Effectiveness of Switching CGRP Monoclonal Antibodies in Non-Responder Patients in the UAE: A Retrospective Study
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
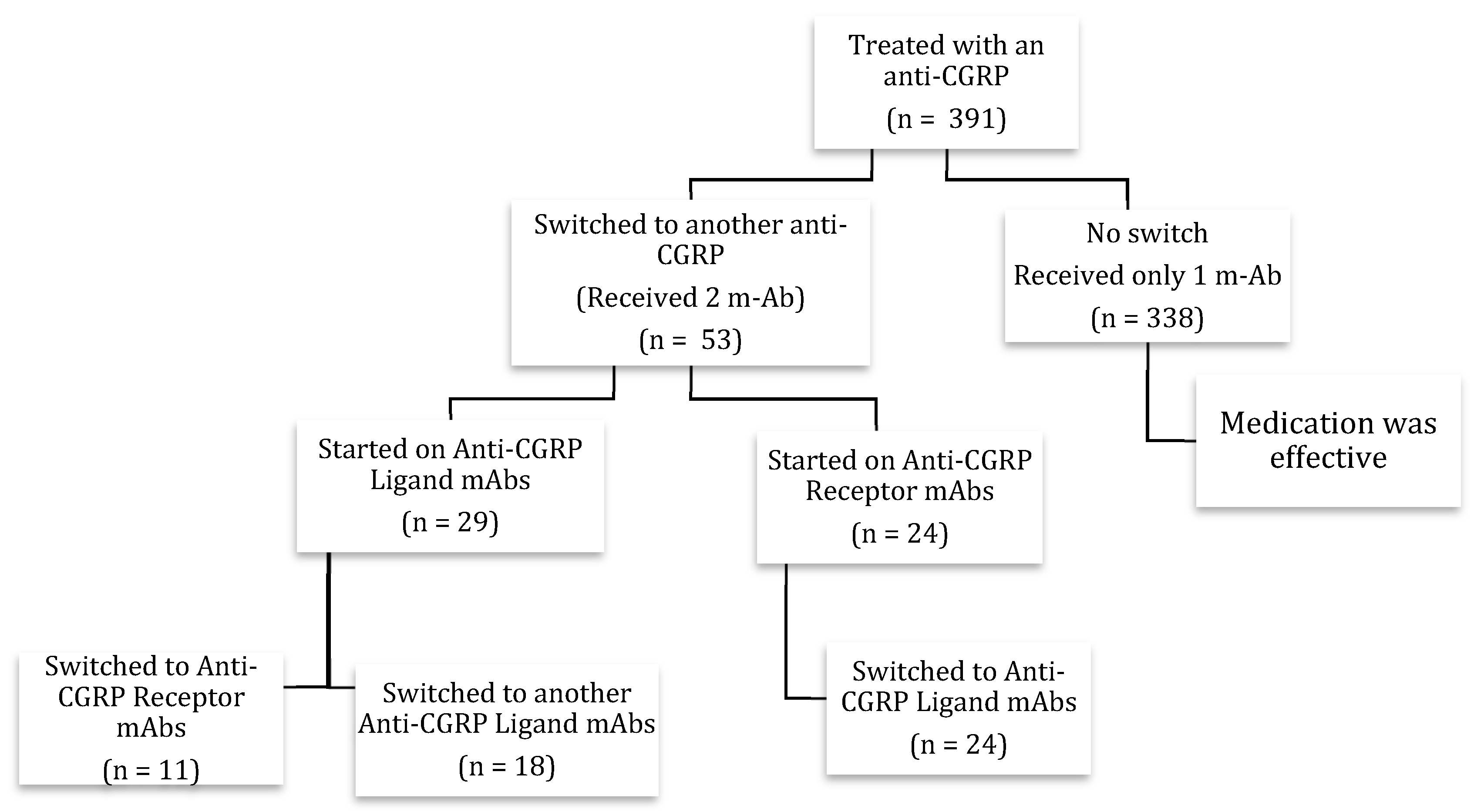
3. Results
3.1. Demographics and Baseline Characteristics
3.2. First CGRP Monoclonal Antibody
3.3. Second CGRP Monoclonal Antibody
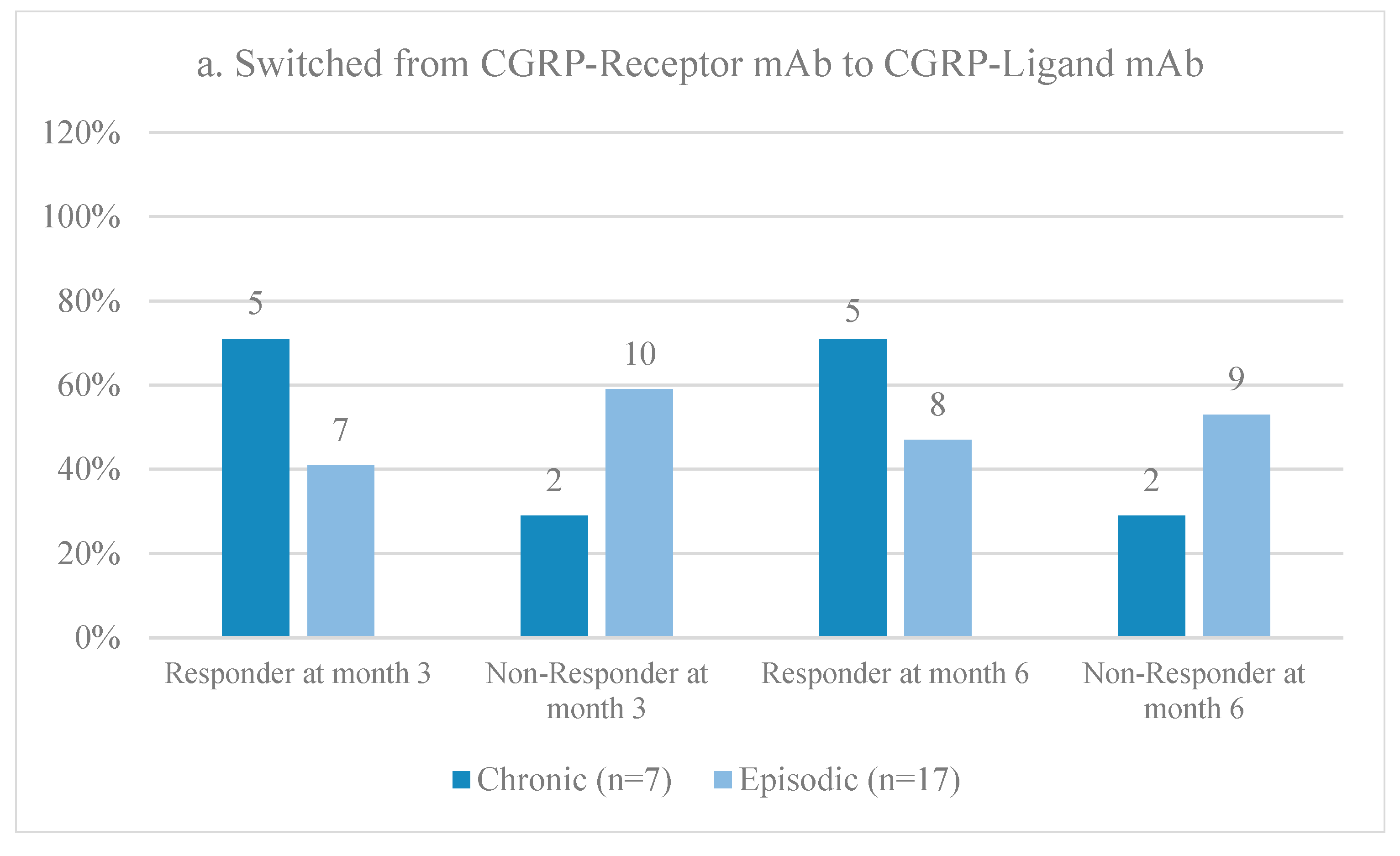
3.4. First versus Second CGRP Monoclonal Antibody
3.5. Exposure and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Pourfathi, H.; Eagan, A.; Mansournia, M.A.; Khodayari, M.T.; Sullman, M.J.; Kaufman, J.; Collins, G.; Dai, H.; Bragazzi, N.L. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain 2022, 163, e293–e309. [Google Scholar] [CrossRef]
- On behalf of Lifting the Burden: The Global Campaign against Headache; Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [CrossRef]
- Buse, D.C.; Scher, A.I.; Dodick, D.W.; Reed, M.L.; Fanning, K.M.; Adams, A.M.; Lipton, R.B. Impact of Migraine on the Family: Perspectives of People with Migraine and Their Spouse/Domestic Partner in the CaMEO Study. Mayo Clin. Proc. 2016, 91, 596–611. [Google Scholar] [CrossRef]
- Wattiez, A.S.; Sowers, L.P.; Russo, A.F. Calcitonin gene-related peptide (CGRP): Role in migraine pathophysiology and therapeutic targeting. Expert Opin Ther Targets 2020, 24, 91–100. [Google Scholar] [CrossRef]
- Shi, M.; Guo, J.; Li, Z.; Sun, H.; Yang, X.; Yang, D.; Zhao, H. Network meta-analysis on efficacy and safety of different anti-CGRP monoclonal antibody regimens for prophylaxis and treatment of episodic migraine. Neurol. Res. 2021, 43, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Schuster, N.M.; Rapoport, A.M. New strategies for the treatment and prevention of primary headache disorders. Nat. Rev. Neurol. 2016, 12, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, B.; Reuter, U. The Biology of Monoclonal Antibodies: Focus on Calcitonin Gene-Related Peptide for Prophylactic Migraine Therapy. Neurotherapeutics 2018, 15, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.J.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.D.; Winner, P.; Zhang, F.; Cheng, S.; Mikol, D.D.; et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: Results from a 52-week, open-label extension study. Cephalalgia 2020, 40, 543–553. [Google Scholar] [CrossRef]
- Kanaan, S.; Hettie, G.; Loder, E.; Burch, R. Real-world effectiveness and tolerability of erenumab: A retrospective cohort study. Cephalalgia 2020, 40, 1511–1522. [Google Scholar] [CrossRef]
- Ruff, D.D.; Ford, J.H.; Tockhorn-Heidenreich, A.; Stauffer, V.L.; Govindan, S.; Aurora, S.K.; Terwindt, G.M.; Goadsby, P.J. Efficacy of galcanezumab in patients with episodic migraine and a history of preventive treatment failure: Results from two global randomized clinical trials. Eur. J. Neurol. 2020, 27, 609–618. [Google Scholar] [CrossRef]
- Dhillon, S. Eptinezumab: First Approval. Drugs 2020, 80, 733–739. [Google Scholar] [CrossRef]
- Winner, P.K.; McAllister, P.; Chakhava, G.; Ailani, J.; Ettrup, A.; Josiassen, M.K.; Lindsten, A.; Mehta, L.; Cady, R. Effects of Intravenous Eptinezumab vs Placebo on Headache Pain and Most Bothersome Symptom When Initiated During a Migraine Attack: A Randomized Clinical Trial. JAMA 2021, 325, 2348. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Goadsby, P.J.; Silberstein, S.D.; Lipton, R.B.; Olesen, J.; Ashina, M.; Wilks, K.; Kudrow, D.; Kroll, R.; Kohrman, B.; et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014, 13, 1100–1107. [Google Scholar] [CrossRef]
- Alex, A.; Vaughn, C.; Rayhill, M. Safety and Tolerability of 3 CGRP Monoclonal Antibodies in Practice: A Retrospective Cohort Study. Headache J. Head Face Pain 2020, 60, 2454–2462. [Google Scholar] [CrossRef]
- López Moreno, Y.; Castro Sánchez, M.V.; García Trujillo, L.; Serrano Castro, P.J. Fracaso de un anticuerpo monoclonal anti-CGRP en el tratamiento de la migraña. ¿Tiene sentido probar otro? Rev. Neurol. 2022, 75, 87. [Google Scholar] [CrossRef] [PubMed]
- Briceño-Casado, M.D.P.; Gil-Sierra, M.D.; De-La-Calle-Riaguas, B. Switching of monoclonal antibodies against calcitonin gene-related peptide in chronic migraine in clinical practice: A case series. Eur. J. Hosp. Pharm. 2021, 30, e19. [Google Scholar] [CrossRef]
- Patier Ruiz, I.; Sánchez-Rubio Ferrández, J.; Cárcamo Fonfría, A.; Molina García, T. Early Experiences in Switching between Monoclonal Antibodies in Patients with Nonresponsive Migraine in Spain: A Case Series. Eur. Neurol. 2022, 85, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Ziegeler, C.; May, A. Non-Responders to Treatment with Antibodies to the CGRP-Receptor May Profit from a Switch of Antibody Class. Headache J. Head Face Pain 2020, 60, 469–470. [Google Scholar] [CrossRef]
- Overeem, L.H.; Peikert, A.; Hofacker, M.D.; Kamm, K.; Ruscheweyh, R.; Gendolla, A.; Raffaelli, B.; Reuter, U.; Neeb, L. Effect of antibody switch in non-responders to a CGRP receptor antibody treatment in migraine: A multi-center retrospective cohort study. Cephalalgia 2022, 42, 291–301. [Google Scholar] [CrossRef]
- Sacco, S.; Amin, F.M.; Ashina, M.; Bendtsen, L.; Deligianni, C.I.; Gil-Gouveia, R.; Katsarava, Z.; MaassenVanDenBrink, A.; Martelletti, P.; Mitsikostas, D.D.; et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J. Headache Pain 2022, 23, 67. [Google Scholar] [CrossRef]
- Alsaadi, T.; Kayed, D.M.; Al-Madani, A.; Hassan, A.M.; Krieger, D.; Riachi, N.; Sarathchandran, P.; Al-Rukn, S. Acute Treatment of Migraine: Expert Consensus Statements from the United Arab Emirates (UAE). Neurol. Ther. 2024, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 627–808. [Google Scholar]
- Kaltseis, K.; Filippi, V.; Frank, F.; Eckhardt, C.; Schiefecker, A.; Broessner, G. Monoclonal antibodies against CGRP (R): Non-responders and switchers: Real world data from an austrian case series. BMC Neurol. 2023, 23, 174. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Aurilia, C.; Egeo, G.; Proietti, S.; D’Onofrio, F.; Torelli, P.; Aguggia, M.; Bertuzzo, D.; Finocchi, C.; Trimboli, M. Ultra-late response (>24 weeks) to anti-CGRP monoclonal antibodies in migraine: A multicenter, prospective, observational study. J. Neurol. 2024. [Google Scholar] [CrossRef]




| Drug | Mechanism | Indication | Dosing | FDA Approved | Availability in the UAE |
|---|---|---|---|---|---|
| Erenumab | Blocks CGRP receptor | Prophylactic | Monthly, subcutaneous | 2018 | Yes |
| Eptinezumab | Binds to CGRP ligand | Prophylactic | Quarterly, intravenous | 2020 | Yes |
| Galcanezumab | Binds to CGRP ligand | Prophylactic | Monthly, subcutaneous | 2018 | Yes |
| Fremanezumab | Binds to CGRP ligand | Prophylactic | Monthly or quarterly, subcutaneous, but intravenous load for cluster headache | 2018 | No |
| Total Cohort | |
|---|---|
| (n = 53) | |
| Demographics | |
| Age (years), mean +/− SD. | 39.2 +/− 11.0 |
| Sex female, n (%) | 42 (79.2%) |
| Migraine features | |
| Chronic migraine, n (%) | 20 (37.7%) |
| Pain intensity, mild, n (%) | 0 (0.0%) |
| Pain intensity, moderate, n (%) | 48 (90.6%) |
| Pain intensity, severe, n (%) | 5 (9.4%) |
| Patients with daily headaches, n (%) | 10 (18.9%) |
| Age at migraine diagnosis, mean +/− SD | 27.56 +/− 10.62 |
| Migraine duration (years), mean +/− SD | 11.6 +/− 11.3 |
| Other features | |
| Positive family history of migraine, n (%) | 16 (30%) |
| Received prior preventive treatment, n (%) | 16 (30%) |
| Psychiatric comorbidity, n (%) | 24 (45.0%) |
| First monoclonal antibody | |
| CGRP/L mAb, n (%) | 29 (55.0%) |
| Galcanezumab, n (%) | 28 (53) |
| Eptinezumab, n (%) | 1 (2.0%) |
| CGRP/R mAb (erenumab), n (%) | 24 (45.0%) |
| Second monoclonal antibody | |
| CGRP/L mAb, n | 42 |
| Galcanezumab, n | 7 |
| Eptinezumab, n | 35 |
| CGRP/R mAb (erenumab), n | 11 |
| Switching profile | |
| CGRP/L to CGRP/R, n | 11 |
| CGRP/L to another CGRP/L, n | 18 |
| CGRP/R to CGRP/L, n | 24 |
| Drug | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Pre-switch BL MMD | 12.92 | 1.0 | 28.0 | 8.23 |
| Pre-switch M3 MMD | 5.53 | 0.0 | 28.0 | 5.73 |
| Pre-switch M6 MMD | 5.92 | 0.0 | 28.0 | 6.69 |
| Post-switch BL MMD | 10.21 | 2.0 | 28.0 | 5.42 |
| Post-switch M3 MMD | 5.74 | 0.0 | 15.0 | 4.32 |
| Post-switch M6 MMD | 5.42 | 0.0 | 28.0 | 4.98 |
| Median Difference (IQR) 6 Month | N | |
|---|---|---|
| All patients p-value | −5.1 (5.5) <0.001 | 53 |
| Anti-CGRP/L before switching to anti-CGRP/R p-value | −2.2 (7.0) 0.025 | 11 |
| Anti-CGRP/R before switching to anti-CGRP/L p-value | −3.15 (4.5) 0.002 | 24 |
| Anti-CGRP/L before switching to another anti-CGRP/L p-value | −3.21 (5.25) 0.001 | 18 |
| Median Difference (IQR) 6 Month | N | |
|---|---|---|
| All patients p-value | −5.0 (5.0) <0.001 | 53 |
| Anti-CGRP/R after anti-CGRP/L p-value | −1.60 (9.0) 0.109 | 11 |
| Anti-CGRP/L after CGRP/R p-value | −3.73 (3.75) <0.001 | 24 |
| Anti-CGRP/L after another anti-CGRP/L p-value | −3.47 (7.25) 0.001 | 18 |
| Switching Profile | Q1 Frequency (%) | Q2 Frequency (%) | Q3 Frequency (%) | Q4 Frequency (%) |
|---|---|---|---|---|
| Ligand–Receptor | 6.0 (66.7) | 2.0 (22.2) | 0.0 (0.0) | 1.0 (11.1) |
| Receptor–Ligand | 15.0 (71.4) | 2.0 (9.5) | 1.0 (4.8) | 3.0 (14.3) |
| Ligand–Ligand | 8.0 (44.4) | 4.0 (22.2) | 3.0 (16.7) | 3.0 (16.7) |
| Pain Severity | Initial n (%) | Final (%) |
|---|---|---|
| Severe | 5 (9.4%) | 3 (5.7%) |
| Moderate | 48 (90.6%) | 39 (73.6%) |
| Mild | 0 (0.0%) | 11 (20.8%) |
| Status | Number of Patients | Percentage |
|---|---|---|
| Improved | 37 | 69.8% |
| No improvement | 15 | 28.3% |
| Worsened | 1 | 1.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suliman, R.; Santos, V.; Al Qaisi, I.; Aldaher, B.; Al Fardan, A.; Al Barrawy, H.; Bader, Y.; Supena, J.L.; Alejandro, K.; Alsaadi, T. Effectiveness of Switching CGRP Monoclonal Antibodies in Non-Responder Patients in the UAE: A Retrospective Study. Neurol. Int. 2024, 16, 274-288. https://doi.org/10.3390/neurolint16010019
Suliman R, Santos V, Al Qaisi I, Aldaher B, Al Fardan A, Al Barrawy H, Bader Y, Supena JL, Alejandro K, Alsaadi T. Effectiveness of Switching CGRP Monoclonal Antibodies in Non-Responder Patients in the UAE: A Retrospective Study. Neurology International. 2024; 16(1):274-288. https://doi.org/10.3390/neurolint16010019
Chicago/Turabian StyleSuliman, Reem, Vanessa Santos, Ibrahim Al Qaisi, Batool Aldaher, Ahmed Al Fardan, Hajir Al Barrawy, Yazan Bader, Jonna Lyn Supena, Kathrina Alejandro, and Taoufik Alsaadi. 2024. "Effectiveness of Switching CGRP Monoclonal Antibodies in Non-Responder Patients in the UAE: A Retrospective Study" Neurology International 16, no. 1: 274-288. https://doi.org/10.3390/neurolint16010019
APA StyleSuliman, R., Santos, V., Al Qaisi, I., Aldaher, B., Al Fardan, A., Al Barrawy, H., Bader, Y., Supena, J. L., Alejandro, K., & Alsaadi, T. (2024). Effectiveness of Switching CGRP Monoclonal Antibodies in Non-Responder Patients in the UAE: A Retrospective Study. Neurology International, 16(1), 274-288. https://doi.org/10.3390/neurolint16010019





