Analysis of Upper Facial Weakness in Central Facial Palsy Following Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
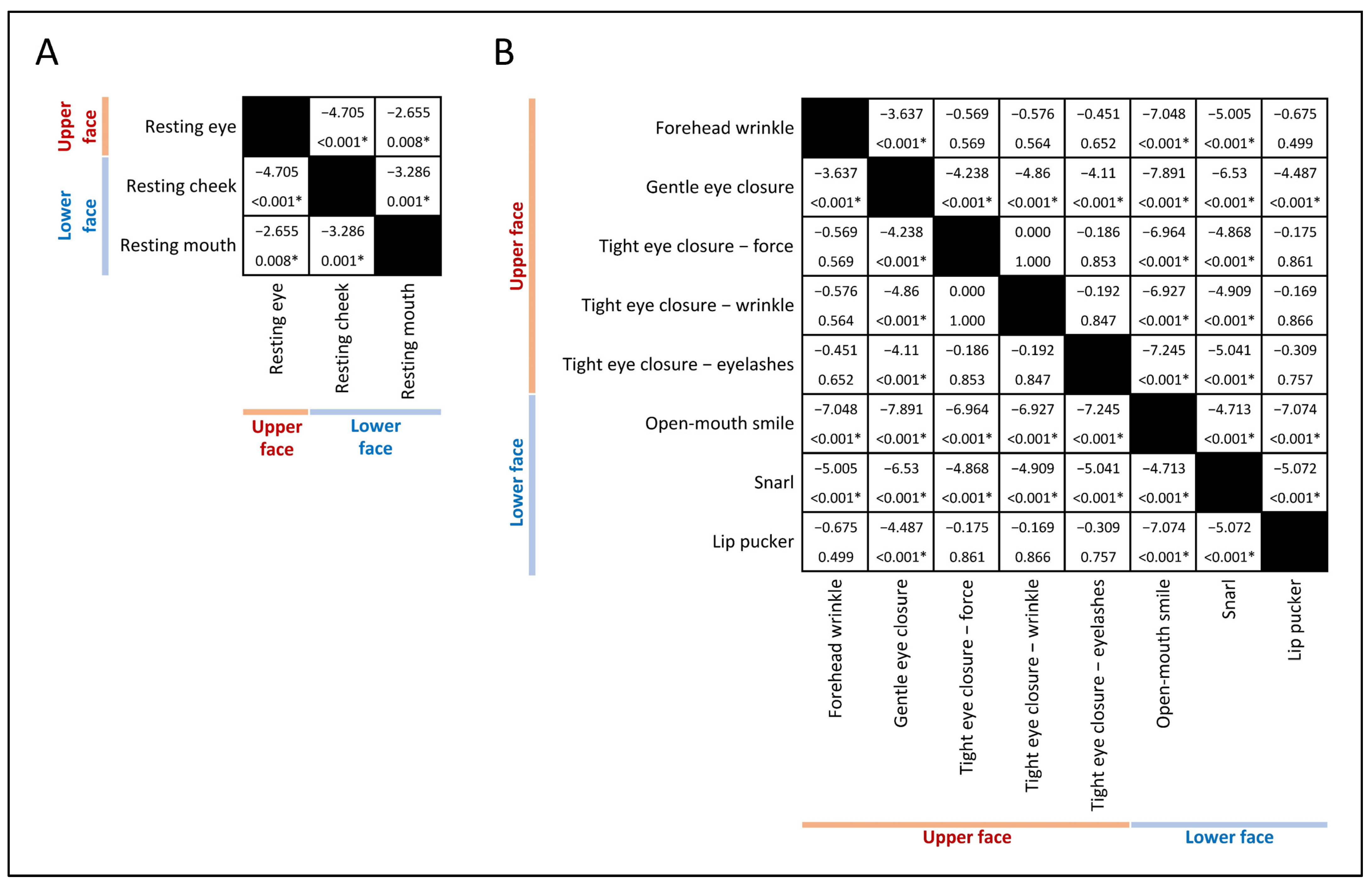
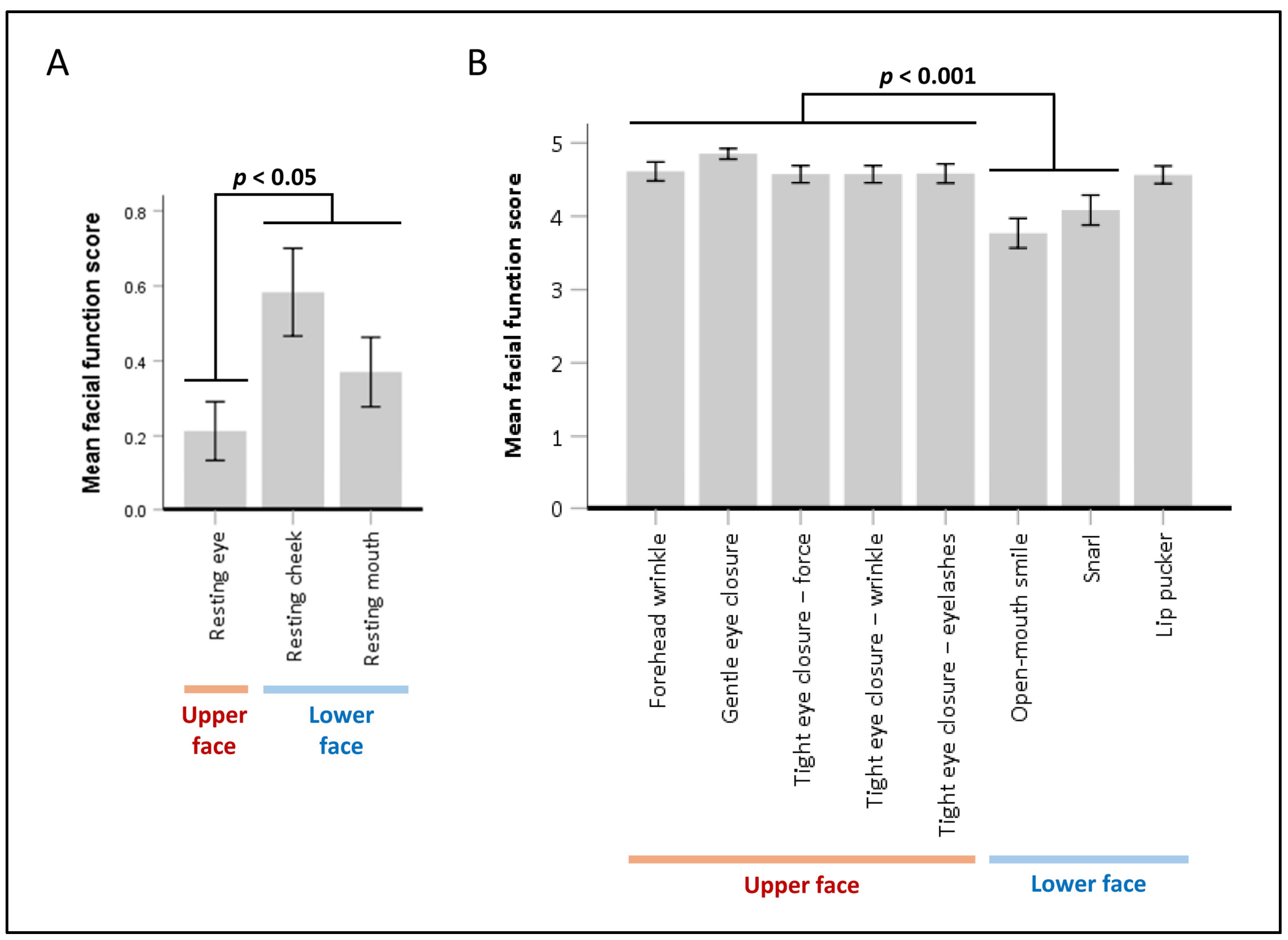
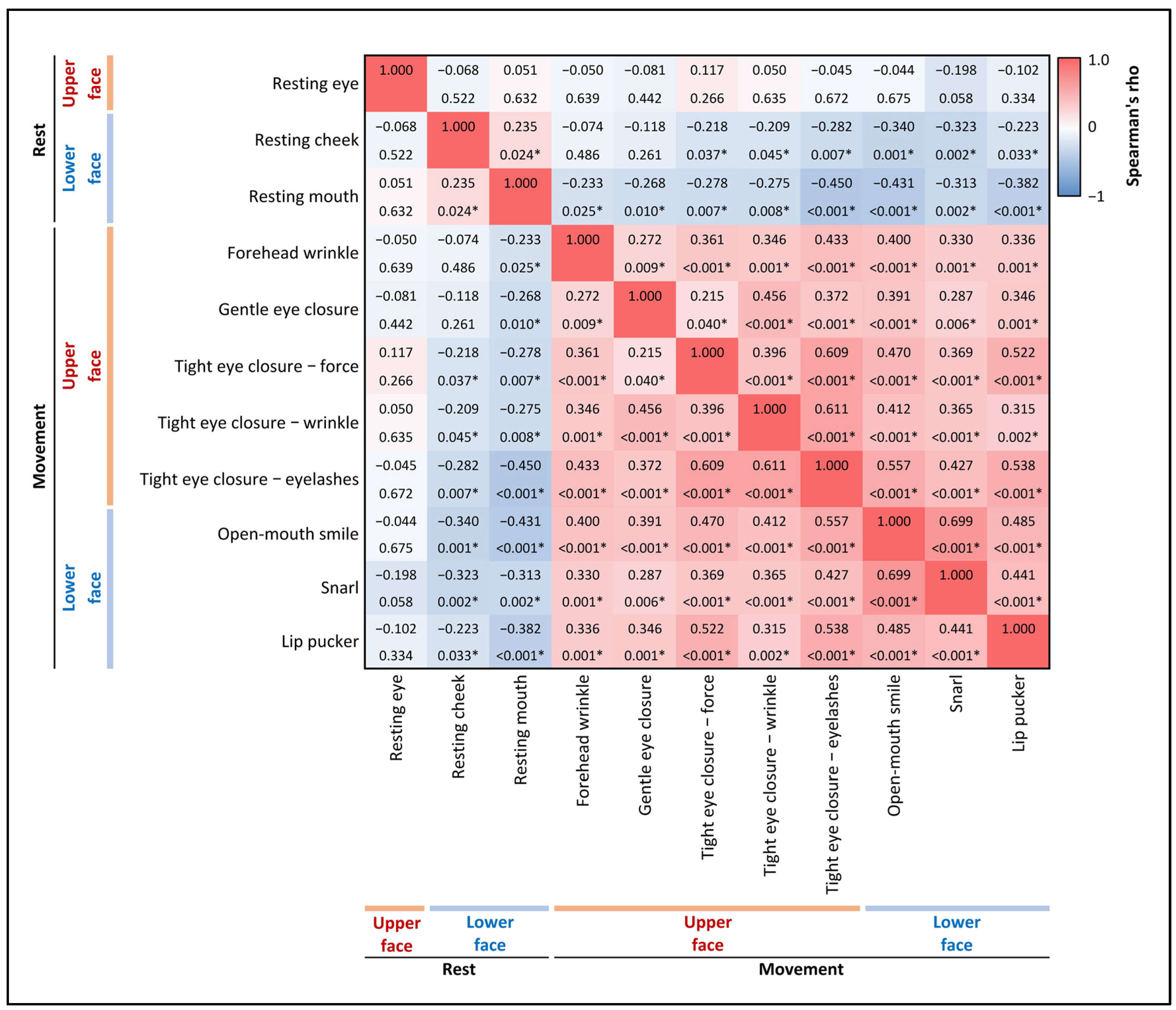
2.2. Facial Muscle Function Assessment
- Resting Symmetry: This component evaluates the symmetry of three facial features at rest: the palpebral fissure, nasolabial fold, and mouth corner.
- Symmetry of Voluntary Movement: Five standard facial expressions are assessed: forehead wrinkles, gentle eye closure, open-mouth smile, snarl, and lip pucker.
2.3. Stroke Lesion Localization
2.4. Clinical Data
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Prevalence and Severity of Upper Facial Weakness
3.3. Factors Associated with Upper Facial Weakness
4. Discussion
4.1. Key Findings
4.2. Comparison with Previous Studies
4.3. Facial Motor Control Pathways and Correlated Findings
4.4. Limitations
4.5. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ropper, A.H.; Samuels, M.A.; Klein, J.P.; Prasad, S. Paralysis and Weakness. In Adams and Victor’s Principles of Neurology, 12th ed.; McGraw-Hill Education: New York, NY, USA, 2023. [Google Scholar]
- Lin, J.; Chen, Y.; Wen, H.; Yang, Z.; Zeng, J. Weakness of eye closure with central facial paralysis after unilateral hemispheric stroke predicts a worse outcome. J. Stroke Cerebrovasc. Dis. 2017, 26, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Hebant, B.; Costentin, G.; Slama, M.; Guegan-Massardier, E. Precentral gyrus infarct presenting as isolated contralateral peripheral-type facial palsy. Neurology 2018, 91, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Can, F.Y.; Çetin, B.N.; Poyraz, U.; Önder, H. Examination of Supranuclear Lesion Localizations of Peripheral Facial Palsy Induced by Acute Stroke. Ank. Univ. Tip Fak. Mecm.=J. Ank. Univ. Fac. Med. 2022, 75, 226. [Google Scholar]
- Cattaneo, L.; Saccani, E.; De Giampaulis, P.; Crisi, G.; Pavesi, G. Central facial palsy revisited: A clinical-radiological study. Ann. Neurol. 2010, 68, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, E.W.; Anderson, N.E. Lower cranial nerve motor function in unilateral vascular lesions of the cerebral hemisphere. Br. Med. J. (Clin. Res. Ed.) 1984, 289, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.G.; Fradet, G.; Nedzelski, J.M. Development of a sensitive clinical facial grading system. Otolaryngol.—Head Neck Surg. 1996, 114, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M.; Group, A.S. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Puetz, V.; Sylaja, P.; Coutts, S.B.; Hill, M.D.; Dzialowski, I.; Mueller, P.; Becker, U.; Urban, G.; O’Reilly, C.; Barber, P.A. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008, 39, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K. PACS and Imaging Informatics: Basic Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Morone, G.; La Greca, F.M.; Marchegiani, V.; Palomba, A.; Iosa, M.; Musto, G.; Simonelli, M. Sunnybrook Facial Grading System reliability in subacute stroke patients. Eur. J. Phys. Rehabil. Med. 2021, 57, 685–690. [Google Scholar] [CrossRef]
- Patten, J.P. The Brain Stem. In Neurological Differential Diagnosis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Morecraft, R.J.; Stilwell–Morecraft, K.S.; Rossing, W.R. The motor cortex and facial expression:: New insights from neuroscience. Neurol. 2004, 10, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Jenny, A.B.; Saper, C.B. Organization of the facial nucleus and corticofacial projection in the monkey: A reconsideration of the upper motor neuron facial palsy. Neurology 1987, 37, 930–939. [Google Scholar] [CrossRef]
- Kuypers, H.G.J.M. Corticobulbar connexions to the pons and lower brain-stem in man: An anatomical study. Brain 1958, 81, 364–388. [Google Scholar] [CrossRef]
- Morecraft, R.J.; Louie, J.L.; Herrick, J.L.; Stilwell-Morecraft, K.S. Cortical innervation of the facial nucleus in the non-human primate: A new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain 2001, 124, 176–208. [Google Scholar] [CrossRef] [PubMed]
- Morecraft, R.; Binneboese, A.; Stilwell-Morecraft, K.; Ge, J. Localization of orofacial representation in the corona radiata, internal capsule and cerebral peduncle in Macaca mulatta. J. Comp. Neurol. 2017, 525, 3429–3457. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Total (N = 108) | Upper Facial Weakness (N = 70) | No Upper Facial Weakness (N = 38) | Measure of Effect † | Unadjusted Effect Size (95% CI) † | p-Value |
|---|---|---|---|---|---|---|
| Age—yr | 63.1 ± 11.8 | 63.7 ± 11.5 | 62.1 ± 12.5 | Mean difference | 1.59 (−6.33–3.15) | 0.507 |
| Male sex—no. (%) | 55 (50.9) | 38 (54.3) | 17 (44.7) | Odds ratio | 1.47 (0.66–3.24) | 0.343 |
| Median prestroke mRS (IQR) ‡ | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | r | −0.08 | 0.432 |
| Median NIHSS score on the day of facial recording (IQR) § | 2.0 (1.0−4.0) | 3.0 (1.0–5.0) | 2.0 (1.0–3.0) | r | −0.33 | 0.001 |
| Risk factor for ischemic stroke—no. (%) | ||||||
| Hypertension | 86 (79.6) | 55 (78.6) | 31 (81.6) | Odds ratio | 0.83 (0.31–2.25) | 0.711 |
| Dyslipidemia | 67 (62.0) | 43 (61.4) | 24 (63.2) | Odds ratio | 0.93 (0.41–2.10) | 0.860 |
| Diabetes mellitus | 45 (41.7) | 32 (45.7) | 13 (34.2) | Odds ratio | 1.62 (0.71–3.67) | 0.247 |
| Smoking | 34 (31.5) | 23 (32.9) | 11 (28.9) | Odds ratio | 1.20 (0.51–2.84) | 0.676 |
| Chronic kidney disease | 14 (13.0) | 8 (11.4) | 6 (15.8) | Odds ratio | 0.69 (0.22–2.15) | 0.519 |
| Atrial fibrillation | 8 (7.4) | 5 (7.1) | 3 (7.9) | Odds ratio | 0.90 (0.20–3.98) | 0.887 |
| Coronary artery disease | 7 (6.5) | 3 (4.3) | 4 (10.5) | Odds ratio | 0.38 (0.08–1.80) | 0.208 |
| Dilated cardiomyopathy | 2 (1.9) | 0 (0.0) | 2 (5.3) | NA | NA | 0.053 |
| Rheumatic heart disease | 2 (1.9) | 2 (2.9) | 0 (0.0) | NA | NA | 0.293 |
| Left ventricular thrombus | 1 (0.9) | 0 (0.0) | 1 (2.6) | NA | NA | 0.173 |
| Intravenous thrombolysis—no. (%) | 15 (13.9) | 10 (14.3) | 5 (13.2) | Odds ratio | 1.10 (0.35–3.49) | 0.871 |
| Imaging for localization—no. (%) | ||||||
| CT | 96 (88.9) | 63 (90.0) | 33 (86.8) | Odds ratio | 1.36 (0.40–4.63) | 0.618 |
| MRI | 12 (11.1) | 7 (10.0) | 5 (13.2) | |||
| Side of lesions—no. (%) ¶ | ||||||
| Right | 32 (33.3) | 23 (37.1) | 9 (26.5) | Odds ratio | 1.64 (0.65–4.11) | 0.291 |
| Left | 64 (66.7) | 39 (62.9) | 25 (73.5) | |||
| Localization by tentorium cerebelli—no. (%) ¶ | ||||||
| Supratentorium | 84 (87.5) | 56 (90.3) | 28 (82.4) | Odds ratio | 2.00 (0.59–6.77) | 0.259 |
| Infratentorium | 12 (12.5) | 6 (9.7) | 6 (17.6) | |||
| Localization by cerebral cortex | ||||||
| Cortical areas | 17 (20.2) | 9 (16.1) | 8 (28.6) | Odds ratio | 0.48 (0.16–1.42) | 0.179 |
| Subcortical areas | 67 (79.8) | 47 (83.9) | 20 (71.4) | |||
| Localization by the ASPECTS system—no. (%) ¶ǁ | ||||||
| M1 | 7 (6.5) | 6 (8.6) | 1 (2.6) | Odds ratio | 3.47 (0.40–29.9) | 0.231 |
| M2 | 3 (2.8) | 2 (2.9) | 1 (2.6) | Odds ratio | 1.09 (0.10–12.4) | 0.946 |
| M3 | 5 (4.6) | 4 (5.7) | 1 (2.6) | Odds ratio | 2.24 (0.24–20.81) | 0.467 |
| M4 | 6 (5.6) | 5 (7.1) | 1 (2.6) | Odds ratio | 2.85 (0.32–25.30) | 0.328 |
| M5 | 47 (43.5) | 35 (50.0) | 12 (31.6) | Odds ratio | 2.17 (0.95–4.96) | 0.065 |
| M6 | 8 (7.4) | 5 (7.1) | 3 (7.9) | Odds ratio | 0.90 (0.20–3.98) | 0.887 |
| I | 12 (11.1) | 7 (10.0) | 5 (13.2) | Odds ratio | 0.73 (0.22–2.49) | 0.618 |
| C | 3 (2.8) | 2 (2.9) | 1 (1.6) | Odds ratio | 1.09 (0.10–12.41) | 0.946 |
| L | 14 (13.0) | 12 (17.1) | 2 (5.3) | Odds ratio | 3.72 (0.79–17.61) | 0.079 |
| IC | 27 (25.0) | 20 (28.6) | 7 (18.4) | Odds ratio | 1.77 (0.67–4.68) | 0.245 |
| A | 2 (1.9) | 1 (1.4) | 1 (2.6) | Odds ratio | 0.54 (0.03–8.82) | 0.658 |
| P | 4 (3.7) | 2 (2.9) | 2 (5.3) | Odds ratio | 0.53 (0.07–3.92) | 0.527 |
| Thalamus | 7 (6.5) | 3 (4.3) | 4 (10.5) | Odds ratio | 0.38 (0.08–1.80) | 0.208 |
| Midbrain | 1 (0.9) | 0 (0.0) | 1 (2.6) | NA | NA | 0.173 |
| Pons | 8 (7.4) | 7 (10.0) | 1 (2.6) | Odds ratio | 4.11 (0.49–34.74) | 0.163 |
| Cerebellum | 8 (7.4) | 2 (2.9) | 6 (15.8) | Odds ratio | 0.16 (0.03–0.82) | 0.014 |
| Median interval between stroke onset and facial assessment recording (IQR)—hour | 62.5 (30.6−96.0) | 61.5 (28.0–96.0) | 64.8 (38.9–109.0) | r | −0.05 | 0.578 |
| Lower face weakness—no. (%) | 88 (81.5) | 66 (94.3) | 22 (57.9) | Odds ratio | 12.0 (3.62–39.73) | <0.001 |
| Facial Function | Number (%) † (N = 92) | Median Score ‡ (IQR) |
|---|---|---|
| Resting Symmetry | ||
| Palpebral fissure | 0.0 (0.0−0.8) | |
| Normal [0] § | 69 (75.0) | |
| Abnormal | 23 (25.0) | |
| Wide [1] | 23 (25.0) | |
| Nasolabial fold | 1.0 (0.0−1.0) | |
| Normal [0] | 36 (39.1) | |
| Abnormal | 56 (60.9) | |
| Less pronounced [1] | 49 (53.3) | |
| Absent [2] | 7 (7.6) | |
| Mouth corner | 0.0 (0.0−1.0) | |
| Normal [0] | 52 (56.5) | |
| Abnormal | 40 (43.5) | |
| Corner dropped/pull up [1] | 40 (43.5) | |
| Voluntary Movement Symmetry | ||
| Forehead wrinkles | 5.0 (4.0−5.0) | |
| Normal symmetry [5] § | 60 (65.2) | |
| Abnormal | 32 (34.8) | |
| Mild asymmetry [4] | 24 (26.1) | |
| Moderate symmetry [3] | 6 (6.5) | |
| Severe asymmetry [2] | 2 (2.2) | |
| Gross asymmetry [1] | 0 (0.0) | |
| Gentle eye closure | 5.0 (5.0−5.0) | |
| Normal symmetry [5] | 77 (83.7) | |
| Abnormal | 15 (16.3) | |
| Mild asymmetry [4] | 14 (15.2) | |
| Moderate symmetry [3] | 1 (1.1) | |
| Severe asymmetry [2] | 0 (0.0) | |
| Gross asymmetry [1] | 0 (0.0) | |
| Tight eye closure—force | 5.0 (4.0−5.0) | |
| Normal symmetry [5] | 53 (57.6) | |
| Abnormal | 39 (42.4) | |
| Mild asymmetry [4] | 32 (34.8) | |
| Moderate symmetry [3] | 7 (7.6) | |
| Severe asymmetry [2] | 0 (0.0) | |
| Gross asymmetry [1] | 0 (0.0) | |
| Tight eye closure—wrinkles | 5.0 (4.0−5.0) | |
| Normal symmetry [5] | 53 (57.6) | |
| Abnormal | 39 (42.4) | |
| Mild asymmetry [4] | 32 (34.8) | |
| Moderate symmetry [3] | 7 (7.6) | |
| Severe asymmetry [2] | 0 (0.0) | |
| Gross asymmetry [1] | 0 (0.0) | |
| Tight eye closure—eyelashes | 5.0 (4.0−5.0) | |
| Normal symmetry [5] | 58 (63.0) | |
| Abnormal | 34 (37.0) | |
| Mild asymmetry [4] | 24 (26.1) | |
| Moderate symmetry [3] | 9 (9.8) | |
| Severe asymmetry [2] | 1 (1.1) | |
| Gross asymmetry [1] | 0 (0.0) | |
| Open mouth smile | 4.0 (3.0−4.0) | |
| Normal symmetry [5] | 13 (14.1) | |
| Abnormal | 79 (85.9) | |
| Mild asymmetry [4] | 42 (45.7) | |
| Moderate symmetry [3] | 24 (26.1) | |
| Severe asymmetry [2] | 9 (9.8) | |
| Gross asymmetry [1] | 4 (4.3) | |
| Snarl | 4.0 (3.3−5.0) | |
| Normal symmetry [5] | 31 (33.7) | |
| Abnormal | 61 (66.3) | |
| Mild asymmetry [4] | 38 (41.3) | |
| Moderate symmetry [3] | 12 (13.0) | |
| Severe asymmetry [2] | 7 (7.6) | |
| Gross asymmetry [1] | 4 (4.3) | |
| Lip pucker | 5.0 (4.0−5.0) | |
| Normal symmetry [5] | 53 (57.6) | |
| Abnormal | 39 (42.4) | |
| Mild asymmetry [4] | 31 (33.7) | |
| Moderate symmetry [3] | 8 (8.7) | |
| Severe asymmetry [2] | 0 (0.0) | |
| Gross asymmetry [1] | 0 (0.0) | |
| Predictor Variable | Upper Facial Weakness (N = 70) | No Upper Facial Weakness (N = 38) | Adjusted OR (95%CI) | p-Value |
|---|---|---|---|---|
| Median NIHSS score (IQR) | 3.0 (1.0–5.0) | 2.0 (1.0–3.0) | 1.42 (1.07−1.88) † | 0.016 |
| Lower face weakness—no. (%) | 66 (94.3) | 22 (57.9) | 6.56 (1.85−23.29) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongwandee, M.; Hongdusit, K. Analysis of Upper Facial Weakness in Central Facial Palsy Following Acute Ischemic Stroke. Neurol. Int. 2025, 17, 12. https://doi.org/10.3390/neurolint17010012
Wongwandee M, Hongdusit K. Analysis of Upper Facial Weakness in Central Facial Palsy Following Acute Ischemic Stroke. Neurology International. 2025; 17(1):12. https://doi.org/10.3390/neurolint17010012
Chicago/Turabian StyleWongwandee, Monton, and Kantham Hongdusit. 2025. "Analysis of Upper Facial Weakness in Central Facial Palsy Following Acute Ischemic Stroke" Neurology International 17, no. 1: 12. https://doi.org/10.3390/neurolint17010012
APA StyleWongwandee, M., & Hongdusit, K. (2025). Analysis of Upper Facial Weakness in Central Facial Palsy Following Acute Ischemic Stroke. Neurology International, 17(1), 12. https://doi.org/10.3390/neurolint17010012





