Thecaloscopy Reduces the Risk of Recurrent Perineural (Tarlov) Cysts after Microsurgical Resection
Abstract
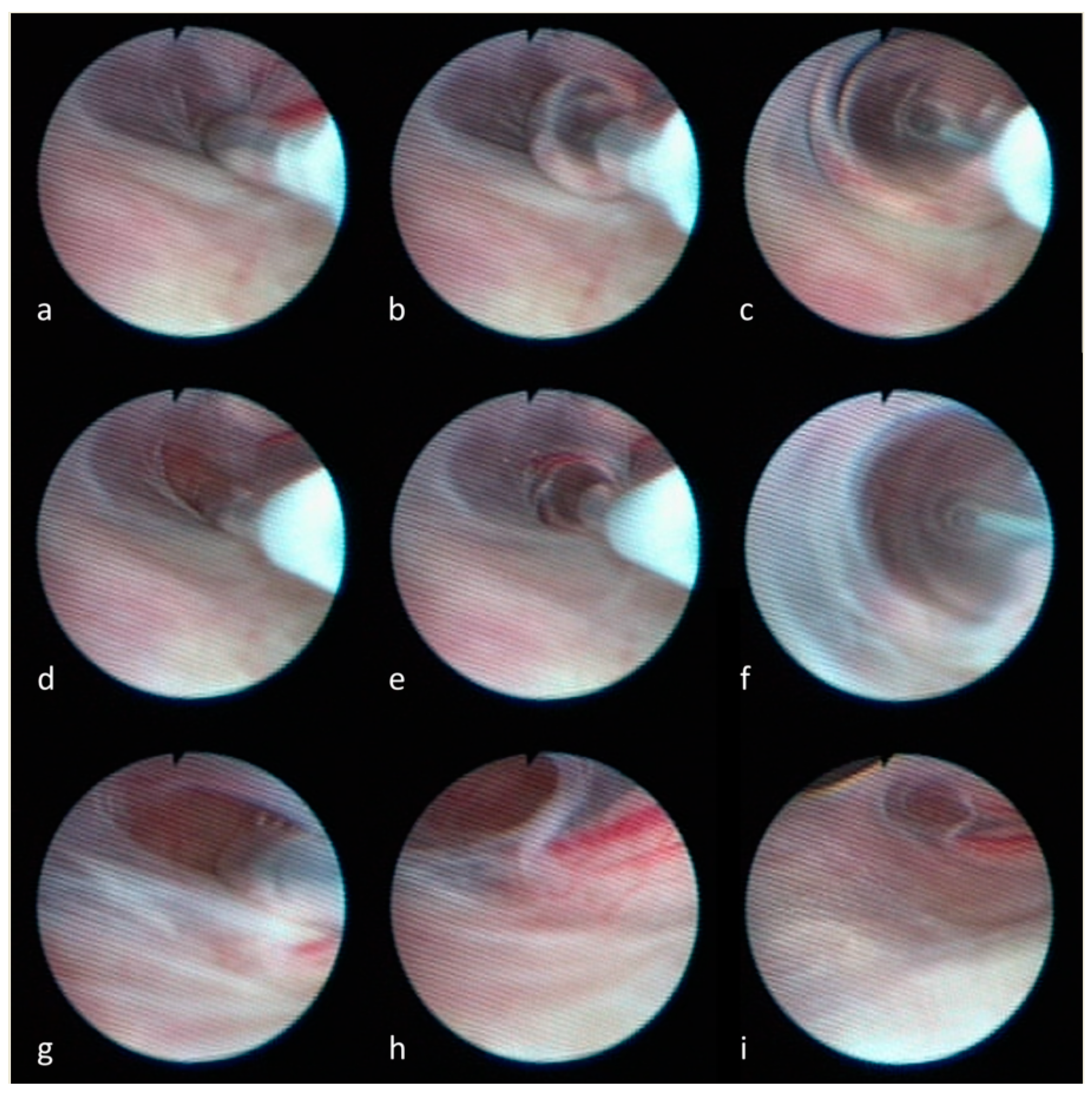
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarlov, I.M. Cysts, perineurial, of the sacral roots; another cause, removable, of sciatic pain. J. Am. Med. Assoc. 1948, 138, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Tarlov, I.M. Spinal perineurial and meningeal cysts. J. Neurol. Neurosurg. Psychiatry 1970, 33, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Klepinowski, T.; Orbik, W.; Sagan, L. Global incidence of spinal perineural Tarlov’s cysts and their morphological characteristics: A meta-analysis of 13,266 subjects. Surg. Radiol. Anat. 2021, 43, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Shoyab, M. Tarlov cysts in back pain patients: Prevalence, measurement method and reporting points. Br. J. Radiol. 2021, 94, 20210505. [Google Scholar] [CrossRef] [PubMed]
- Kameda-Smith, M.M.; Fathalla, Z.; Ibrahim, N.; Astaneh, B.; Farrokhyar, F. A systematic review of the efficacy of surgical intervention in the management of symptomatic Tarlov cysts: A meta-analysis. Br. J. Neurosurg. 2021, 38, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hulens, M.; Dankaerts, W.; Rasschaert, R.; Bruyninckx, F.; Stalmans, I.; Vansant, G.; De Mulder, P. Hydrocephalus associated with multiple Tarlov cysts. Med. Hypotheses 2019, 130, 109293. [Google Scholar] [CrossRef] [PubMed]
- Hulens, M.; Rasschaert, R.; Bruyninckx, F.; Dankaerts, W.; Stalmans, I.; De Mulder, P.; Vansant, G. Symptomatic Tarlov cysts are often overlooked: Ten reasons why-a narrative review. Eur. Spine J. 2019, 28, 2237–2248. [Google Scholar] [CrossRef]
- Burke, J.F.; Thawani, J.P.; Berger, I.; Nayak, N.R.; Stephen, J.H.; Farkas, T.; Aschyan, H.J.; Pierce, J.; Kanchwala, S.; Long, D.M.; et al. Microsurgical treatment of sacral perineural (Tarlov) cysts: Case series and review of the literature. J. Neurosurg. Spine 2016, 24, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Oaklander, A.L.; Elias, G.; Kathuria, S.; Long, D.M. Treatment of 213 Patients with Symptomatic Tarlov Cysts by CT-Guided Percutaneous Injection of Fibrin Sealant. AJNR Am. J. Neuroradiol. 2016, 37, 373–379. [Google Scholar] [CrossRef]
- Sharma, M.; SirDeshpande, P.; Ugiliweneza, B.; Dietz, N.; Boakye, M. A systematic comparative outcome analysis of surgical versus percutaneous techniques in the management of symptomatic sacral perineural (Tarlov) cysts: A meta-analysis. J. Neurosurg. Spine 2019, 30, 623–634. [Google Scholar] [CrossRef]
- Sugawara, T.; Higashiyama, N.; Tamura, S.; Endo, T.; Shimizu, H. Novel wrapping surgery for symptomatic sacral perineural cysts. J. Neurosurg. Spine 2021, 36, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, L.E.; Clement, F.; Coward, S.; Lorenzetti, D.L.; Noseworthy, T.; Sevick, L.; Spackman, A.E. Effectiveness of Surgical Treatment for Tarlov Cysts: A Systematic Review of Published Literature. Clin. Spine Surg. 2018, 31, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.P.; Rodrigues, M.A.S.; Suriano, I.C.; Zymberg, S.T. Idiopathic Intracranial Hypertension Associated with Symptomatic Perineural Cysts: Presentation of 2 Cases. World Neurosurg. 2018, 119, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.I.; Rinehart, C.D.; McShane, B.J.; Hitti, F.L.; Welch, W.C. Growth of Lumbosacral Perineural (Tarlov) Cysts: A Natural History Analysis. Neurosurgery 2020, 86, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S. Surgical Approaches in Symptomatic Tarlov Cysts. World Neurosurg. 2016, 86, 20–21. [Google Scholar] [CrossRef]
- Lucantoni, C.; Than, K.D.; Wang, A.C.; Valdivia-Valdivia, J.M.; Maher, C.O.; La Marca, F.; Park, P. Tarlov cysts: A controversial lesion of the sacral spine. Neurosurg. Focus 2011, 31, E14. [Google Scholar] [CrossRef]
- Murphy, K.J.; Nussbaum, D.A.; Schnupp, S.; Long, D. Tarlov cysts: An overlooked clinical problem. Semin. Musculoskelet. Radiol. 2011, 15, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Luchtmann, M.; Klammer, A.; Iova, M.A.; Roth, A.; Mawrin, C.; Warnke, J.P. Combined endoscopic and microsurgical treatment of Tarlov cysts. Interdiscip. Neurosurg. 2023, 36, 101925. [Google Scholar] [CrossRef]
- Potts, M.B.; McGrath, M.H.; Chin, C.T.; Garcia, R.M.; Weinstein, P.R. Microsurgical Fenestration and Paraspinal Muscle Pedicle Flaps for the Treatment of Symptomatic Sacral Tarlov Cysts. World Neurosurg. 2016, 86, 233–242. [Google Scholar] [CrossRef]
- Godel, T.; Pham, M.; Heiland, S.; Bendszus, M.; Baumer, P. Human dorsal-root-ganglion perfusion measured in-vivo by MRI. Neuroimage 2016, 141, 81–87. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakahara, S.; Ito, Y.; Nakanishi, K.; Sugimoto, Y.; Ikuma, H.; Ozaki, T. Surgical results of sacral perineural (Tarlov) cysts. Acta Med. Okayama 2006, 60, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shu, K.; Chen, R.; Ke, C.; Zhu, Y.; Lei, T. Microsurgical treatment of symptomatic sacral perineurial cysts. Neurosurgery 2007, 60, 1059–1065, discussion 1065–1056. [Google Scholar] [CrossRef] [PubMed]
- Neulen, A.; Kantelhardt, S.R.; Pilgram-Pastor, S.M.; Metz, I.; Rohde, V.; Giese, A. Microsurgical fenestration of perineural cysts to the thecal sac at the level of the distal dural sleeve. Acta Neurochir. 2011, 153, 1427–1434, discussion 1434. [Google Scholar] [CrossRef] [PubMed]
- Gortvai, P. Extradural cysts of the spinal canal. J. Neurol. Neurosurg. Psychiatry 1963, 26, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.N.P.; Pickard, J.D.; Lever, A.M.L. Chronic fatigue syndrome and idiopathic intracranial hypertension: Different manifestations of the same disorder of intracranial pressure? Med. Hypotheses 2017, 105, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Shams, P.N.; Goadsby, P.J.; Crockard, H.A.; Casey, A.T.; Plant, G.T. Paroxysmal raised intracranial pressure associated with spinal meningeal cysts. J. Neurol. 2005, 252, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Takemori, T.; Kakutani, K.; Maeno, K.; Akisue, T.; Kurosaka, M.; Nishida, K. Symptomatic perineural cyst: Report of two cases treated with cyst-subarachnoid shunts. Eur. Spine J. 2014, 23 (Suppl. S2), 267–270. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, M.; Kurtcuoglu, V.; Schmid Daners, M. Is posture-related craniospinal compliance shift caused by jugular vein collapse? A theoretical analysis. Fluids Barriers CNS 2017, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Carluccio, M.A.; Di Donato, I.; Sicurelli, F.; Chini, E.; Di Toro Mammarella, L.; Rossi, F.; Rubegni, A.; Federico, A. Tarlov cysts: Clinical evaluation of an italian cohort of patients. Neurol. Sci. 2013, 34, 1679–1682. [Google Scholar] [CrossRef]
- Henderson, F.C., Sr.; Austin, C.; Benzel, E.; Bolognese, P.; Ellenbogen, R.; Francomano, C.A.; Ireton, C.; Klinge, P.; Koby, M.; Long, D.; et al. Neurological and spinal manifestations of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 195–211. [Google Scholar] [CrossRef]
- Hoshino, Y.; Edakuni, H.; Shimada, H.; Hayashi, S.; Machida, M.; Shimano, S.; Taya, T.; Ohki, I.; Takahashi, A.; Kurihara, T.; et al. Sacral arachnoid cyst associated with marfan syndrome. Intern. Med. 2005, 44, 271–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Weerasuriya, A.; Mizisin, A.P. The blood-nerve barrier: Structure and functional significance. Methods Mol. Biol. 2011, 686, 149–173. [Google Scholar] [CrossRef] [PubMed]
| Recurrent cyst | |||||
| ∑ | Yes | No | |||
| N (% column) | N (% row) | N (% row) | p-value | ||
| Thecaloscopy | Yes | 48 (61.5) | 5 (10.4) | 43 (89.6) | 0.0368 |
| No | 30 (38.5) | 9 (30.0) | 21 (70.0) | ||
| ∑ | 78 | 14 (17.9) | 64 (82.1) | ||
| ∑ | Thecaloscopy | No Thecaloscopy | p-Value | Recurrent Cyst | No Recurrent Cyst | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%)/Mean ± SD | N (%)/Mean ± SD | N (%)/Mean ± SD | N (%)/Mean ± SD | |||||
| Demographic information | |||||||||
| Age [years] | 78 | 48/50.0 ± 11.4 | 30/54.4 ± 9.0 | 0.077 | 14/53.1 ± 7.6 | 64/51.4 ± 11.3 | 0.590 | ||
| Gender | 78 | ||||||||
| female | 63 (80.8) | 38 (60.3) | 25 (39.7) | 0.772 | 10 (15.9) | 53 (84.1) | 0.453 | ||
| male | 15 (19.2) | 10 (66.7) | 5 (33.3) | 4 (26.7) | 11 (73.3) | ||||
| Height [cm] | 78 | 48/169.9 ± 7.3 | 30/179.6 ± 7.6 | 0.689 | 14/175.0 ± 7.2 | 64/169.2 ± 7.1 | 0.007 | ||
| Weight [kg] | 78 | 48/66.8 ± 13.2 | 30/71.1 ± 13.8 | 0.168 | 14/73.4 ± 13.7 | 64/67.4 ± 13.4 | 0.136 | ||
| Body mass index | 78 | 48/22.7 ± 3.7 | 30/23.9 ± 3.9 | 0.189 | 14/23.6 ± 3.5 | 64/23.1 ± 3.9 | 0.674 | ||
| Clinical parameters | |||||||||
| Symptoms duration [years] | 77 | 47/6.8 ± 9.9 | 30/2.5 ± 3.3 | 0.025 | 14/3.5 ± 4.5 | 63/5.5 ± 8.8 | 0.422 | ||
| Pain level (VAS) | |||||||||
| pre-OP | 78 | 48/8.0 ± 2.0 | 30/8.0 ± 1.3 | 0.960 | 14/8.4 ± 0.9 | 64/7.9 ± 1.9 | 0.420 | ||
| Follow up | 78 | 48/4.5 ± 2.7 | 30/5.2 ± 2.7 | 0.280 | 14/6.4 ± 2.2 | 64/4.4 ± 2.7 | 0.010 | ||
| Bladder or bowl dysfunction | 78 | ||||||||
| Yes | 46 (59.0) | 29 (63.0) | 17 (37.0) | 0.815 | 5 (10.9) | 41 (89.1) | 0.072 | ||
| No | 32 (41.0) | 19 (59.4) | 13 (40.6) | 9 (28.1) | 23 (71.8) | ||||
| Dysesthesia | 78 | ||||||||
| Yes | 59 (75.6) | 34 (57.6) | 25 (42.4) | 0.282 | 10 (16.9) | 49 (83.1) | 0.735 | ||
| No | 19 (24.4) | 14 (73.7) | 5 (26.3) | 4 (21.1) | 15 (78.9) | ||||
| Sexual dysfunction | 78 | ||||||||
| Yes | 8 (10.3) | 5 (62.5) | 3 (37.5) | 1.000 | 2 (25.0) | 6 (75.0) | 0.629 | ||
| No | 70 (89.7) | 43 (55.1) | 27 (44.9) | 12 (17.1) | 58 (82.9) | ||||
| Symptoms after surgery | 78 | ||||||||
| Improved | 55 (70.5) | 35 (63.6) | 20 (36,4) | 0.582 | 5 (9.1) | 50 (90.9) | 0.006 | ||
| Equal | 18 (23.1) | 11 (61.1) | 7 (38.9) | 7 (41.2) | 10 (58.8) | ||||
| Worsened | 5 (6.4) | 2 (40.0) | 3 (60.0) | 2 (33.3) | 4 (66.7) | ||||
| Residual symptoms | |||||||||
| Yes | 63 (80.8) | 37 (58.7) | 26 (41.3) | 0.383 | 11 (17.5) | 52 (82.5) | 1.000 | ||
| No | 15 (19.2) | 11 (73.3) | 4 (26.7) | 3 (20.0) | 12 (80.0) | ||||
| Cyst characteristics | |||||||||
| Number | 78 | 48/2.1 ± 1.2 | 30/2.6 ± 1.6 | 0.143 | 14/2.9 ± 1.5 | 64/2.2 ± 1.3 | 0.103 | ||
| Size [mm] | |||||||||
| max | 75 | 47/23.0 ± 15.2 | 28/26.3 ± 14.0 | 0.357 | 14/25.9 ± 8.0 | 61/23.0 ± 16.0 | 0.637 | ||
| mean | 75 | 47/20.0 ± 15.8 | 28/22.3 ± 16.4 | 0.592 | 14/18.4 ± 6.0 | 61/21.3 ± 17.1 | 0.136 | ||
| Bilateral | 78 | ||||||||
| Yes | 34 (43.6) | 26 (76.5) | 8 (23.5) | 0.020 | 13 (27.1) | 35 (72.9) | 0.002 | ||
| No | 44 (56.4) | 22 (50.0) | 22 (50.0) | 1 (3.3) | 29 (96.7) | ||||
| Multisegmental | 77 | ||||||||
| Yes | 43 (55.8) | 24 (55.8) | 19 (44.2) | 0.238 | 10 (23.3) | 33 (76.7) | 0.129 | ||
| No | 34 (44.2) | 24 (70.6) | 10 (29.4) | 4 (11.8) | 31 (88.2) | ||||
| Bone erosion | 78 | ||||||||
| Yes | 54 (43.6) | 37 (68.8) | 17 (31.5) | 0.078 | 8 (14.8) | 46 (85.2) | 0.342 | ||
| No | 24 (56.4) | 11 (45.8) | 13 (54.2) | 6 (25.0) | 18 (75.0) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchtmann, M.; Klammer, A.; Iova, M.-A.; Roth, A.; Chanamolu, V.K.; Mawrin, C.; Warnke, J.-P. Thecaloscopy Reduces the Risk of Recurrent Perineural (Tarlov) Cysts after Microsurgical Resection. Neurol. Int. 2024, 16, 450-458. https://doi.org/10.3390/neurolint16020033
Luchtmann M, Klammer A, Iova M-A, Roth A, Chanamolu VK, Mawrin C, Warnke J-P. Thecaloscopy Reduces the Risk of Recurrent Perineural (Tarlov) Cysts after Microsurgical Resection. Neurology International. 2024; 16(2):450-458. https://doi.org/10.3390/neurolint16020033
Chicago/Turabian StyleLuchtmann, Michael, Angelika Klammer, Mircea-Alin Iova, André Roth, Vijay Kumar Chanamolu, Christian Mawrin, and Jan-Peter Warnke. 2024. "Thecaloscopy Reduces the Risk of Recurrent Perineural (Tarlov) Cysts after Microsurgical Resection" Neurology International 16, no. 2: 450-458. https://doi.org/10.3390/neurolint16020033
APA StyleLuchtmann, M., Klammer, A., Iova, M.-A., Roth, A., Chanamolu, V. K., Mawrin, C., & Warnke, J.-P. (2024). Thecaloscopy Reduces the Risk of Recurrent Perineural (Tarlov) Cysts after Microsurgical Resection. Neurology International, 16(2), 450-458. https://doi.org/10.3390/neurolint16020033





