Anterograde Intramedullary Nailing without Bone Grafting for Humeral Shaft Nonunion Associated with Early Exploration of Secondary Radial Nerve Palsy: A Case Report
Abstract
1. Introduction
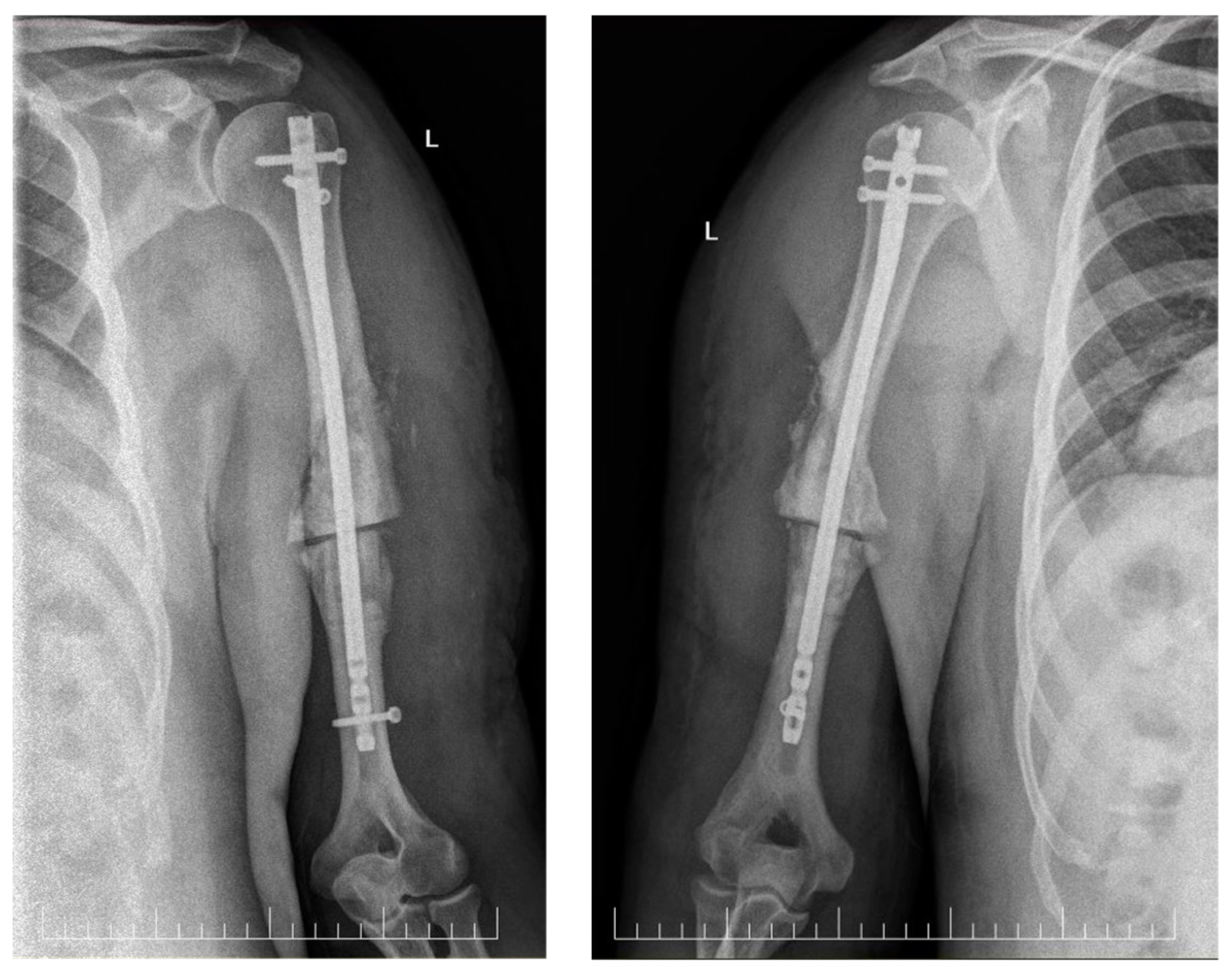
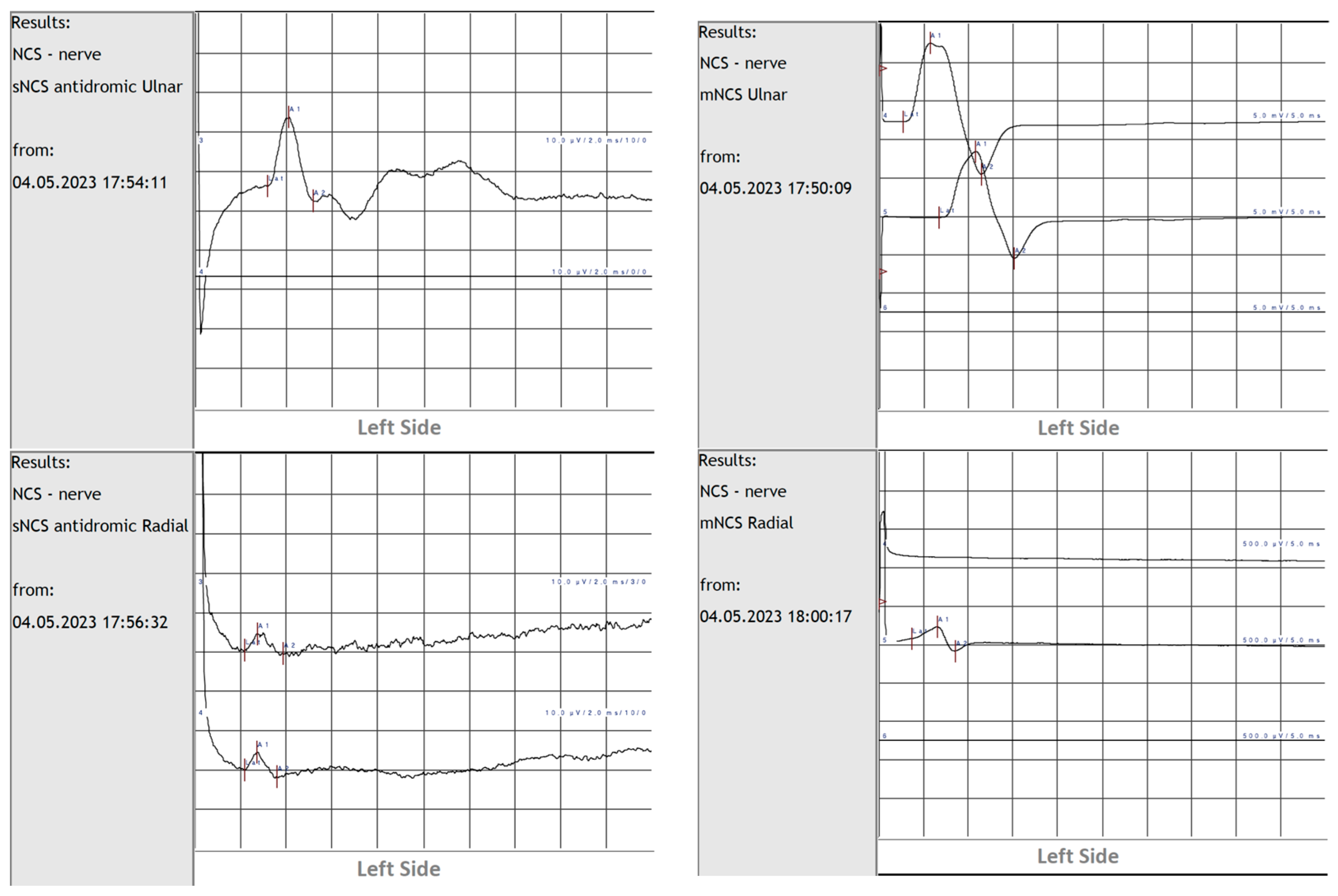
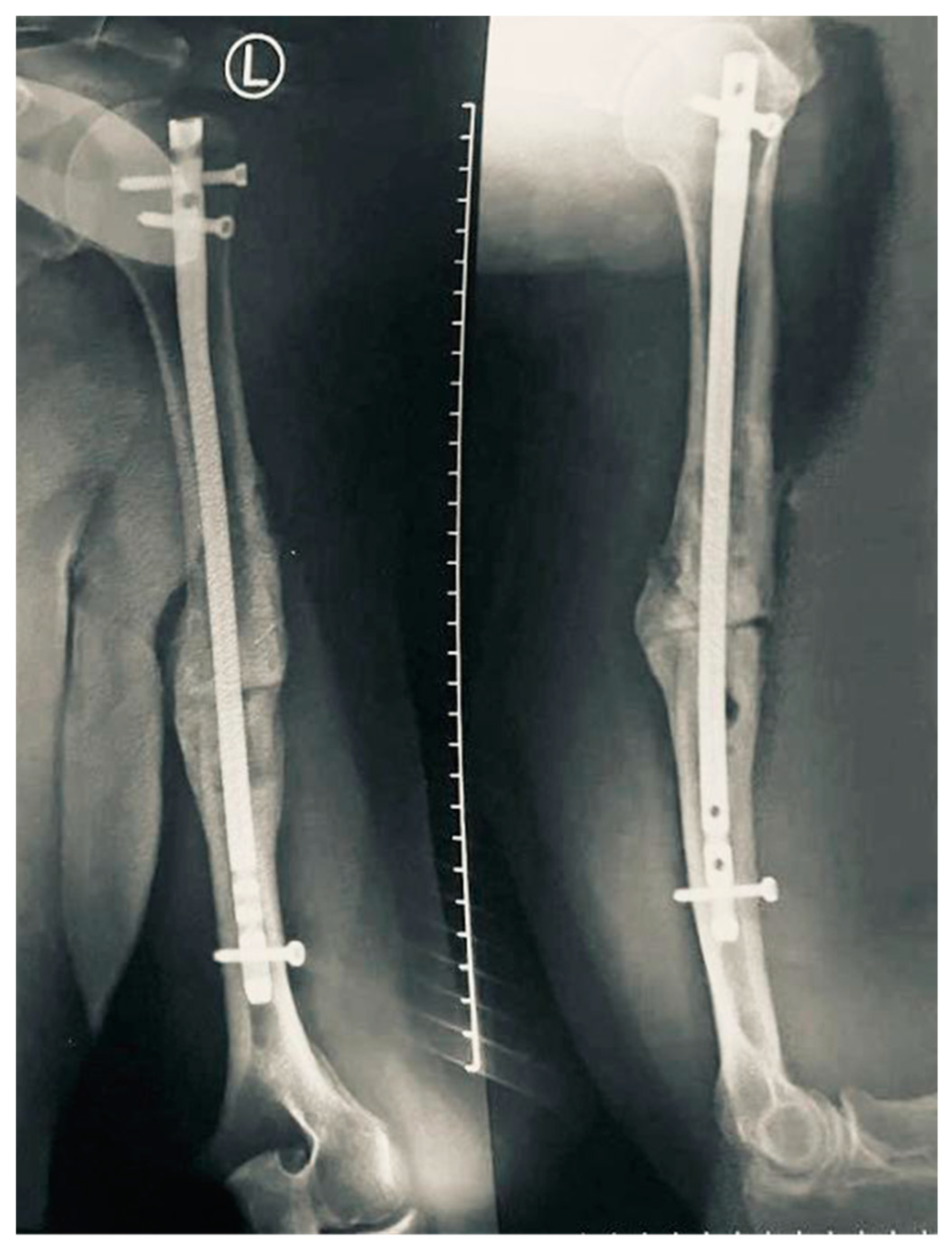
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallusser, N.; Barimani, B.; Vauclair, F. Humeral shaft fractures. EFORT Open Rev. 2021, 6, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, E.H.; McKee, M.D. Approach to Humeral Shaft Nonunion: Evaluation and Surgical Techniques. J. Am. Acad. Orthop. Surg. 2022, 30, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.J.; Entezari, V.; Vallier, H.A. Risk factors for nonunion after traumatic humeral shaft fractures in adults. JSES Int. 2020, 4, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Papasoulis, E.; Drosos, G.I.; Ververidis, A.N.; Verettas, D.A. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury 2010, 41, e21–e27. [Google Scholar] [CrossRef]
- Rämö, L.; Sumrein, B.O.; Lepola, V.; Lähdeoja, T.; Ranstam, J.; Paavola, M.; Järvinen, T.; Taimela, S.; FISH Investigators. Effect of Surgery vs Functional Bracing on Functional Outcome among Patients with Closed Displaced Humeral Shaft Fractures: The FISH Randomized Clinical Trial. JAMA 2020, 323, 1792–1801. [Google Scholar] [CrossRef]
- Entezari, V.; Olson, J.J.; Vallier, H.A. Predictors of traumatic nerve injury and nerve recovery following humeral shaft fracture. J. Shoulder Elbow. Surg. 2021, 30, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.M.; Mangan, J.J.; Graham, J. Radial Nerve Palsy Recovery with Fractures of the Humerus: An Updated Systematic Review. J. Am. Acad. Orthop. Surg. 2020, 28, e263–e269. [Google Scholar] [CrossRef]
- Van Bergen, S.H.; Mahabier, K.C.; Van Lieshout, E.M.M.; Van der Torre, T.; Notenboom, C.A.W.; Jawahier, P.A.; Verhofstad, M.H.J.; Den Hartog, D. Humeral shaft fracture: Systematic review of non-operative and operative treatment. Arch. Orthop. Trauma. Surg. 2023, 143, 5035–5054. [Google Scholar] [CrossRef]
- Leiblein, M.; Verboket, R.; Marzi, I.; Wagner, N.; Nau, C. Nonunions of the humerus—Treatment concepts and results of the last five years. Chin. J. Traumatol. 2019, 22, 187–195. [Google Scholar] [CrossRef]
- Oliver, W.M.; Searle, H.K.C.; Ng, Z.H.; Molyneux, S.G.; White, T.O.; Clement, N.D.; Duckworth, A.D. Factors associated with humeral shaft nonunion. J. Shoulder Elbow. Surg. 2021, 30, 2283–2295. [Google Scholar] [CrossRef]
- Beeres, F.J.P.; van Veelen, N.; Houwert, R.M.; Link, B.C.; Heng, M.; Knobe, M.; Groenwold, R.H.H.; Babst, R.; van de Wall, B.J.M. Open plate fixation versus nailing for humeral shaft fractures: A meta-analysis and systematic review of randomised clinical trials and observational studies. Eur. J. Trauma Emerg. Surg. 2022, 48, 2667–2682. [Google Scholar] [CrossRef] [PubMed]
- Reahl, G.B.; Gerstenfeld, L.; Kain, M. Epidemiology, Clinical Assessments, and Current Treatments of Nonunions. Curr Osteoporos. Rep. 2020, 18, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Vincken, L.; van der Broeck, L.; Geurts, J.; Qiu Shao, S.S.; Poeze, M.; Blokhuis, T.J. The effect of post-traumatic long bone non-unions on health-related quality of life. Injury 2023, 54 (Suppl. 5), 110929. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.G.; Oldrich, C.; Konstam, P.G. Pseudoarthrosis: Pathophysiology, Biomechanics, Therapy, Results; Hans Huber Publishers: Bern, Switzerland, 1976; p. 323. [Google Scholar]
- Solomin, L.N.; Semenistyy, A.A.; Komarov, A.V.; Khominets, V.V.; Sheridan, G.A.; Rozbruch, S.R. Universal Long Bone Nonunion Classification. Strateg. Trauma Limb Reconstr. 2023, 18, 169–173. [Google Scholar] [CrossRef]
- Peters, R.M.; Claessen, F.M.; Doornberg, J.N.; Kolovich, G.P.; Diercks, R.L.; van den Bekerom, M.P. Union rate after operative treatment of humeral shaft nonunion—A systematic review. Injury 2015, 46, 2314–2324. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. Bone Healing Materials in the Treatment of Recalcitrant Nonunions and Bone Defects. Int. J. Mol. Sci. 2022, 23, 3352. [Google Scholar] [CrossRef]
- Rocchi, M.; Tarallo, L.; Mugnai, R.; Adani, R. Humerus shaft fracture complicated by radial nerve palsy: Is surgical exploration necessary? Musculoskelet. Surg. 2016, 100 (Suppl. 1), 53–60. [Google Scholar] [CrossRef]
- Shao, Y.C.; Harwood, P.; Grotz, M.R.; Limb, D.; Giannoudis, P.V. Radial nerve palsy associated with fractures of the shaft of the humerus: A systematic review. J. Bone Joint Surg. Br. 2005, 87, 1647–1652. [Google Scholar] [CrossRef]
- Niver, G.E.; Ilyas, A.M. Management of radial nerve palsy following fractures of the humerus. Orthop. Clin. N. Am. 2013, 44, 419–424. [Google Scholar] [CrossRef]
- Bumbasirevic, M.; Palibrk, T.; Lesic, A.; Atkinson, H. Radial nerve palsy. EFORT Open Rev. 2017, 1, 286–294. [Google Scholar] [CrossRef]
- Reichert, P.; Wnukiewicz, W.; Witkowski, J.; Bocheńska, A.; Mizia, S.; Gosk, J.; Zimmer, K. Causes of Secondary Radial Nerve Palsy and Results of Treatment. Med. Sci. Monit. 2016, 22, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Ilyas, A.M. Radial Nerve Palsy after Humeral Shaft Fractures: The Case for Early Exploration and a New Classification to Guide Treatment and Prognosis. Hand Clin. 2018, 34, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Dahlin, L.B. Anatomy, function, and pathophysiology of peripheral nerves and nerve compression. Hand Clin. 1996, 12, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2000, 23, 863–873. [Google Scholar] [CrossRef]
- Kamble, N.; Shukla, D.; Bhat, D. Peripheral Nerve Injuries: Electrophysiology for the Neurosurgeon. Neurol. India 2019, 67, 1419–1422. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflammation 2011, 8, 109. [Google Scholar] [CrossRef]
- Bage, T.; Power, D.M. Iatrogenic peripheral nerve injury: A guide to management for the orthopaedic limb surgeon. EFORT Open Rev. 2021, 6, 607–617. [Google Scholar] [CrossRef]
- Belayneh, R.; Lott, A.; Haglin, J.; Konda, S.; Leucht, P.; Egol, K. Final outcomes of radial nerve palsy associated with humeral shaft fracture and nonunion. J. Orthop. Traumatol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Angst, F.; Schwyzer, H.K.; Aeschlimann, A.; Simmen, B.R.; Goldhahn, J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis. Care Res. 2011, 63 (Suppl. 11), S174–S188. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. 4), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Schmal, H.; Brix, M.; Bue, M.; Ekman, A.; Ferreira, N.; Gottlieb, H.; Kold, S.; Taylor, A.; Toft Tengberg, P.; Ban, I.; et al. Nonunion—Consensus from the 4th annual meeting of the Danish Orthopaedic Trauma Society. EFORT Open Rev. 2020, 5, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Bégué, T.; Mouchantaf, M.; Aurégan, J.C. Aseptic humeral shaft nonunion. Orthop. Traumatol. Surg. Res. 2023, 109, 103462. [Google Scholar] [CrossRef] [PubMed]
- Micic, I.; Kholinne, E.; Kwak, J.M.; Sun, Y.; Nanda, A.; Kim, H.; Koh, K.H.; Jeon, I.H. Humeral Diaphyseal Fracture Nonunion: An Audit of the Outcome from Intramedullary Nailing and DCP Plating. Biomed. Res. Int. 2019, 2019, 9107898. [Google Scholar] [CrossRef]
- Amer, K.M.; Kurland, A.M.; Smith, B.; Abdo, Z.; Amer, R.; Vosbikian, M.M.; Ahmed, I.H. Intramedullary Nailing Versus Plate Fixation for Humeral Shaft Fractures: A Systematic Review and Meta-Analysis. Arch. Bone Jt. Surg. 2022, 10, 661–667. [Google Scholar] [CrossRef]
- Singh, A.K.; Arun, G.R.; Narsaria, N.; Srivastava, A. Treatment of non-union of humerus diaphyseal fractures: A prospective study comparing interlocking nail and locking compression plate. Arch. Orthop. Trauma. Surg. 2014, 134, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.; Kerschbaum, M.; Pfeifer, C.; Popp, D.; Freigang, V.; Hinterberger, T.; Alt, V.; Rupp, M. Long-term patient-related quality of life after successfully treated aseptic non-unions of the long bones. Injury 2021, 52, 1880–1885. [Google Scholar] [CrossRef]
- Temiz, N.C.; Doğan, A.; Kırık, A.; Yaşar, S.; Durmaz, M.O.; Kutlay, A.M. Radial nerve injuries and outcomes: Our surgical experience. Radial sinir yaralanmaları ve sonuçları: Cerrahi deneyimimiz. Ulus. Travma Acil Cerrahi Derg. 2021, 27, 690–696. [Google Scholar] [CrossRef]
- Carlan, D.; Pratt, J.; Patterson, J.M.; Weiland, A.J.; Boyer, M.I.; Gelberman, R.H. The radial nerve in the brachium: An anatomic study in human cadavers. J. Hand Surg. Am. 2007, 32, 1177–1182. [Google Scholar] [CrossRef]
- Bono, C.M.; Grossman, M.G.; Hochwald, N.; Tornetta, P., 3rd. Radial and axillary nerves. Anatomic considerations for humeral fixation. Clin. Orthop. Relat. Res. 2000, 373, 259–264. [Google Scholar] [CrossRef]
- Koh, J.; Tornetta, P., 3rd; Walker, B.; Jones, C.; Sharmaa, T.; Sems, S.; Ringenbach, K.; Boateng, H.; Bellevue, K.; Firoozabadi, R.; et al. What is the Real Rate of Radial Nerve Injury After Humeral Nonunion Surgery? J. Orthop. Trauma 2020, 34, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Kandel, I.S.; Agarwal, A.K.; Vijay, V.; Vaish, A.; Acharya, K. Is early exploration of secondary radial nerve injury in patients with humerus shaft fracture justified? J. Clin. Orthop. Trauma 2019, 10, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Moon, D.K.; Gwark, J.Y.; Park, H.B. Need for early exploration of radial nerve in humeral shaft fractures with radial nerve palsy. Arch. Orthop. Trauma Surg. 2021, 141, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Rasulić, L.; Savić, A.; Vitošević, F.; Samardžić, M.; Živković, B.; Mićović, M.; Baščarević, V.; Puzović, V.; Joksimović, B.; Novakovic, N.; et al. Iatrogenic Peripheral Nerve Injuries-Surgical Treatment and Outcome: 10 Years’ Experience. World Neurosurg. 2017, 103, 841–851.e6. [Google Scholar] [CrossRef]
- Steenbeek, E.D.; Pondaag, W.; Tannemaat, M.R.; Van Zwet, E.W.; Malessy, M.J.A.; Groen, J.L. Optimal timing of needle electromyography to diagnose lesion severity in traumatic radial nerve injury. Muscle Nerve 2023, 67, 314–319. [Google Scholar] [CrossRef]
- Şahin, F.; Atalay, N.Ş.; Akkaya, N.; Ercidoğan, Ö.; Başakçi, B.; Kuran, B. The correlation of neurophysiological findings with clinical and functional status in patients following traumatic nerve injury. J. Hand Surg. Eur. Vol. 2014, 39, 199–206. [Google Scholar] [CrossRef]
- Wade, M.D.; McDowell, A.R.; Ziermann, J.M. Innervation of the Long Head of the Triceps Brachii in Humans—A Fresh Look. Anat. Rec. 2018, 301, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jeng, C.L.; Rosenblatt, M.A. Intraneural injections and regional anesthesia: The known and the unknown. Minerva Anestesiol. 2011, 77, 54–58. [Google Scholar]
- Jeng, C.L.; Torrillo, T.M.; Rosenblatt, M.A. Complications of peripheral nerve blocks. Br. J. Anaesth. 2010, 105 (Suppl. 1), i97–i107. [Google Scholar] [CrossRef]
- Schwaiger, K.; Abed, S.; Russe, E.; Koeninger, F.; Wimbauer, J.; Kholosy, H.; Hitzl, W.; Wechselberger, G. Management of Radial Nerve Lesions after Trauma or Iatrogenic Nerve Injury: Autologous Grafts and Neurolysis. J. Clin. Med. 2020, 9, 3823. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, D.V.; Melinte, R.M.; von Mengershausen, R. Anterograde Intramedullary Nailing without Bone Grafting for Humeral Shaft Nonunion Associated with Early Exploration of Secondary Radial Nerve Palsy: A Case Report. Neurol. Int. 2024, 16, 1014-1025. https://doi.org/10.3390/neurolint16050077
Nistor DV, Melinte RM, von Mengershausen R. Anterograde Intramedullary Nailing without Bone Grafting for Humeral Shaft Nonunion Associated with Early Exploration of Secondary Radial Nerve Palsy: A Case Report. Neurology International. 2024; 16(5):1014-1025. https://doi.org/10.3390/neurolint16050077
Chicago/Turabian StyleNistor, Dan Viorel, Răzvan Marian Melinte, and Romana von Mengershausen. 2024. "Anterograde Intramedullary Nailing without Bone Grafting for Humeral Shaft Nonunion Associated with Early Exploration of Secondary Radial Nerve Palsy: A Case Report" Neurology International 16, no. 5: 1014-1025. https://doi.org/10.3390/neurolint16050077
APA StyleNistor, D. V., Melinte, R. M., & von Mengershausen, R. (2024). Anterograde Intramedullary Nailing without Bone Grafting for Humeral Shaft Nonunion Associated with Early Exploration of Secondary Radial Nerve Palsy: A Case Report. Neurology International, 16(5), 1014-1025. https://doi.org/10.3390/neurolint16050077






