The Beneficial Outcome of Subsequent Treatment with Anakinra during the Chronic Phase of Febrile Infection-Related Epilepsy Syndrome (FIRES): A Case Report
Abstract
:1. Introduction
2. Methods
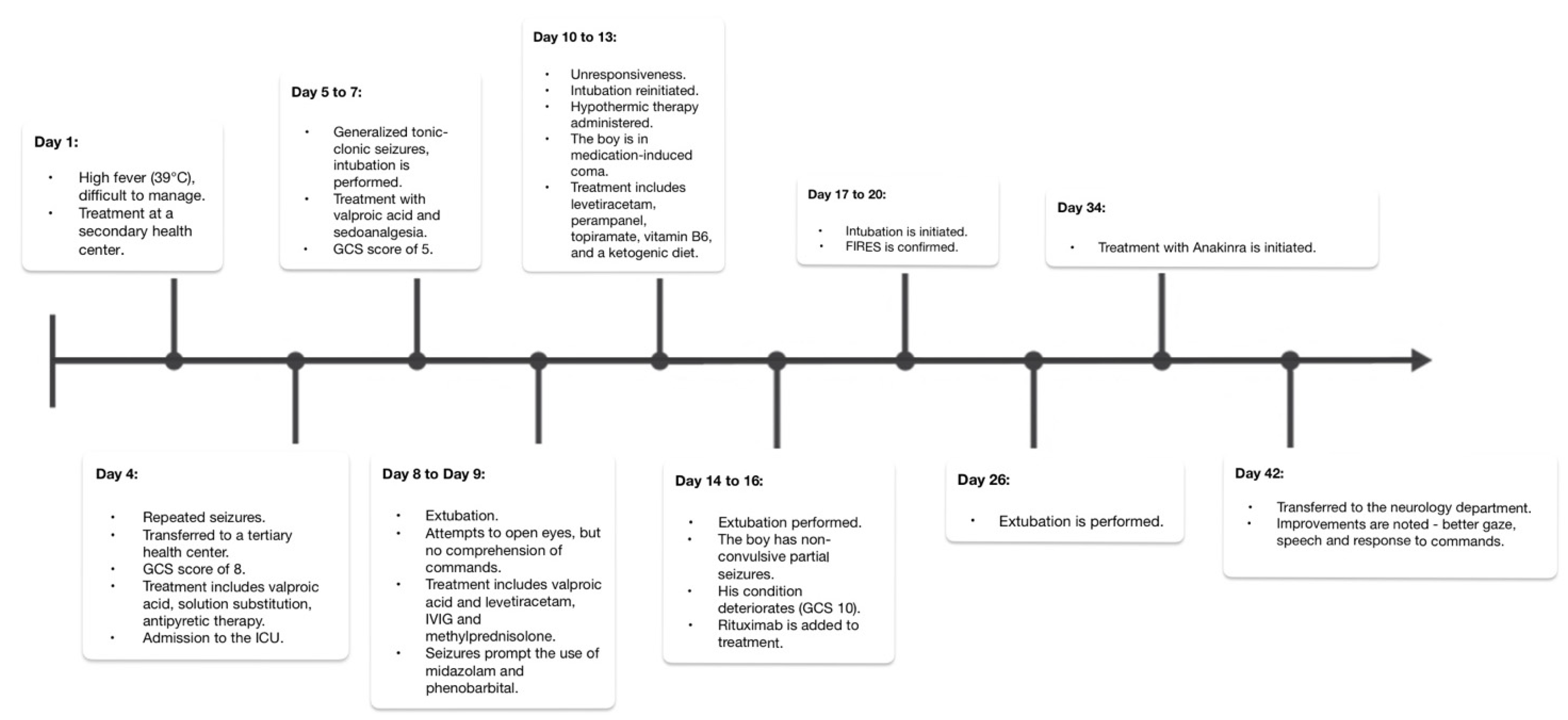
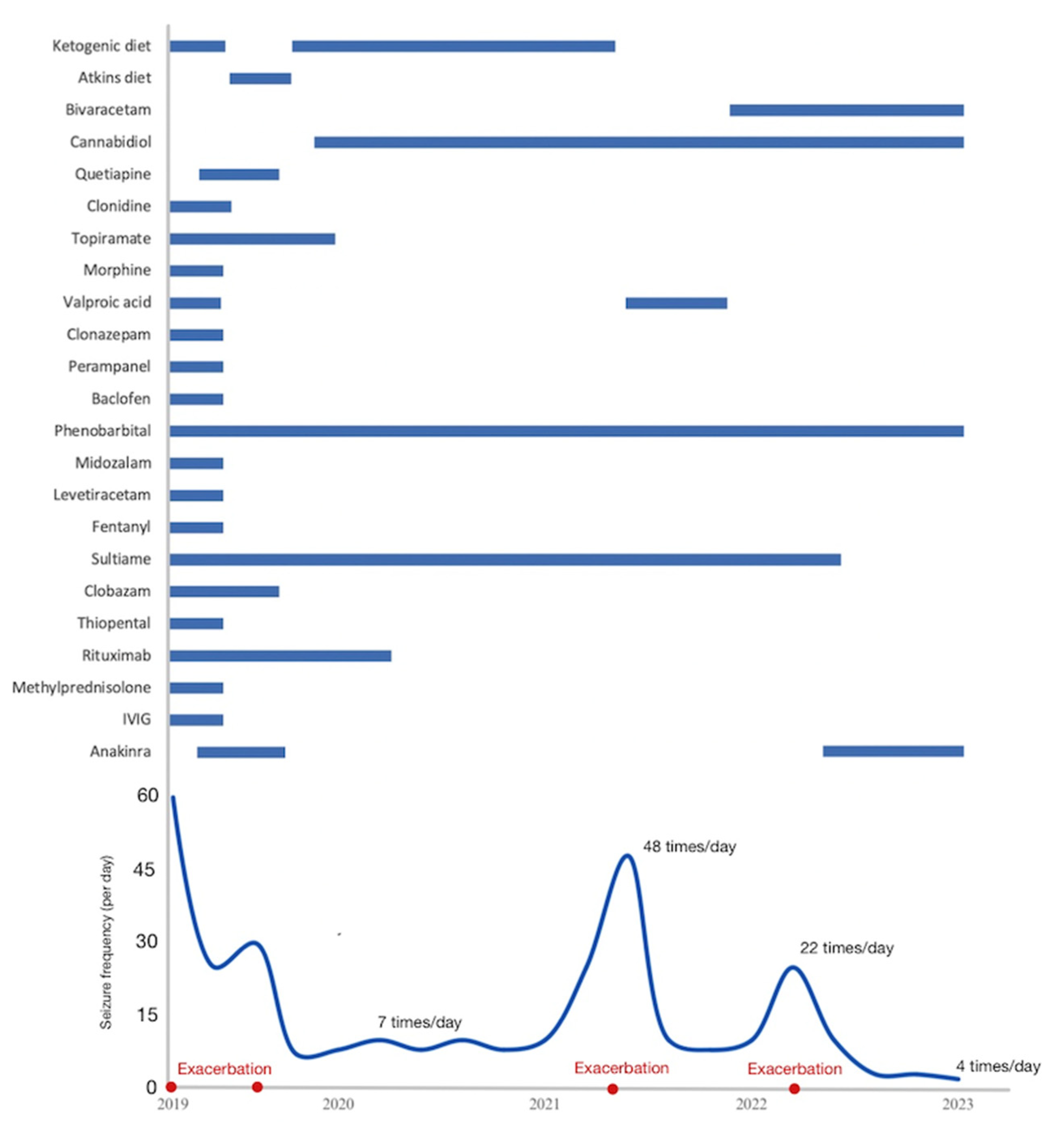
3. Case Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serino, D.; Santarone, M.E.; Caputo, D.; Fusco, L. Febrile infection-related epilepsy syndrome (FIRES): Prevalence, impact and management strategies. Neuropsychiatr. Dis. Treat. 2019, 15, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Pavone, P.; Corsello, G.; Raucci, U.; Lubrano, R.; Parano, E.; Ruggieri, M.; Greco, F.; Marino, S.; Falsaperla, R. Febrile infection-related Epilepsy Syndrome (FIRES): A severe encephalopathy with status epilepticus. Literature review and presentation of two new cases. Ital. J. Pediatr. 2022, 48, 199. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Wells, M.E.; Tennison, M.; Vaughn, B. Febrile Infection-Related Epilepsy Syndrome (FIRES): A Literature Review and Case Study. Neurodiagnostic J. 2017, 57, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Wickström, R.; Taraschenko, O.; Dilena, R.; Payne, E.T.; Specchio, N.; Nabbout, R.; Koh, S.; Gaspard, N.; Hirsch, L.J.; Auvin, S.; et al. International consensus recommendations for management of new onset refractory status epilepticus (NORSE) including febrile infection-related epilepsy syndrome (FIRES): Summary and clinical tools. Epilepsia 2022, 63, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Reppucci, D.; Datta, A.N. FIRES—Pathophysiology, Therapeutical Approach, and Outcome. Z. Epileptol. 2022, 35, 322–331. [Google Scholar] [CrossRef]
- Koh, S.; Wirrell, E.; Vezzani, A.; Nabbout, R.; Muscal, E.; Kaliakatsos, M.; Wickström, R.; Riviello, J.J.; Brunklaus, A.; Payne, E.; et al. Proposal to optimize evaluation and treatment of Febrile infection-related epilepsy syndrome (FIRES): A Report from FIRES workshop. Epilepsia Open 2021, 6, 62–72. [Google Scholar] [CrossRef]
- Farias-Moeller, R.; Bartolini, L.; Staso, K.; Schreiber, J.M.; Carpenter, J.L. Early ictal and interictal patterns in FIRES: The sparks before the blaze. Epilepsia 2017, 58, 1340–1348. [Google Scholar] [CrossRef]
- Kramer, U.; Chi, C.S.; Lin, K.L.; Specchio, N.; Sahin, M.; Olson, H.; Nabbout, R.; Kluger, G.; Lin, J.J.; van Baalen, A. Febrile infection-related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome: A multicenter study on 77 children. Epilepsia 2011, 52, 1956–1965. [Google Scholar] [CrossRef]
- Lam, S.K.; Lu, W.Y.; Weng, W.C.; Fan, P.C.; Lee, W.T. The short-term and long-term outcome of febrile infection-related epilepsy syndrome in children. Epilepsy Behav. 2019, 95, 117–123. [Google Scholar] [CrossRef]
- Lee, Y.-J. Febrile Infection-Related Epilepsy Syndrome: Refractory Status Epilepticus and Management Strategies. Ann. Child Neurol. 2020, 28, 8–15. [Google Scholar] [CrossRef]
- Gofshteyn, J.S.; Wilfong, A.; Devinsky, O.; Bluvstein, J.; Charuta, J.; Ciliberto, M.A.; Laux, L.; Marsh, E.D. Cannabidiol as a Potential Treatment for Febrile Infection-Related Epilepsy Syndrome (FIRES) in the Acute and Chronic Phases. J. Child Neurol. 2017, 32, 35–40. [Google Scholar] [CrossRef]
- Fetta, A.; Crotti, E.; Campostrini, E.; Bergonzini, L.; Cesaroni, C.A.; Conti, F.; Di Pisa, V.; Gentile, V.; Mondardini, M.C.; Vezzoli, C.; et al. Cannabidiol in the acute phase of febrile infection-related epilepsy syndrome (FIRES). Epilepsia Open 2023, 8, 685–691. [Google Scholar] [CrossRef]
- Peng, P.; Peng, J.; Yin, F.; Deng, X.; Chen, C.; He, F.; Wang, X.; Guang, S.; Mao, L. Ketogenic Diet as a Treatment for Super-Refractory Status Epilepticus in Febrile Infection-Related Epilepsy Syndrome. Front. Neurol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Muscal, E.; Wells, E.; Shukla, N.; Eschbach, K.; Lee, K.H.; Kaliakatsos, M.; Desai, N.; Wickström, R.; Viri, M.; et al. Anakinra usage in febrile infection related epilepsy syndrome: An international cohort. Ann. Clin. Transl. Neurol. 2020, 7, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Risen, S.; Erklauer, J.; Lai, Y.C.; Riviello, J.; Muscal, E. Anakinra (IL-1 blockade) Use in Children with Suspected FIRES: A Single Institution Experience (P4.346). Neurology 2018, 90 (Suppl. S15), P4.346. [Google Scholar] [CrossRef]
- Sakuma, H.; Horino, A.; Kuki, I. Neurocritical care and target immunotherapy for febrile infection-related epilepsy syndrome. Biomed. J. 2020, 43, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.B.; Katanyuwong, K.; Mackay, M.T.; Bailey, C.A.; Scheffer, I.E.; Freeman, J.L.; Berkovic, S.F.; Harvey, A.S. Long-Term Follow-up of Febrile Infection-Related Epilepsy Syndrome. Epilepsia 2011, 53, 101–110. [Google Scholar] [CrossRef]
- Rachfalska, N.; Pietruszewski, J.; Paprocka, J. Dramatic Course of Paediatric Cryptogenic Febrile Infection-Related Epilepsy Syndrome with Unusual Chronic Phase Presentation—A Case Report with Literature Study. Brain Sci. 2021, 11, 1030. [Google Scholar] [CrossRef]
- Operto, F.F.; Pastorino, G.M.G.; Viggiano, A.; Dell’Isola, G.B.; Verrotti, A.; Coppola, G. Epilepsy and Cognitive Impairment in Childhood and Adolescence: A Mini-Review. Curr. Neuropharmacol. 2023, 21, 1646–1665. [Google Scholar] [CrossRef]
- Koh, S.; Kim, T.; Lim, T.; Lee, J.S.; Kim, B.G.; Park, S.A.; Huh, K.; Choi, J.Y. Pentobarbital coma therapy for super-refractory status epilepticus and in-hospital mortality: An observational study. Epileptic Disord. 2021, 23, 833–842. [Google Scholar] [CrossRef]
- Yamanaka, G.; Ishida, Y.; Kanou, K.; Suzuki, S.; Watanabe, Y.; Takamatsu, T.; Morichi, S.; Go, S.; Oana, S.; Yamazaki, T.; et al. Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy. Int. J. Mol. Sci. 2021, 22, 6282. [Google Scholar] [CrossRef] [PubMed]
- Aledo-Serrano, A.; Hariramani, R.; Gonzalez-Martinez, A.; Álvarez-Troncoso, J.; Toledano, R.; Bayat, A.; Garcia-Morales, I.; Becerra, J.L.; Villegas-Martínez, I.; Beltran-Corbellini, A.; et al. Anakinra and Tocilizumab in the Chronic Phase of Febrile Infection-Related Epilepsy Syndrome (FIRES): Effectiveness and Safety from a Case-Series. Seizure 2022, 100, 51–55. [Google Scholar] [CrossRef]
- Dilena, R.; Mauri, E.; Aronica, E.; Bernasconi, P.; Bana, C.; Cappelletti, C.; Carrabba, G.; Ferrero, S.; Giorda, R.; Guez, S.; et al. Therapeutic Effect of Anakinra in the Relapsing Chronic Phase of Febrile Infection–Related Epilepsy Syndrome. Epilepsia Open 2019, 4, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Nataraj, S.; Sattar, S. Successful Treatment of Pediatric FIRES With Anakinra. Clin. Lett. 2020, 114, P60–P61. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.; Subramaniam, T.; Seagren, R.M.; Tarula, E.; Co, D.; Furstenberg-Knauff, M.; Wallace, A.; Hsu, D.; Payne, E. Febrile Infection-Related Epilepsy Syndrome Treated Successfully with Anakinra in a 21-Year-Old Woman. WMJ 2019, 118, 135–139. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cupane, T.L.; Strautmanis, J.; Setlere, S.; Diriks, M.; Auzenbaha, M. The Beneficial Outcome of Subsequent Treatment with Anakinra during the Chronic Phase of Febrile Infection-Related Epilepsy Syndrome (FIRES): A Case Report. Neurol. Int. 2023, 15, 1489-1496. https://doi.org/10.3390/neurolint15040097
Cupane TL, Strautmanis J, Setlere S, Diriks M, Auzenbaha M. The Beneficial Outcome of Subsequent Treatment with Anakinra during the Chronic Phase of Febrile Infection-Related Epilepsy Syndrome (FIRES): A Case Report. Neurology International. 2023; 15(4):1489-1496. https://doi.org/10.3390/neurolint15040097
Chicago/Turabian StyleCupane, Tina Luize, Jurgis Strautmanis, Signe Setlere, Mikus Diriks, and Madara Auzenbaha. 2023. "The Beneficial Outcome of Subsequent Treatment with Anakinra during the Chronic Phase of Febrile Infection-Related Epilepsy Syndrome (FIRES): A Case Report" Neurology International 15, no. 4: 1489-1496. https://doi.org/10.3390/neurolint15040097
APA StyleCupane, T. L., Strautmanis, J., Setlere, S., Diriks, M., & Auzenbaha, M. (2023). The Beneficial Outcome of Subsequent Treatment with Anakinra during the Chronic Phase of Febrile Infection-Related Epilepsy Syndrome (FIRES): A Case Report. Neurology International, 15(4), 1489-1496. https://doi.org/10.3390/neurolint15040097





