagnosed FTD patients was 5.17% (3/58). Yield was higher among those with a positive family history (3/16, 18.75%) compared with those without (0/42, 0.00%; Fisher’s exact test, p = 0.018; risk difference 18.75%, 95% CI 3.98–43.01).
No significant demographic or clinical differences were observed between FH+ and FH− patients. Age at onset (U = 247.00, Z = −1.55, p = 0.121), disease duration (U = 324.50, Z = −0.20, p = 0.840), years of education (U = 281.00, Z = −1.00, p = 0.316), and sex distribution (χ2(1) = 0.276, p = 0.599) were similar across groups. MMSE (U = 233.50, Z = −1.79, p = 0.074) and FAB scores (U = 119.00, Z = −1.93, p = 0.054) tended to be lower in FH+ patients, but did not reach statistical significance.
4. Discussion
This study represents the first analysis of FTD genetics in a hospital-based tertiary care cohort from southeastern Serbia. Among 58 clinically diagnosed patients within the FTD spectrum, we identified pathogenic mutations in three individuals (5.2%), comprising two MAPT carriers and one C9orf72 expansion carrier, while no GRN mutations were detected. Four unaffected relatives were also identified as MAPT mutation carriers. These findings may add to the evidence suggesting lower frequencies of pathogenic variants in the Balkans than in Northern and Western Europe, a notion supported by several other hospital-based cohorts across Europe.
In Northern Europe, particularly in Scandinavia, C9orf72 has emerged as the predominant genetic cause of FTD. The initial Finnish observations [20] were later confirmed in Sweden, where mutations were found in 45 of 132 patients (34%). C9orf72 accounted for more than a quarter of the entire cohort (26.5%), whereas GRN (6.8%) and MAPT (0.8%) were much less frequent. In Sweden, mutations were detected in 76% of patients with a strong family history but also in 20% of those classified as apparently sporadic, highlighting how differences in family history assessment can influence observed yields [21]. By contrast, in our series the overall yield was substantially lower (5.2%), with pathogenic variants confined to FH+ cases (18.8%), and none detected among sporadic patients.
In Central and Western Europe, somewhat different patterns have been observed. In a large German multicenter study [22], pathogenic variants were found in 18% overall and in 75% of FH+ cases, again dominated by C9orf72 (51%), followed by GRN (28%) and MAPT (12%). In Belgium, 22% overall and 34% of FH+ cases carried a pathogenic variant, with C9orf72 and GRN contributing equally (~10% each) and MAPT being less frequent (3%) [23]. In the UK, 19% of patients harbored mutations, with C9orf72 (35%) and GRN (33%) both common, but MAPT unusually prominent (29% of genetic cases) [24], a finding that may in part be related to a founder effect described in families of Welsh origin [25]. This latter observation is particularly relevant to our study, where MAPT mutation carriers were also identified.
Data from Southern and Southeastern Europe remain limited but are gradually emerging. In Southern Italy, Capozzo et al. examined 65 FTD patients (22 familial, 43 sporadic) and identified only one novel GRN splice-site mutation, corresponding to a very low overall yield of 1.5% (4.5% among familial cases), with no C9orf72 or MAPT variants detected [26]. In Belgrade, Serbia, Stefanova et al. reported pathogenic variants in 7.8% of patients, mainly five C9orf72 expansions and three GRN mutations, but no MAPT mutation carriers [6]. In Greece, Ramos et al. found a yield of 9.3%, with contributions from both C9orf72 and GRN [27], while in a Bulgarian dementia cohort C9orf72 expansions accounted for 3.7% of cases [7]. A study from Istanbul, Turkey, reported a higher overall yield of 17.8%, with pathogenic variants identified in C9orf72, GRN, and MAPT [28]. Although heterogeneous, these Southern and Southeastern European data consistently show lower yields than those reported in Northern, Central and Western Europe, where hospital-based series often range from 16–34%. Our present findings from Niš, Serbia (5.2% overall; 18.8% among FH+ patients) are in line with this pattern, slightly higher than Italy but lower than Turkey and Greece.
Within our series, 27.6% of patients had a positive family history. Although no significant demographic or clinical differences were observed between FH+ and FH− patients, the FH+ group tended to score lower on both MMSE and FAB. While not statistically significant, this pattern may hint at a more aggressive clinical course in familial cases, a possibility that deserves further study in larger cohorts.
To our knowledge, MAPT-positive cases had not previously been reported in Serbia. In the Serbian cohort from Belgrade, Stefanova et al. identified no MAPT mutation carriers [6]. Of particular interest in our series, therefore, is the detection of the MAPT c.1920+16C>T (also known as IVS10+16C>T) variant in two apparently unrelated families from southeastern Serbia. While GRN and C9orf72 mutations are usually more frequent worldwide, MAPT variants remain relatively rare and often show geographic clustering. The intronic MAPT variant c.1920+16C>T shifts pre-mRNA splicing toward the inclusion of exon 10. Because exon 10 encodes one of the four microtubule-binding repeats of tau, its preferential inclusion increases the proportion of 4-repeat (4R) tau isoforms relative to 3-repeat (3R) tau [29]. This isoform imbalance promotes tau aggregation and represents a well-recognized pathogenic mechanism in FTLD-tau [30,31]. Together with the exonic variants c.837C>A (p.Asn279Lys, N279K) and c.902C>T (p.Pro301Leu, P301L), c.1920+16C>T is among the most frequently reported MAPT mutations, associated with a wide clinical spectrum including bvFTD, PSP-like, and occasionally AD-like presentations. [32,33]. A strong founder effect has been documented in North Wales, where genealogical and haplotype studies traced the origin of this variant to around 23 generations ago [25]. Against this background, the presence of the same mutation in two independent families from Serbia is noteworthy. It expands the known geographic distribution of MAPT c.1920+16C>T into the Balkans and underscores the importance of including MAPT in routine testing for the FTD spectrum in this region. Whether these families share a distant founder haplotype or represent independent mutational events remains an open question, one that could be clarified by future haplotype analyses.
This study has some limitations. The sample size is modest, and as a single-center hospital-based series it may not reflect the full spectrum of FTD genetics in the wider population of southeastern Serbia. We did not perform haplotype analyses, which prevents confirmation of a potential founder status. The small number of genetically confirmed cases also limits genotype–phenotype correlations, and the cognitive differences between FH+ and FH− groups, while suggestive, cannot be interpreted with confidence. As a single-center hospital-based series, the cohort may be subject to selection bias, and we were unable to quantify how representative it is of all regional FTD cases. Finally, as our testing panel focused on the three major genes, pathogenic variants in other FTD-associated genes may have been missed.
5. Conclusions
Despite these limitations, our study provides the first systematic data on FTD genetics from southeastern Serbia and adds to the emerging evidence of regional variation across Europe. We confirm that pathogenic variants account for only a subset of cases, at lower frequencies than in Northern and Western Europe, with an absence of GRN mutations in our cohort. The detection of the MAPT c.1920+16C>T mutation in two families illustrates how rare founder variants may also be present in the Balkans, highlighting the importance of systematic genetic testing in clinical practice. Larger collaborative studies, ideally including haplotype analysis, will be essential to clarify the origins of these variants and to improve genetic counseling, patient management, and research in the region.
Author Contributions
Conceptualization, V.M. and J.B.; methodology, V.M. and J.B.; formal analysis, V.M.; investigation, V.M., E.A. and M.M.; resources, M.S. (Marija Semnic) and M.S. (Milan Stoiljković); data curation, V.M., E.A., M.S. (Marija Semnic) and M.M.; writing—original draft preparation, V.M.; writing—review and editing, V.M., J.B., M.S. (Marija Semnic), E.A., M.M. and M.S. (Milan Stoiljković); supervision, V.M., J.B. and M.S. (Milan Stoiljković); project administration, V.M.; funding acquisition, V.M., J.B. and M.S. (Milan Stoiljković). All authors have read and agreed to the published version of the manuscript.
Funding
Genetic testing was conducted as part of the prescreening procedure for participation in the multicenter clinical trial DNLI-H-0001 (NCT05262023), sponsored by Denali Therapeutics Inc. and co-developed with Takeda Pharmaceutical Company Limited.
Institutional Review Board Statement
The study was approved by the Ethics Committee of the Republic of Serbia (approval no. 515-20-26891-22-002, 12 January 2023; and approval no. 515-20-05578-23-003, 7 August 2023), the Medicines and Medical Devices Agency of Serbia (ALIMS; approval no. 515-04-27124-22-1, 23 March 2023; and approval no. 515-04-05872-2023-4, 31 August 2023). All procedures were conducted in accordance with the Declaration of Helsinki and relevant national regulations. Genetic testing was performed as part of a clinical trial DNLI-H-0001 (NCT05262023), sponsored by Denali Therapeutics Inc. No eligible patients were identified for subsequent screening and enrollment, and therefore the Serbian site was later marked as withdrawn on ClinicalTrials.gov. Genetic data were anonymized and processed in compliance with applicable data protection regulations.
Informed Consent Statement
All participants or their legal representatives provided written informed consent prior to testing.
Data Availability Statement
The data presented in this study were generated as part of the pre-screening procedure for the multicenter clinical trial DNLI-H-0001 (NCT05262023). Due to ethical and legal restrictions, including patient privacy and data protection regulations, the datasets are not publicly available. Anonymized data may be made available from the corresponding author upon reasonable request and with appropriate institutional and regulatory approvals.
Acknowledgments
We thank Denali Therapeutics Inc. and Takeda Pharmaceutical Company Limited for granting permission to publish prescreening genetic results. We also gratefully acknowledge the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (Grant No. 451-03-137/2025-03/200113) for supporting our work. Finally, we thank all patients and their families for their invaluable participation and contribution to this study.
Conflicts of Interest
Vuk Milošević has served as the principal investigator at the University Clinical Center Niš for the multicenter clinical trial DNLI-H-0001 (NCT05262023), sponsored by Denali Therapeutics Inc. and co-developed with Takeda Pharmaceutical Company Limited. At the same site, Eva Antić has served as sub-investigator and study coordinator, and Marina Malobabić as sub-investigator. The sponsor had no role in the design of this analysis, data interpretation, or manuscript preparation. The authors declare no additional conflicts of interest related to this work.
Abbreviations
The following abbreviations are used in this manuscript:
| ACMG | American College of Medical Genetics and Genomics |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| bvFTD | Behavioral Variant Frontotemporal Dementia |
| CBS | Corticobasal Syndrome |
| CI | Confidence Interval |
| CLIA | Clinical Laboratory Improvement Amendments |
| CNV | Copy number variants |
| CSF | Cerebrospinal Fluid |
| CT | Computed Tomography |
| FAB | Frontal Assessment Battery |
| FH | Family History |
| FH+ | Positive Family History |
| FH− | Negative Family History |
| FTD | Frontotemporal Dementia |
| FTD-ALS | Frontotemporal Dementia with Amyotrophic Lateral Sclerosis |
| FTLD | Frontotemporal Lobar Degeneration |
| GRN | Progranulin |
| HGVS | Human Genome Variation Society |
| MAPT | Microtubule-Associated Protein Tau |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic Resonance Imaging |
| nfvPPA | Non-Fluent/Agrammatic Variant Primary Progressive Aphasia |
| NGS | Next-Generation Sequencing |
| PPA | Primary Progressive Aphasia |
| PSP | Progressive Supranuclear Palsy |
| SD | Standard Deviation |
| SNV | Single Nucleotide Variant |
| svPPA | Semantic Variant Primary Progressive Aphasia |
| TMEM106B | Transmembrane Protein 106B |
| VUS | Variant of Uncertain Significance |
References
- Ulugut, H.; Pijnenburg, Y.A. Frontotemporal Dementia: Past, Present, and Future. Alzheimer’s Dement. 2023, 19, 5253–5263. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Bonvicini, C.; Scassellati, C.; Carrara, M.; Maj, C.; Fostinelli, S.; Binetti, G.; Ghidoni, R.; Benussi, L. The Missing Heritability of Sporadic Frontotemporal Dementia: New Insights from Rare Variants in Neurodegenerative Candidate Genes. Int. J. Mol. Sci. 2019, 20, 3903. [Google Scholar] [CrossRef] [PubMed]
- Van Mossevelde, S.; Engelborghs, S.; van der Zee, J.; Van Broeckhoven, C. Genotype–Phenotype Links in Frontotemporal Lobar Degeneration. Nat. Rev. Neurol. 2018, 14, 363–378. [Google Scholar] [CrossRef]
- Costa, B.; Manzoni, C.; Bernal-Quiros, M.; Kia, D.A.; Aguilar, M.; Alvarez, I.; Alvarez, V.; Andreassen, O.; Anfossi, M.; Bagnoli, S. C9orf72, Age at Onset, and Ancestry Help Discriminate Behavioral from Language Variants in FTLD Cohorts. Neurology 2020, 95, e3288–e3302. [Google Scholar] [CrossRef]
- Greaves, C.V.; Rohrer, J.D. An Update on Genetic Frontotemporal Dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, E.; Marjanović, A.; Dobričić, V.; Mandić-Stojmenović, G.; Stojković, T.; Branković, M.; Šarčević, M.; Novaković, I.; Kostić, V.S. Frequency of C9orf72, GRN, and MAPT Pathogenic Variants in Patients Recruited at the Belgrade Memory Center. Neurogenetics 2024, 25, 193–200. [Google Scholar] [CrossRef]
- Mehrabian, S.; Thonberg, H.; Raycheva, M.; Lilius, L.; Stoyanova, K.; Forsell, C.; Cavallin, L.; Nesheva, D.; Westman, E.; Toncheva, D. Phenotypic Variability and Neuropsychological Findings Associated with C9orf72 Repeat Expansions in a Bulgarian Dementia Cohort. PLoS ONE 2018, 13, e0208383. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F. Classification of Primary Progressive Aphasia and Its Variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M. Criteria for the Diagnosis of Corticobasal Degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L. Clinical Diagnosis of Progressive Supranuclear Palsy: The Movement Disorder Society Criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Shefner, J.M.; Al-Chalabi, A.; Baker, M.R.; Cui, L.-Y.; de Carvalho, M.; Eisen, A.; Grosskreutz, J.; Hardiman, O.; Henderson, R.; Matamala, J.M. A Proposal for New Diagnostic Criteria for ALS. Clin. Neurophysiol. 2020, 131, 1975–1978. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at Bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Goldman, J.; Farmer, J.; Wood, E.; Johnson, J.; Boxer, A.; Neuhaus, J.; Lomen-Hoerth, C.; Wilhelmsen, K.; Lee, V.-Y.; Grossman, M. Comparison of Family Histories in FTLD Subtypes and Related Tauopathies. Neurology 2005, 65, 1817–1819. [Google Scholar] [CrossRef] [PubMed]
- Cleary, E.M.; Pal, S.; Azam, T.; Moore, D.J.; Swingler, R.; Gorrie, G.; Stephenson, L.; Colville, S.; Chandran, S.; Porteous, M. Improved PCR Based Methods for Detecting C9orf72 Hexanucleotide Repeat Expansions. Mol. Cell. Probes 2016, 30, 218–224. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Majounie, E.; Renton, A.E.; Mok, K.; Dopper, E.G.; Waite, A.; Rollinson, S.; Chiò, A.; Restagno, G.; Nicolaou, N.; Simon-Sanchez, J. Frequency of the C9orf72 Hexanucleotide Repeat Expansion in Patients with Amyotrophic Lateral Sclerosis and Frontotemporal Dementia: A Cross-Sectional Study. Lancet Neurol. 2012, 11, 323–330. [Google Scholar] [CrossRef]
- Öijerstedt, L.; Chiang, H.-H.; Björkström, J.; Forsell, C.; Lilius, L.; Lindström, A.-K.; Thonberg, H.; Graff, C. Confirmation of High Frequency of C9orf72 Mutations in Patients with Frontotemporal Dementia from Sweden. Neurobiol. Aging 2019, 84, 241.e21–241.e25. [Google Scholar] [CrossRef]
- Wagner, M.; Lorenz, G.; Volk, A.E.; Brunet, T.; Edbauer, D.; Berutti, R.; Zhao, C.; Anderl-Straub, S.; Bertram, L.; Danek, A. Clinico-Genetic Findings in 509 Frontotemporal Dementia Patients. Mol. Psychiatry 2021, 26, 5824–5832. [Google Scholar] [CrossRef]
- Van Langenhove, T.; Van Der Zee, J.; Gijselinck, I.; Engelborghs, S.; Vandenberghe, R.; Vandenbulcke, M.; De Bleecker, J.; Sieben, A.; Versijpt, J.; Ivanoiu, A. Distinct Clinical Characteristics of C9orf72 Expansion Carriers Compared with GRN, MAPT, and Nonmutation Carriers in a Flanders-Belgian FTLD Cohort. JAMA Neurol. 2013, 70, 365–373. [Google Scholar]
- Mahoney, C.J.; Beck, J.; Rohrer, J.D.; Lashley, T.; Mok, K.; Shakespeare, T.; Yeatman, T.; Warrington, E.K.; Schott, J.M.; Fox, N.C. Frontotemporal Dementia with the C9ORF72 Hexanucleotide Repeat Expansion: Clinical, Neuroanatomical and Neuropathological Features. Brain 2012, 135, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Tavian, D.; Baker, M.C.; Richardson, A.M.; Snowden, J.S.; Neary, D.; Mann, D.M.; Pickering-Brown, S.M. Recent Origin and Spread of a Common Welsh MAPT Splice Mutation Causing Frontotemporal Lobar Degeneration. Neurogenetics 2009, 10, 313–318. [Google Scholar] [CrossRef]
- Capozzo, R.; Sassi, C.; Hammer, M.B.; Arcuti, S.; Zecca, C.; Barulli, M.R.; Tortelli, R.; Gibbs, J.R.; Crews, C.; Seripa, D. Clinical and Genetic Analyses of Familial and Sporadic Frontotemporal Dementia Patients in Southern Italy. Alzheimer’s Dement. 2017, 13, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.M.; Koros, C.; Dokuru, D.R.; Van Berlo, V.; Kroupis, C.; Wojta, K.; Wang, Q.; Andronas, N.; Matsi, S.; Beratis, I.N. Frontotemporal Dementia Spectrum: First Genetic Screen in a Greek Cohort. Neurobiol. Aging 2019, 75, 224.e1–224.e8. [Google Scholar] [CrossRef]
- Guven, G.; Lohmann, E.; Bras, J.; Gibbs, J.R.; Gurvit, H.; Bilgic, B.; Hanagasi, H.; Rizzu, P.; Heutink, P.; Emre, M. Mutation Frequency of the Major Frontotemporal Dementia Genes, MAPT, GRN and C9ORF72 in a Turkish Cohort of Dementia Patients. PLoS ONE 2016, 11, e0162592. [Google Scholar] [CrossRef] [PubMed]
- Sposito, T.; Preza, E.; Mahoney, C.J.; Setó-Salvia, N.; Ryan, N.S.; Morris, H.R.; Arber, C.; Devine, M.J.; Houlden, H.; Warner, T.T. Developmental Regulation of Tau Splicing Is Disrupted in Stem Cell-Derived Neurons from Frontotemporal Dementia Patients with the 10+ 16 Splice-Site Mutation in MAPT. Hum. Mol. Genet. 2015, 24, 5260–5269. [Google Scholar] [CrossRef]
- Hutton, M.; Lendon, C.L.; Rizzu, P.; Baker, M.; Froelich, S.; Houlden, H.; Pickering-Brown, S.; Chakraverty, S.; Isaacs, A.; Grover, A. Association of Missense and 5′-Splice-Site Mutations in Tau with the Inherited Dementia FTDP-17. Nature 1998, 393, 702–705. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Murrell, J.R.; Goedert, M.; Farlow, M.R.; Klug, A.; Ghetti, B. Mutation in the Tau Gene in Familial Multiple System Tauopathy with Presenile Dementia. Proc. Natl. Acad. Sci. USA 1998, 95, 7737–7741. [Google Scholar] [CrossRef] [PubMed]
- Pickering-Brown, S.; Richardson, A.; Snowden, J.; McDonagh, A.; Burns, A.; Braude, W.; Baker, M.; Liu, W.; Yen, S.; Hardy, J. Inherited Frontotemporal Dementia in Nine British Families Associated with Intronic Mutations in the Tau Gene. Brain 2002, 125, 732–751. [Google Scholar] [CrossRef] [PubMed]
- Ghetti, B.; Oblak, A.L.; Boeve, B.F.; Johnson, K.A.; Dickerson, B.C.; Goedert, M. Invited Review: Frontotemporal Dementia Caused by Microtubule-Associated Protein Tau Gene (MAPT) Mutations: A Chameleon for Neuropathology and Neuroimaging. Neuropathol. Appl. Neurobiol. 2015, 41, 24–46. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).