Atrial Fibrillation and Reperfusion Therapy in Acute Ischaemic Stroke Patients: Prevalence and Outcomes—A Comprehensive Systematic Review and Meta-Analysis
Abstract
:1. Introduction
- What is the prevalence of AF among AIS patients treated with each type of reperfusion therapy?
- Is there an association between AF and a favourable 90-day functional outcome in AIS patients treated with each type of reperfusion therapy?
- Does AF correlate with the occurrence of sICH in AIS patients treated with each type of reperfusion therapy?
- Is AF associated with 90-day mortality in AIS patients treated with each type of reperfusion therapy?
2. Methods
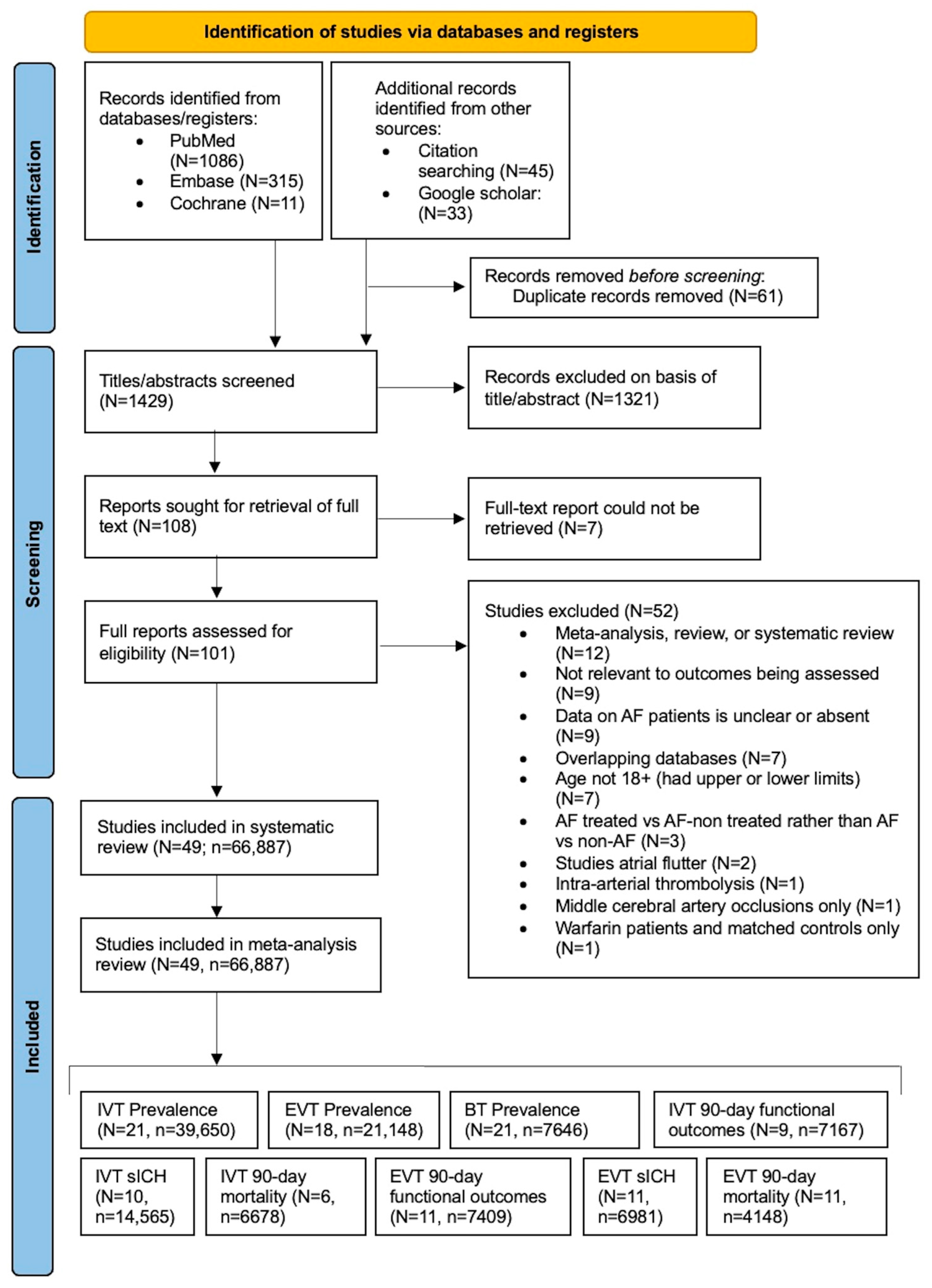
2.1. Literature Search: Study Identification and Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Assessment of Methodological Quality in Included Studies
2.5. Statistical Analysis
3. Results
3.1. Description of Included Studies
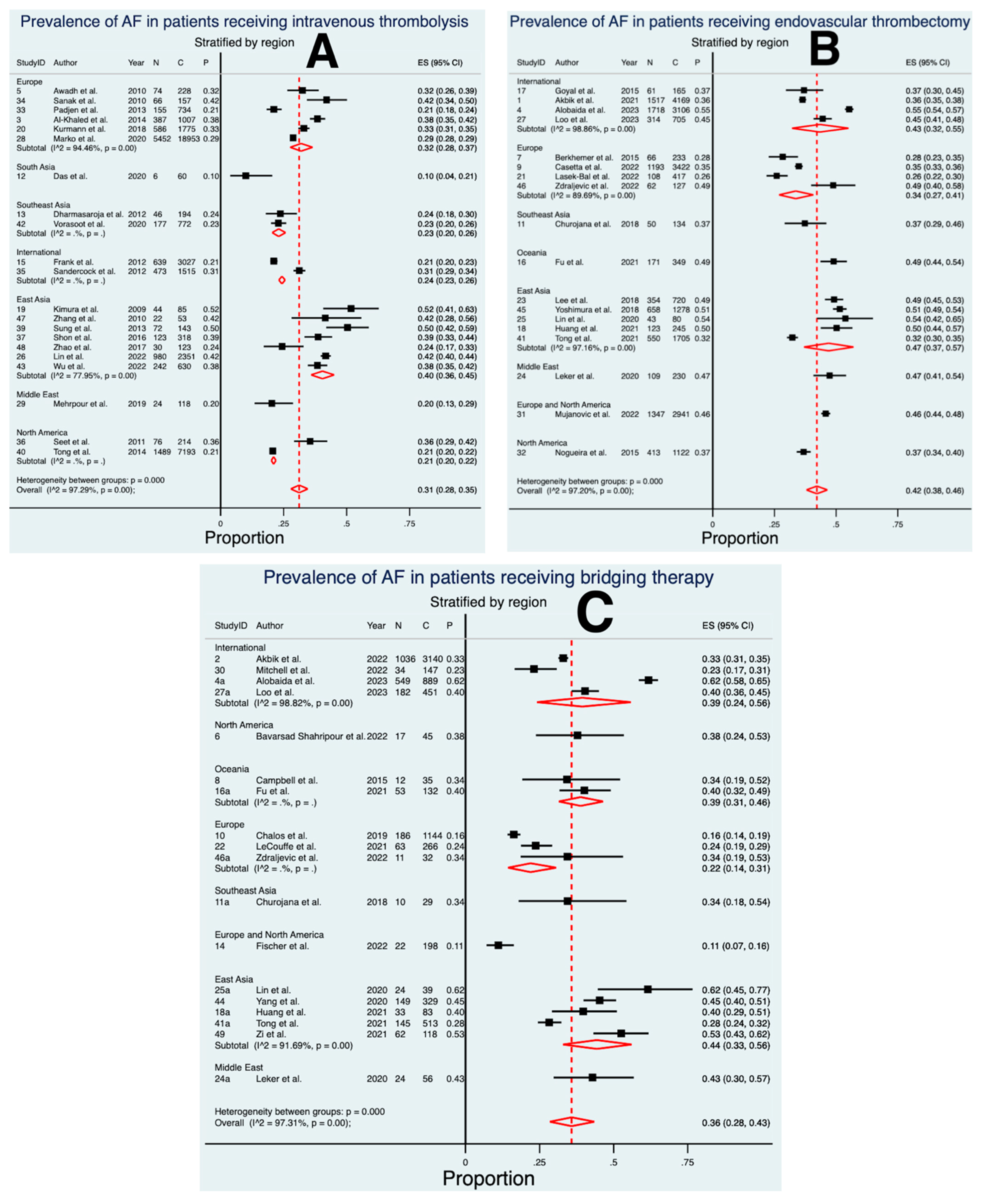
3.2. Prevalence of AF in Patients Treated with IVT
3.3. Prevalence of AF in Patients Treated with EVT
3.4. Prevalence of AF in Patients Treated with BT
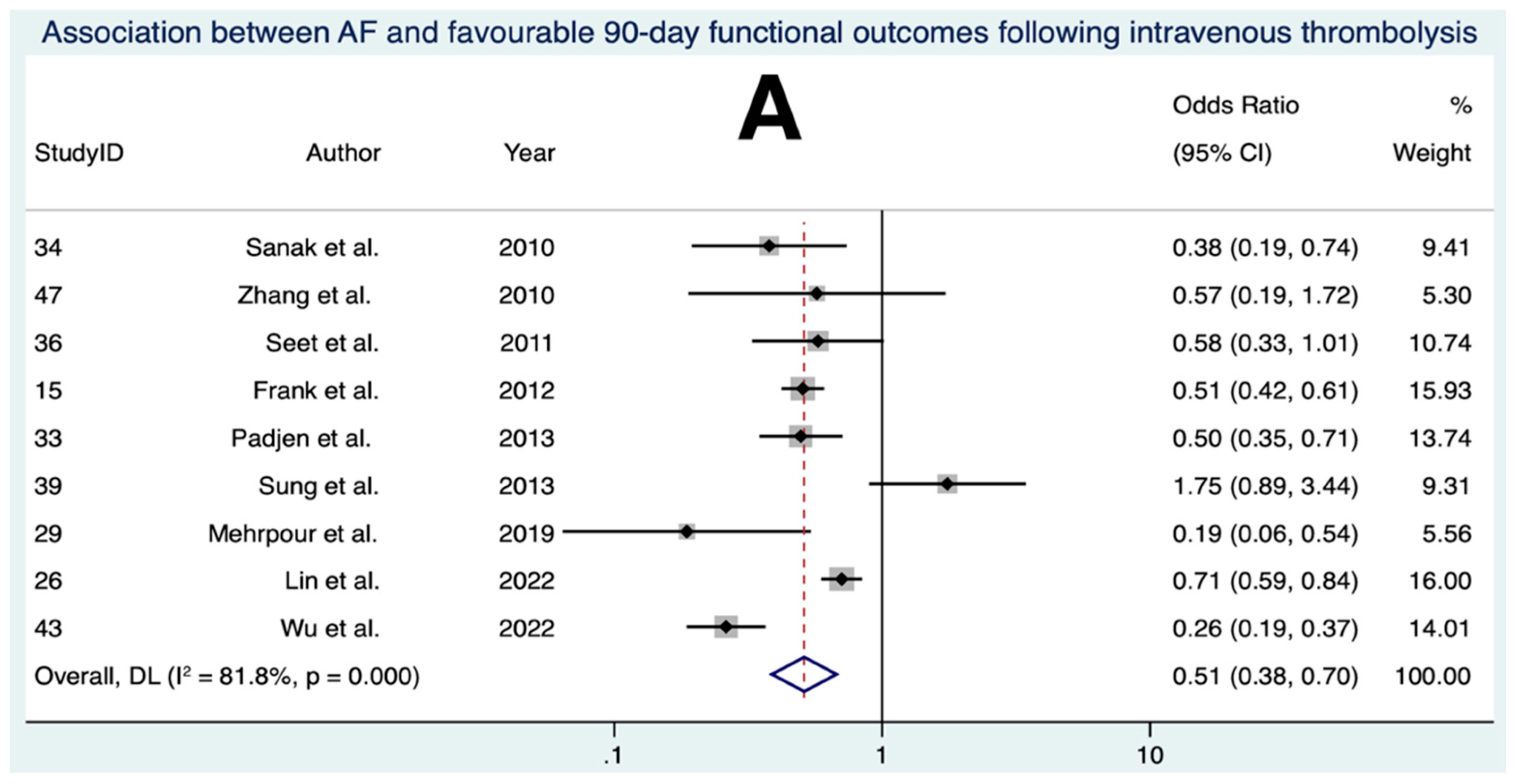
3.5. Association between AF and Favourable 90-Day Functional Outcomes Following IVT
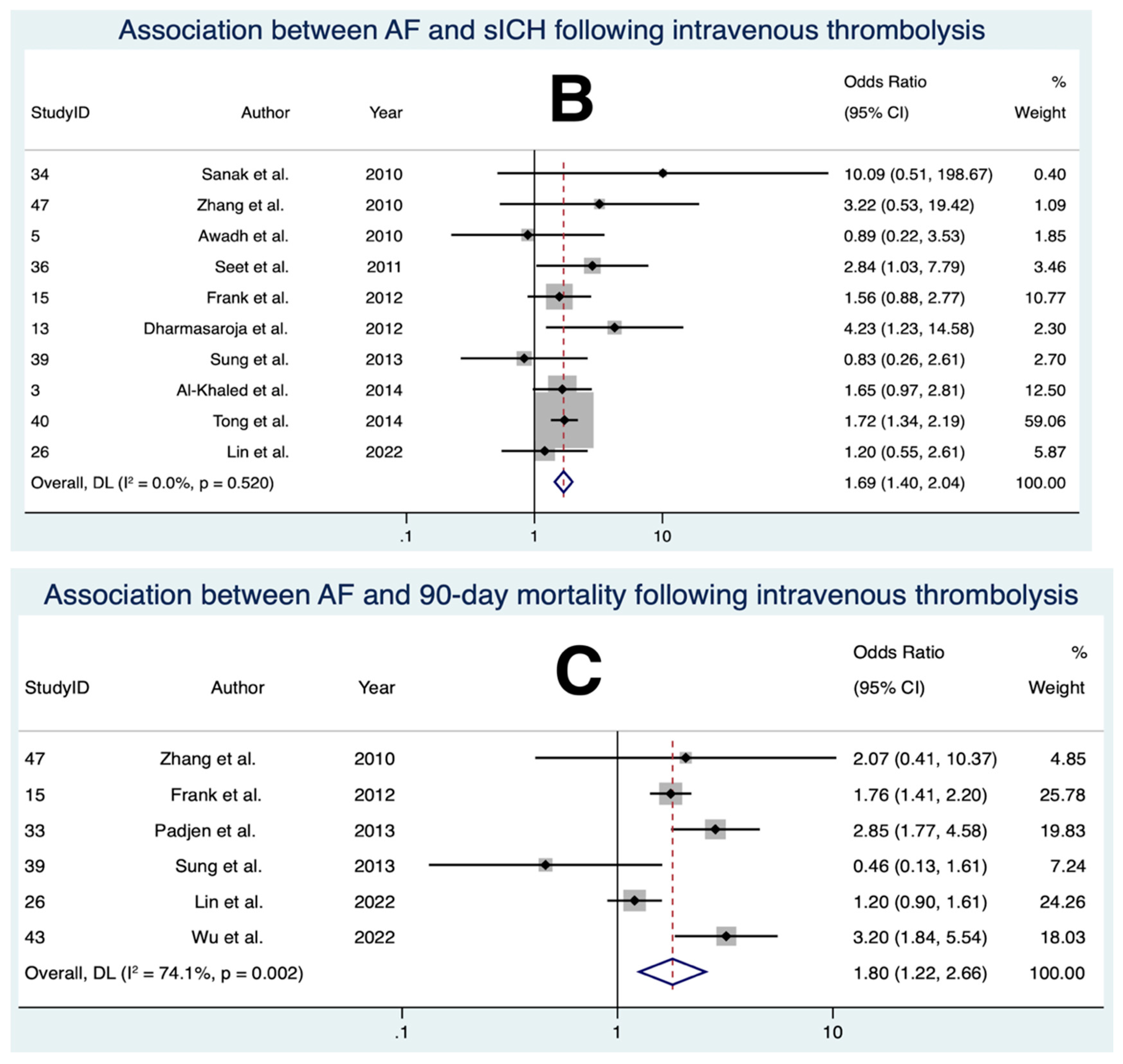
3.6. Association between AF and sICH Following IVT
3.7. Association between AF and 90-Day Mortality Following IVT
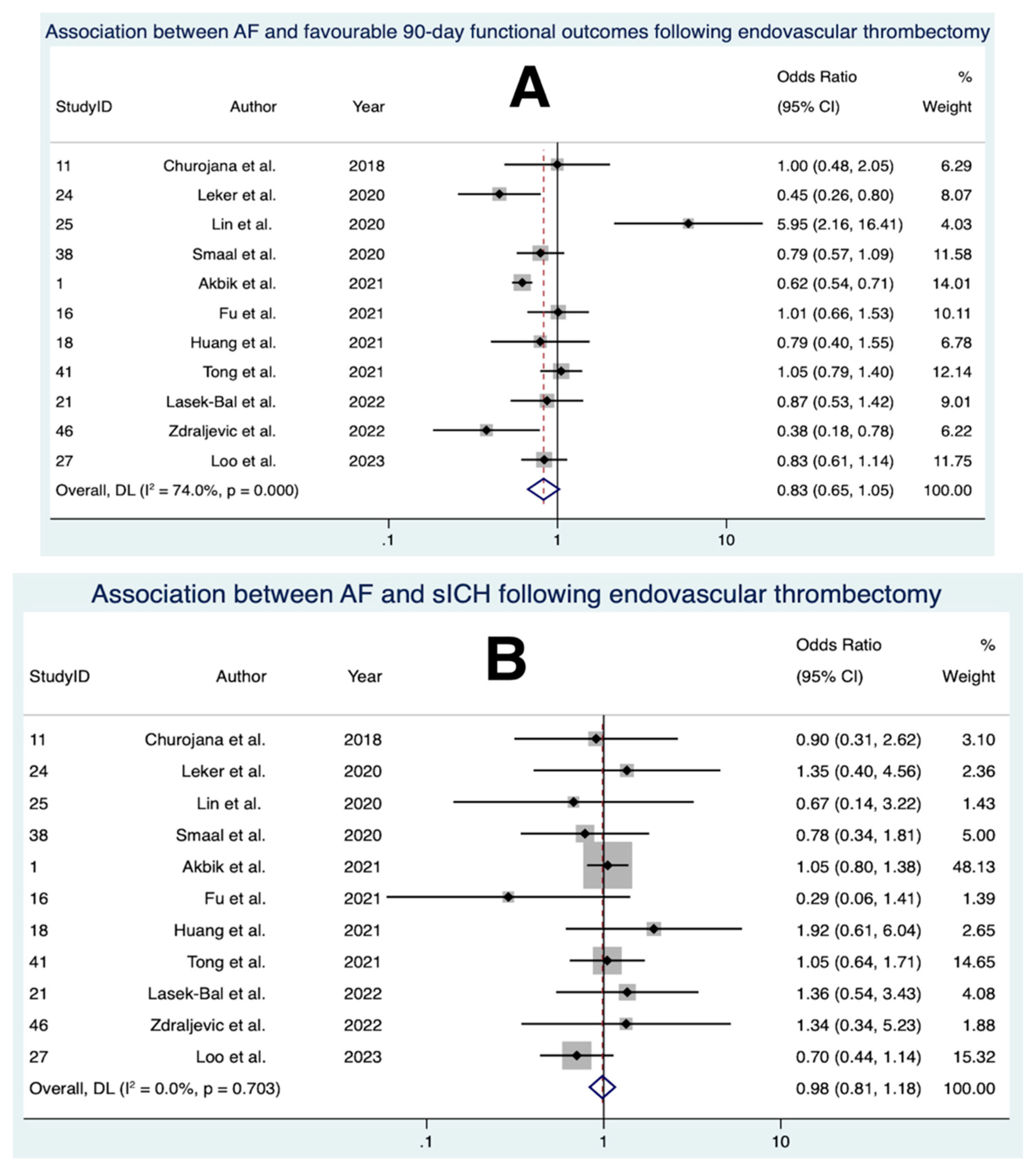
3.8. Association between AF and Favourable 90-Day Functional Outcomes Following EVT
3.9. Association between AF and sICH Following EVT
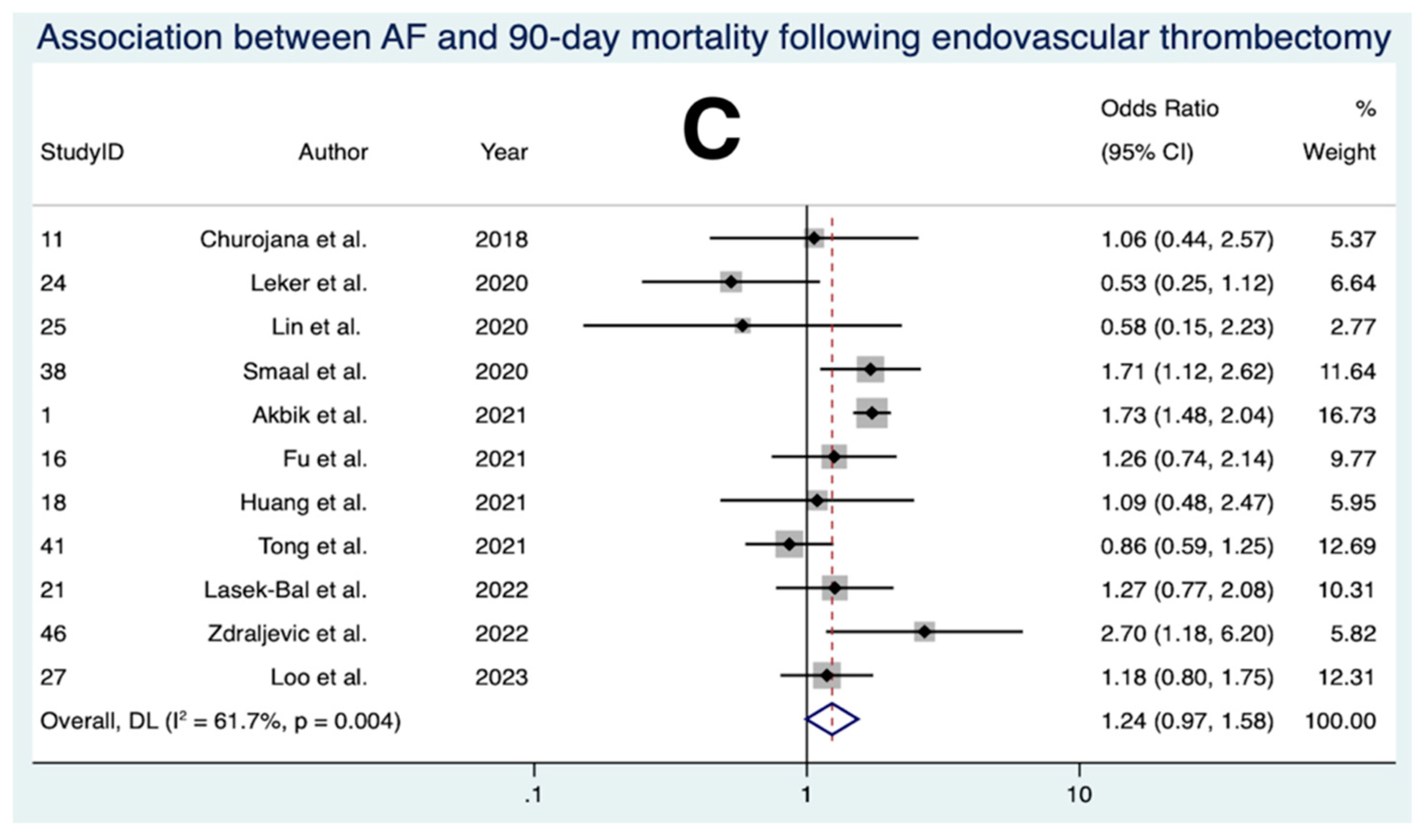
3.10. Association between AF and 90-Day Mortality Following EVT
3.11. Association between AF and Favourable 90-Day Functional Outcomes Following BT
3.12. Association between AF and sICH Following BT
3.13. Association between AF and 90-Day Mortality Following BT
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, X.J.; Wang, B.B.; Hou, F.F.; Jiao, Y.; Li, H.W.; Lv, S.P.; Li, F.H. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019. Europace 2023, 25, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, X.; Liang, Y.; Bai, X.; Liu-Huo, W.S.; Tang, C.; Chen, W.; Zhao, L. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990–2019: Results from a global burden of disease study, 2019. BMC Public Health 2022, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.S.; Maddison, R.; Uddin, R.; Ball, K.; Livingstone, K.M.; Khan, A.; Salmon, J.; Ackerman, I.N.; Adair, T.; Adegboye, O.A.; et al. The burden and trend of diseases and their risk factors in Australia, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2023, 8, e585–e599. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Seslar, S.P.; Sloan, L.A.; Hansen, R.N. Health care resource utilization and costs associated with atrial fibrillation and rural-urban disparities. J. Manag. Care Spec. Pharm. 2022, 28, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Choi, E.K.; Han, K.D.; Lee, H.; Choe, W.S.; Lee, S.R.; Cha, M.J.; Lim, W.H.; Kim, Y.J.; Oh, S. Mortality and causes of death in patients with atrial fibrillation: A nationwide population-based study. PLoS ONE 2018, 13, e0209687. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Okin, P.M.; Elkind, M.S.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef]
- Hald, E.M.; Rinde, L.B.; Lochen, M.L.; Mathiesen, E.B.; Wilsgaard, T.; Njolstad, I.; Braekkan, S.K.; Hansen, J.B. Atrial Fibrillation and Cause-Specific Risks of Pulmonary Embolism and Ischemic Stroke. J. Am. Heart Assoc. 2018, 7, e006502. [Google Scholar] [CrossRef]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Ruiz Vargas, E.; Riccio, P.M.; Hachinski, V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Bui, E.; Fang, J.; Laupacis, A.; Lindsay, M.P.; Tu, J.V.; Silver, F.L.; Kapral, M.K. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 2009, 40, 235–240. [Google Scholar] [CrossRef]
- Carnicelli, A.P.; Hong, H.; Connolly, S.J.; Eikelboom, J.; Giugliano, R.P.; Morrow, D.A.; Patel, M.R.; Wallentin, L.; Alexander, J.H.; Cecilia Bahit, M.; et al. Direct Oral Anticoagulants versus Warfarin in Patients with Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials with Interaction Testing by Age and Sex. Circulation 2022, 145, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.; Koga, M.; Itabashi, R.; Yamagami, H.; Todo, K.; Yoshimura, S.; Kimura, K.; Sato, S.; Terasaki, T.; Inoue, M.; et al. Prior Anticoagulation and Short- or Long-Term Clinical Outcomes in Ischemic Stroke or Transient Ischemic Attack Patients with Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2019, 8, e010593. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Perez, I.; Yu, G.; Kalra, L. Should stroke subtype influence anticoagulation decisions to prevent recurrence in stroke patients with atrial fibrillation? Stroke 2001, 32, 2828–2832. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Horne, B.D.; Stevens, S.M.; Grove, A.S.; Barton, S.; Nicholas, Z.P.; Kahn, S.F.; May, H.T.; Samuelson, K.M.; Muhlestein, J.B.; et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007, 116, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Wańkowicz, P.; Staszewski, J.; Dębiec, A.; Nowakowska-Kotas, M.; Szylińska, A.; Rotter, I. Ischemic Stroke Risk Factors in Patients with Atrial Fibrillation Treated with New Oral Anticoagulants. J. Clin. Med. 2021, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.J.F.; Viana, S.M.N.; Santos, A.S. Mechanical thrombectomy for acute ischemic stroke: Systematic review and meta-analysis. Einstein 2022, 20, eRW6642. [Google Scholar] [CrossRef]
- Kimura, K.; Iguchi, Y.; Yamashita, S.; Shibazaki, K.; Kobayashi, K.; Inoue, T. Atrial fibrillation as an independent predictor for no early recanalization after IV-t-PA in acute ischemic stroke. J. Neurol. Sci. 2008, 267, 57–61. [Google Scholar] [CrossRef]
- Brinjikji, W.; Madalina Mereuta, O.; Dai, D.; Kallmes, D.F.; Savastano, L.; Liu, Y.; Nimjee, S.M.; Nogueira, R.G.; Abbasi, M.; Kadirvel, R. Mechanisms of fibrinolysis resistance and potential targets for thrombolysis in acute ischaemic stroke: Lessons from retrieved stroke emboli. Stroke Vasc. Neurol. 2021, 6, 658–667. [Google Scholar] [CrossRef]
- Huu An, N.; Dang Luu, V.; Duy Ton, M.; Anh Tuan, T.; Quang Anh, N.; Hoang Kien, L.; Tat Thien, N.; Viet Phuong, D.; Minh Duc, N. Thrombectomy Alone versus Bridging Therapy in Acute Ischemic Stroke: Preliminary Results of an Experimental Trial. Clin. Ter. 2022, 173, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sanak, D.; Herzig, R.; Kral, M.; Bartkova, A.; Zapletalova, J.; Hutyra, M.; Skoloudik, D.; Vlachova, I.; Veverka, T.; Horak, D.; et al. Is atrial fibrillation associated with poor outcome after thrombolysis? J. Neurol. 2010, 257, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Zhang, Y.; Wijdicks, E.F.; Rabinstein, A.A. Relationship between chronic atrial fibrillation and worse outcomes in stroke patients after intravenous thrombolysis. Arch. Neurol. 2011, 68, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Mehrpour, M.; Afrakhte, M.; Shojaei, S.F.; Sohrabi, A.; Ashayeri, R.; Esmaeili, S.; Bahadori, M. Factors predicting the outcome of intravenous thrombolysis in stroke patients before rt-PA administration. Casp. J. Intern. Med. 2019, 10, 424–430. [Google Scholar] [CrossRef]
- Tong, X.; Li, S.; Liu, W.; Ren, Z.; Liu, R.; Jia, B.; Zhang, X.; Huo, X.; Luo, G.; Ma, G.; et al. Endovascular treatment for acute ischemic stroke in patients with versus without atrial fibrillation: A matched-control study. BMC Neurol. 2021, 21, 377. [Google Scholar] [CrossRef] [PubMed]
- Churojana, A.; Mongkolratnan, A.; Sangpetngam, B.; Aurboonyawat, T.; Chankaew, E.; Withayasuk, P.; Songsaeng, D.; Cognard, C. A Comparison of Mechanical Thrombectomy for Large Vessel Occlusion in Acute Ischemic Stroke between Patients with and without Atrial Fibrillation. Siriraj Med. J. 2018, 70, 278–283. [Google Scholar]
- Fu, J.; Cappelen-Smith, C.; Edwards, L.; Cheung, A.; Mannin, N.; Wenderoth, J.; Parsons, M.; Cordato, D. Comparison of functional outcomes after endovascular thrombectomy in patients with and without atrial fibrillation. Vessel Plus 2021, 5, 33. [Google Scholar] [CrossRef]
- Akbik, F.; Alawieh, A.; Dimisko, L.; Howard, B.M.; Cawley, C.M.; Tong, F.C.; Nahab, F.; Samuels, O.B.; Maier, I.; Feng, W.; et al. Bridging thrombolysis in atrial fibrillation stroke is associated with increased hemorrhagic complications without improved outcomes. J. Neurointerv. Surg. 2022, 14, 979–984. [Google Scholar] [CrossRef]
- Zi, W.; Qiu, Z.; Li, F.; Sang, H.; Wu, D.; Luo, W.; Liu, S.; Yuan, J.; Song, J.; Shi, Z.; et al. Effect of Endovascular Treatment Alone vs Intravenous Alteplase Plus Endovascular Treatment on Functional Independence in Patients with Acute Ischemic Stroke: The DEVT Randomized Clinical Trial. JAMA 2021, 325, 234–243. [Google Scholar] [CrossRef]
- Fischer, U.; Kaesmacher, J.; Strbian, D.; Eker, O.; Cognard, C.; Plattner, P.S.; Butikofer, L.; Mordasini, P.; Deppeler, S.; Pereira, V.M.; et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: An open-label, blinded-outcome, randomised non-inferiority trial. Lancet 2022, 400, 104–115. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Q.F.; Sheng, C.S.; Zhang, W.; Shao, S.; Wang, D.; Cheng, Y.B.; Wang, Y.; Guo, Q.H.; Zhang, D.Y.; et al. Detection rate and treatment gap for atrial fibrillation identified through screening in community health centers in China (AF-CATCH): A prospective multicenter study. PLoS Med. 2020, 17, e1003146. [Google Scholar] [CrossRef] [PubMed]
- Orchard, J.; Li, J.; Freedman, B.; Webster, R.; Salkeld, G.; Hespe, C.; Gallagher, R.; Patel, A.; Kamel, B.; Neubeck, L.; et al. Atrial Fibrillation Screen, Management, and Guideline-Recommended Therapy in the Rural Primary Care Setting: A Cross-Sectional Study and Cost-Effectiveness Analysis of eHealth Tools to Support All Stages of Screening. J. Am. Heart Assoc. 2020, 9, e017080. [Google Scholar] [CrossRef]
- Alobaida, M.; Harrison, S.L.; Lane, D.A.; Underhill, P.; Hill, A.; Lip, G.Y.H. Outcomes in patients with ischaemic stroke undergoing endovascular thrombectomy: Impact of atrial fibrillation. J. Stroke Cerebrovasc. Dis. 2023, 32, 106917. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ji, C. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: A meta-analysis. BMC Neurol. 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Vinding, N.E.; Kristensen, S.L.; Rorth, R.; Butt, J.H.; Ostergaard, L.; Olesen, J.B.; Torp-Pedersen, C.; Gislason, G.H.; Kober, L.; Kruuse, C.; et al. Ischemic Stroke Severity and Mortality in Patients with and without Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e022638. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.R.; Austin, T.M. Using MeSH (medical subject headings) to enhance PubMed search strategies for evidence-based practice in physical therapy. Phys. Ther. 2012, 92, 124–132. [Google Scholar] [CrossRef]
- Nunn, A.; Bath, P.M.; Gray, L.J. Analysis of the Modified Rankin Scale in Randomised Controlled Trials of Acute Ischaemic Stroke: A Systematic Review. Stroke Res. Treat. 2016, 2016, 9482876. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Romano, L.A.; Smith, E.E.; Campo-Bustillo, I.; Khan, Y.; Tai, S.; Riley, N.; Sacco, R.L.; Khatri, P.; Alger, H.M.; et al. Functional status at 30 and 90 days after mild ischaemic stroke. Stroke Vasc. Neurol. 2022, 7, 375–380. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Shen, Y.W.; Zhang, X.M.; Lv, M.; Chen, L.; Qin, T.J.; Wang, F.; Yang, J.; Liu, P.J.; Yang, J. Utility of gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage in premenopausal women with breast cancer: A systematic review and meta-analysis. OncoTargets Ther. 2015, 8, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.T.J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; (updated February 2022); Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Ajabnoor, A.M.; Zghebi, S.S.; Parisi, R.; Ashcroft, D.M.; Rutter, M.K.; Doran, T.; Carr, M.J.; Mamas, M.A.; Kontopantelis, E. Incidence of nonvalvular atrial fibrillation and oral anticoagulant prescribing in England, 2009 to 2019: A cohort study. PLoS Med. 2022, 19, e1004003. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaled, M.; Matthis, C.; Eggers, J. Predictors of in-hospital mortality and the risk of symptomatic intracerebral hemorrhage after thrombolytic therapy with recombinant tissue plasminogen activator in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Awadh, M.; MacDougall, N.; Santosh, C.; Teasdale, E.; Baird, T.; Muir, K.W. Early recurrent ischemic stroke complicating intravenous thrombolysis for stroke: Incidence and association with atrial fibrillation. Stroke 2010, 41, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mondal, G.P.; Bhattacharya, R.; Ghosh, K.C.; Das, S.; Pattem, H.K.; Paul, S.A.; Patra, C. Predictors of Postthrombolysis Outcome and Symptomatic Postthrombolysis Hemorrhage following Intravenous Thrombolysis with Alteplase for Acute Ischemic Stroke. J. Neurosci. Rural Pract. 2020, 11, 315–324. [Google Scholar] [CrossRef]
- Dharmasaroja, P.A.; Muengtaweepongsa, S.; Pattaraarchachai, J.; Dharmasaroja, P. Intracerebral hemorrhage following intravenous thrombolysis in Thai patients with acute ischemic stroke. J. Clin. Neurosci. 2012, 19, 799–803. [Google Scholar] [CrossRef]
- Frank, B.; Fulton, R.; Weimar, C.; Shuaib, A.; Lees, K.R.; Collaborators, V. Impact of atrial fibrillation on outcome in thrombolyzed patients with stroke: Evidence from the Virtual International Stroke Trials Archive (VISTA). Stroke 2012, 43, 1872–1877. [Google Scholar] [CrossRef]
- Kimura, K.; Iguchi, Y.; Shibazaki, K.; Iwanaga, T.; Yamashita, S.; Aoki, J. IV t-PA therapy in acute stroke patients with atrial fibrillation. J. Neurol. Sci. 2009, 276, 6–8. [Google Scholar] [CrossRef]
- Kurmann, R.; Engelter, S.T.; Michel, P.; Luft, A.R.; Wegener, S.; Branscheidt, M.; Eskioglou, E.; Sirimarco, G.; Lyrer, P.A.; Gensicke, H.; et al. Impact of Smoking on Clinical Outcome and Recanalization after Intravenous Thrombolysis for Stroke: Multicenter Cohort Study. Stroke 2018, 49, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.F.; Chen, C.F.; Hu, H.H.; Ho, B.L.; Chen, C.H.; Chan, L.; Lin, H.J.; Sun, Y.; Lin, Y.Y.; Chen, P.L.; et al. Comparison of Different Dosages of Alteplase in Atrial Fibrillation-Related Acute Ischemic Stroke after Intravenous Thrombolysis: A Nationwide, Multicenter, Prospective Cohort Study in Taiwan. J. Am. Heart Assoc. 2022, 11, e023032. [Google Scholar] [CrossRef] [PubMed]
- Marko, M.; Posekany, A.; Szabo, S.; Scharer, S.; Kiechl, S.; Knoflach, M.; Serles, W.; Ferrari, J.; Lang, W.; Sommer, P.; et al. Trends of r-tPA (Recombinant Tissue-Type Plasminogen Activator) Treatment and Treatment-Influencing Factors in Acute Ischemic Stroke. Stroke 2020, 51, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Padjen, V.; Bodenant, M.; Jovanovic, D.R.; Ponchelle-Dequatre, N.; Novakovic, N.; Cordonnier, C.; Beslac-Bumbasirevic, L.; Leys, D. Outcome of patients with atrial fibrillation after intravenous thrombolysis for cerebral ischaemia. J. Neurol. 2013, 260, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.; Wardlaw, J.M.; Lindley, R.I.; Dennis, M.; Cohen, G.; Murray, G.; Innes, K.; Venables, G.; Czlonkowska, A.; Kobayashi, A.; et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 2012, 379, 2352–2363. [Google Scholar] [CrossRef]
- Shon, S.H.; Heo, S.H.; Kim, B.J.; Choi, H.Y.; Kwon, Y.; Yi, S.H.; Lee, J.S.; Kim, Y.S.; Kim, H.Y.; Koh, S.H.; et al. Predictors of Hemorrhage Volume after Intravenous Thrombolysis. J. Stroke Cerebrovasc. Dis. 2016, 25, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.F.; Chen, Y.W.; Tseng, M.C.; Ong, C.T.; Lin, H.J. Atrial fibrillation predicts good functional outcome following intravenous tissue plasminogen activator in patients with severe stroke. Clin. Neurol. Neurosurg. 2013, 115, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; George, M.G.; Yang, Q.; Gillespie, C. Predictors of in-hospital death and symptomatic intracranial hemorrhage in patients with acute ischemic stroke treated with thrombolytic therapy: Paul Coverdell Acute Stroke Registry 2008–2012. Int. J. Stroke 2014, 9, 728–734. [Google Scholar] [CrossRef]
- Vorasoot, N.; Sothornwit, J.; Chomjit, A.; Kasemsap, N.; Tiamkao, S.; Sawanyawisuth, K.; Kongbunkiat, K. Factors associated with poor clinical outcome after intravenous recombinant tissue plasminogen activator (rt-PA) for acute ischemic stroke in Northeastern Thailand. J. Med. Assoc. Thail. 2020, 103, 81–84. [Google Scholar]
- Wu, H.; Liu, Y.; Miao, G.; Ge, J.; You, S.; Zhang, X.; Liu, H.; Zhou, Y.; Lu, T.; Cao, Y.; et al. Effect of the interaction between atrial fibrillation and rt-PA dose on the prognosis of acute ischaemic stroke with intravenous thrombolysis. Postgrad. Med. J. 2022, 99, 588–594. [Google Scholar] [CrossRef]
- Zhang, J.B.; Ding, Z.Y.; Yang, Y.; Sun, W.; Hai, F.; Sui, X.N.; Li, X.Y.; Wang, H.Z.; Wang, X.T.; Zheng, J.L. Thrombolysis with alteplase for acute ischemic stroke patients with atrial fibrillation. Neurol. Res. 2010, 32, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Shan, W.; Liu, L.; Fu, X.; Liu, P.; Hu, Y. Predictors of functional outcome and hemorrhagic complications in acute ischemic stroke patients treated with intravenous thrombolysis—A retrospective analysis. Int. J. Clin. Pharmacol. Ther. 2017, 55, 893–900. [Google Scholar] [CrossRef]
- Akbik, F.; Alawieh, A.; Cawley, C.M.; Howard, B.M.; Tong, F.C.; Nahab, F.; Saad, H.; Dimisko, L.; Mustroph, C.; Samuels, O.B.; et al. Differential effect of mechanical thrombectomy and intravenous thrombolysis in atrial fibrillation associated stroke. J. Neurointerv. Surg. 2021, 13, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Casetta, I.; Fainardi, E.; Pracucci, G.; Saia, V.; Sallustio, F.; da Ros, V.; Nappini, S.; Nencini, P.; Bigliardi, G.; Vinci, S.; et al. Sex differences in outcome after thrombectomy for acute ischemic stroke. A propensity score-matched study. Eur. Stroke J. 2022, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zha, M.; Gao, J.; Du, J.; Liu, R.; Liu, X. Increased intracranial hemorrhage of mechanical thrombectomy in acute ischemic stroke patients with atrial fibrillation. J. Thromb. Thrombolysis 2021, 51, 536–544. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Zak, A.; Binek, L.; Student, S.; Tomalski, W.; Krzan, A.; Puz, P.; Uchwat, U. The effect of atrial fibrillation on the safety and efficacy of mechanical thrombectomy in patients with stroke. Pol. Arch. Intern. Med. 2022, 132, 16148. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.J.; Hong, J.M.; Choi, J.W.; Hong, J.H.; Chang, H.W.; Kim, C.H.; Kim, Y.W.; Kang, D.H.; Kim, Y.S.; et al. Temporal Changes in Care Processes and Outcomes for Endovascular Treatment of Acute Ischemic Stroke: Retrospective Registry Data from Three Korean Centers. Neurointervention 2018, 13, 2–12. [Google Scholar] [CrossRef]
- Leker, R.R.; Farraj, A.; Sacagiu, T.; Honig, A.; ElHasan, H.A.; Gomori, J.M.; Cohen, J.E. Atrial Fibrillation Treatment Adequacy and Outcome after Endovascular Thrombectomy. J. Stroke Cerebrovasc. Dis. 2020, 29, 104948. [Google Scholar] [CrossRef]
- Lin, C.J.; Luo, C.B.; Chien, C.; Chang, F.C.; Lin, C.J.; Lee, I.H.; Hsu, L.C.; Chung, C.P.; Liu, H.Y.; Chi, N.F.; et al. Better endovascular mechanical thrombectomy outcome in atrial fibrillation patients with acute ischemic stroke: A single-center experience. J. Chin. Med. Assoc. 2020, 83, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.H.; Leow, A.S.; Jing, M.; Sia, C.H.; Chan, B.P.; Seet, R.C.; Teoh, H.L.; Meyer, L.; Fiehler, J.; Papanagiotou, P.; et al. Impact of atrial fibrillation on the treatment effect of bridging thrombolysis in ischemic stroke patients undergoing endovascular thrombectomy: A multicenter international cohort study. J. Neurointerv. Surg. 2023, 6. [Google Scholar] [CrossRef] [PubMed]
- Mujanovic, A.; Kurmann, C.C.; Dobrocky, T.; Olive-Gadea, M.; Maegerlein, C.; Pierot, L.; Mendes Pereira, V.; Costalat, V.; Psychogios, M.; Michel, P.; et al. Bridging intravenous thrombolysis in patients with atrial fibrillation. Front. Neurol. 2022, 13, 945338. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Gupta, R.; Jovin, T.G.; Levy, E.I.; Liebeskind, D.S.; Zaidat, O.O.; Rai, A.; Hirsch, J.A.; Hsu, D.P.; Rymer, M.M.; et al. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: A multicenter retrospective analysis of 1122 patients. J. Neurointerv. Surg. 2015, 7, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Sakai, N.; Uchida, K.; Yamagami, H.; Ezura, M.; Okada, Y.; Kitagawa, K.; Kimura, K.; Sasaki, M.; Tanahashi, N.; et al. Endovascular Therapy in Ischemic Stroke with Acute Large-Vessel Occlusion: Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism Japan Registry 2. J. Am. Heart Assoc. 2018, 7, e008796. [Google Scholar] [CrossRef] [PubMed]
- Zdraljevic, M.; Pekmezovic, T.; Stanarcevic, P.; Vukasinovic, I.; Berisavac, I.; Ercegovac, M.; Vitosevic, F.; Nestorovic, D.; Cvetic, V.; Padjen, V.; et al. Atrial fibrillation is associated with poor long-term outcome after mechanical thrombectomy for anterior large vessel occlusion stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106755. [Google Scholar] [CrossRef] [PubMed]
- Smaal, J.A.; de Ridder, I.R.; Heshmatollah, A.; van Zwam, W.H.; Dippel, D.; Majoie, C.B.; Brown, S.; Goyal, M.; Campbell, B.; Muir, K.W.; et al. Effect of atrial fibrillation on endovascular thrombectomy for acute ischemic stroke. A meta-analysis of individual patient data from six randomised trials: Results from the HERMES collaboration. Eur. Stroke J. 2020, 5, 245–251. [Google Scholar] [CrossRef]
- Bavarsad Shahripour, R.; Azarpazhooh, D.; Shifflett, B.; Osouli, S.; Meyer, B.C.; Meyer, D.M. The Impact of Atrial Fibrillation on the Outcome of Ischemic Stroke Treated with Thrombolysis or Endovascular Therapy. J. Neurol. Res. 2022, 12, 121–127. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Chalos, V.; LeCouffe, N.E.; Uyttenboogaart, M.; Lingsma, H.F.; Mulder, M.; Venema, E.; Treurniet, K.M.; Eshghi, O.; van der Worp, H.B.; van der Lugt, A.; et al. Endovascular Treatment with or without Prior Intravenous Alteplase for Acute Ischemic Stroke. J. Am. Heart Assoc. 2019, 8, e011592. [Google Scholar] [CrossRef]
- LeCouffe, N.E.; Kappelhof, M.; Treurniet, K.M.; Rinkel, L.A.; Bruggeman, A.E.; Berkhemer, O.A.; Wolff, L.; van Voorst, H.; Tolhuisen, M.L.; Dippel, D.W.J.; et al. A Randomized Trial of Intravenous Alteplase before Endovascular Treatment for Stroke. N. Engl. J. Med. 2021, 385, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Yan, B.; Churilov, L.; Dowling, R.J.; Bush, S.J.; Bivard, A.; Huo, X.C.; Wang, G.; Zhang, S.Y.; Ton, M.D.; et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4.5 h of stroke onset: An open-label, blinded-endpoint, randomised non-inferiority trial. Lancet 2022, 400, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, Y.; Zhang, L.; Zhang, Y.; Treurniet, K.M.; Chen, W.; Peng, Y.; Han, H.; Wang, J.; Wang, S.; et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N. Engl. J. Med. 2020, 382, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, S.; Palaszewski, B.; Friberg, L.; Bergfeldt, L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: A population-based study. Stroke 2013, 44, 3103–3108. [Google Scholar] [CrossRef]
- Otite, F.O.; Khandelwal, P.; Chaturvedi, S.; Romano, J.G.; Sacco, R.L.; Malik, A.M. Increasing atrial fibrillation prevalence in acute ischemic stroke and TIA. Neurology 2016, 87, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Alqahtani, F.; Aljohani, S.; Alvi, M.; Holmes, D.R. Burden of Atrial Fibrillation-Associated Ischemic Stroke in the United States. JACC Clin. Electrophysiol. 2018, 4, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Guzman, J.; Freixa-Pamias, R.; Garcia-Alegria, J.; Perez Cabeza, A.I.; Roldan-Rabadan, I.; Antolin-Fontes, B.; Rebollo, P.; Llorac, A.; Genis-Girones, M.; Escobar-Cervantes, C. Epidemiology of atrial fibrillation-related ischemic stroke and its association with DOAC uptake in Spain: First national population-based study 2005 to 2018. Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ebeling, M.; Ziegler, L.; Wennberg, A.; Modig, K. Time trends in atrial fibrillation-related stroke during 2001–2020 in Sweden: A nationwide, observational study. Lancet Reg. Health Eur. 2023, 28, 100596. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 574–582. [Google Scholar] [CrossRef]
- Joseph, P.G.; Healey, J.S.; Raina, P.; Connolly, S.J.; Ibrahim, Q.; Gupta, R.; Avezum, A.; Dans, A.L.; Lopez-Jaramillo, P.; Yeates, K.; et al. Global variations in the prevalence, treatment, and impact of atrial fibrillation in a multi-national cohort of 153 152 middle-aged individuals. Cardiovasc. Res. 2021, 117, 1523–1531. [Google Scholar] [CrossRef]
- Kimura, K.; Minematsu, K.; Yamaguchi, T.; Japan Multicenter Stroke Investigators, C. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.Y.; Contreras, R.; Sobnosky, S.; Shah, A.I.; Ichiuji, A.M.; Jorgensen, M.B.; Brar, S.S.; Chen, W. Racial/ethnic differences in the prevalence of atrial fibrillation among older adults--A cross-sectional study. J. Natl. Med. Assoc. 2010, 102, 906–913. [Google Scholar] [CrossRef]
- Dewland, T.A.; Olgin, J.E.; Vittinghoff, E.; Marcus, G.M. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 2013, 128, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.P.; Gbadebo, T.D.; Connolly, S.J.; Van Gelder, I.C.; Capucci, A.; Gold, M.R.; Israel, C.W.; Morillo, C.A.; Siu, C.W.; Abe, H.; et al. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J. Cardiovasc. Electrophysiol. 2013, 24, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Koga, M. More Benefits From Endovascular Thrombectomy in Patients with Atrial Fibrillation? Circ. J. 2018, 82, 2483–2484. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamkhani, W.; Ayetey, H.; Lip, G.Y.H. Atrial fibrillation in the Middle East: Unmapped, underdiagnosed, undertreated. Expert Rev. Cardiovasc. Ther. 2018, 16, 341–348. [Google Scholar] [CrossRef]
- Navar, A.M.; Kolkailah, A.A.; Overton, R.; Shah, N.P.; Rousseau, J.F.; Flaker, G.C.; Pignone, M.P.; Peterson, E.D. Trends in Oral Anticoagulant Use among 436 864 Patients with Atrial Fibrillation in Community Practice, 2011 to 2020. J. Am. Heart Assoc. 2022, 11, e026723. [Google Scholar] [CrossRef]
- Nelson, W.W.; Wang, L.; Baser, O.; Damaraju, C.V.; Schein, J.R. Out-of-range INR values and outcomes among new warfarin patients with non-valvular atrial fibrillation. Int. J. Clin. Pharm. 2015, 37, 53–59. [Google Scholar] [CrossRef]
- Cho, H.J.; Kang, Y.J.; Sung, S.M.; Ahn, S.H.; Jung, Y.H.; Lee, K.Y.; Seo, J.H.; Han, S.W.; Park, J.H.; Choi, H.Y.; et al. Effects of dabigatran and rivaroxaban on stroke severity according to the results of routine coagulation tests. PLoS ONE 2020, 15, e0240483. [Google Scholar] [CrossRef]
- Seachrist, E.J.; Petrone, A.; Nevin, C.; Ranasinghe, T.; Jacob, S.; Ferari, C.; Adcock, A. Incidence of Atrial Fibrillation in Large Vessel Occlusion and Large Embolic Stroke of Undetermined Source. Cureus 2023, 15, e33700. [Google Scholar] [CrossRef]
- Yue, R.; Li, D.; Yu, J.; Li, S.; Ma, Y.; Huang, S.; Zeng, Z.; Zeng, R.; Sun, X. Atrial Fibrillation is Associated with Poor Outcomes in Thrombolyzed Patients with Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e3054. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Gladstone, D.; Raptis, R.; Zhou, L.; Hart, R.G.; Investigators of the Registry of the Canadian Stroke Network; the Stroke Outcomes Research Canada (SORCan) Working Group. Atrial fibrillation in ischemic stroke: Predicting response to thrombolysis and clinical outcomes. Stroke 2013, 44, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Findler, M.; Molad, J.; Bornstein, N.M.; Auriel, E. Worse Outcome in Patients with Acute Stroke and Atrial Fibrillation Following Thrombolysis. Isr. Med. Assoc. J. 2017, 19, 293–295. [Google Scholar] [PubMed]
- Kim, S.K.; Yoon, W.; Kim, T.S.; Kim, H.S.; Heo, T.W.; Park, M.S. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. AJNR Am. J. Neuroradiol. 2015, 36, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Killingsworth, M.C.; Bhaskar, S.M.M. Is Composition of Brain Clot Retrieved by Mechanical Thrombectomy Associated with Stroke Aetiology and Clinical Outcomes in Acute Ischemic Stroke? A Systematic Review and Meta-Analysis. Neurol. Int. 2022, 14, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Mohammaden, M.H.; Stapleton, C.J.; Brunozzi, D.; Hussein, A.E.; Khedr, E.M.; Atwal, G.; Alaraj, A. Predictors of Poor Outcome Despite Successful Mechanical Thrombectomy of Anterior Circulation Large Vessel Occlusions within 6 h of Symptom Onset. Front. Neurol. 2020, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Liu, S.; Cui, C.; Cao, Y.; Jia, Z.; Liu, H.; Wang, C.; Hang, Y.; Ni, H.; Chen, M.; et al. Endovascular thrombectomy for acute ischemic stroke in elderly patients with atrial fibrillation. BMC Neurol. 2022, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Wang, Z.; Shi, H.; Li, Y.; Zhuang, Y.; Xu, J.; Xu, M. Stroke severity modified the effect of chronic atrial fibrillation on the outcome of thrombolytic therapy. Medicine 2022, 101, e29322. [Google Scholar] [CrossRef]
- Lei, Z.; Li, S.; Hu, S.; Ren, L. Effects of Baseline Systolic Blood Pressure on Outcome in Ischemic Stroke Patients with Intravenous Thrombolysis Therapy: A Systematic Review and Meta-Analysis. Neurologist 2020, 25, 62–69. [Google Scholar] [CrossRef]
- Xiaoxi, Z.; Xuan, Z.; Lei, Z.; Zifu, L.; Pengfei, X.; Hongjian, S.; Yongxin, Z.; Weilong, H.; Yihan, Z.; Dongwei, D.; et al. Baseline blood pressure does not modify the effect of intravenous thrombolysis in successfully revascularized patients. Front. Neurol. 2022, 13, 984599. [Google Scholar] [CrossRef]
- Aune, D.; Mahamat-Saleh, Y.; Kobeissi, E.; Feng, T.; Heath, A.K.; Janszky, I. Blood pressure, hypertension and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2023, 38, 145–178. [Google Scholar] [CrossRef] [PubMed]
- Bivard, A.; Zhao, H.; Churilov, L.; Campbell, B.C.V.; Coote, S.; Yassi, N.; Yan, B.; Valente, M.; Sharobeam, A.; Balabanski, A.H.; et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): A phase 2, randomised, open-label trial. Lancet Neurol. 2022, 21, 520–527. [Google Scholar] [CrossRef]
- Kvistad, C.E.; Naess, H.; Helleberg, B.H.; Idicula, T.; Hagberg, G.; Nordby, L.M.; Jenssen, K.N.; Tobro, H.; Rorholt, D.M.; Kaur, K.; et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): A phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022, 21, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Pan, Y.; Li, H.; Parsons, M.W.; Campbell, B.C.V.; Schwamm, L.H.; Fisher, M.; Che, F.; Dai, H.; et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): A phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet 2023, 401, 645–654. [Google Scholar] [CrossRef]
- Kobeissi, H.; Ghozy, S.; Seymour, T.; Gupta, R.; Bilgin, C.; Kadirvel, R.; Rabinstein, A.A.; Kallmes, D.F. Outcomes of Patients with Atrial Fibrillation Following Thrombectomy for Stroke: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2249993. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, B.M.; Heo, J.H.; Kim, D.J.; Nam, H.S.; Kim, Y.D. Outcomes of Endovascular Treatment for Acute Intracranial Atherosclerosis-Related Large Vessel Occlusion. Stroke 2018, 49, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; LeCouffe, N.E.; Zinkstok, S.M.; Compagne, K.C.J.; Eker, R.; Treurniet, K.M.; Tolhuisen, M.L.; van der Worp, H.B.; Jansen, I.G.H.; van Oostenbrugge, R.J.; et al. Collateral Circulation and Outcome in Atherosclerotic versus Cardioembolic Cerebral Large Vessel Occlusion. Stroke 2019, 50, 3360–3368. [Google Scholar] [CrossRef]
- Suzuki, K.; Matsumaru, Y.; Takeuchi, M.; Morimoto, M.; Kanazawa, R.; Takayama, Y.; Kamiya, Y.; Shigeta, K.; Okubo, S.; Hayakawa, M.; et al. Effect of Mechanical Thrombectomy without vs With Intravenous Thrombolysis on Functional Outcome Among Patients with Acute Ischemic Stroke: The SKIP Randomized Clinical Trial. JAMA 2021, 325, 244–253. [Google Scholar] [CrossRef]
- Yaghi, S.; Mistry, E.; de Havenon, A.; Leon Guerrero, C.R.; Nouh, A.; Liberman, A.L.; Giles, J.; Liu, A.; Nagy, M.; Kaushal, A.; et al. Effect of Alteplase Use on Outcomes in Patients with Atrial Fibrillation: Analysis of the Initiation of Anticoagulation after Cardioembolic Stroke Study. J. Am. Heart Assoc. 2021, 10, e020945. [Google Scholar] [CrossRef]
- Lin, L.; Blair, C.; Fu, J.; Cordato, D.; Cappelen-Smith, C.; Cheung, A.; Manning, N.W.; Wenderoth, J.; Chen, C.; Bivard, A.; et al. Prior anticoagulation and bridging thrombolysis improve outcomes in patients with atrial fibrillation undergoing endovascular thrombectomy for anterior circulation stroke. J. Neurointerv. Surg. 2023. [Google Scholar] [CrossRef]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Chapagain, B.; Maharjan, R.; Farah, H.W.; Nazeer, A.; Lootens, R.J.; Rosenfeld, A. Prolonged Cardiac Monitoring to Detect Atrial Fibrillation after Cryptogenic Stroke or Transient Ischemic Attack: A Meta-Analysis of Randomized Controlled Trials. Ann. Noninvasive Electrocardiol. 2016, 21, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Buck, B.H.; Hill, M.D.; Quinn, F.R.; Butcher, K.S.; Menon, B.K.; Gulamhusein, S.; Siddiqui, M.; Coutts, S.B.; Jeerakathil, T.; Smith, E.E.; et al. Effect of Implantable vs Prolonged External Electrocardiographic Monitoring on Atrial Fibrillation Detection in Patients with Ischemic Stroke: The PER DIEM Randomized Clinical Trial. JAMA 2021, 325, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Chang, S.L.; Yeh, Y.H.; Chung, F.P.; Hu, Y.F.; Chou, C.C.; Hung, K.C.; Chang, P.C.; Liao, J.N.; Chan, Y.H.; et al. Enhanced detection of cardiac arrhythmias utilizing 14-day continuous ECG patch monitoring. Int. J. Cardiol. 2021, 332, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tan, S.Y.; Wang, J.K.; Li, J.; Tu, T.M.; Tan, V.H.; Yeo, C. A meta-analysis of extended ECG monitoring in detection of atrial fibrillation in patients with cryptogenic stroke. Open Heart 2022, 9, e002081. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.D.; Guimaraes, R.B.; Stephan, L.S.; Medeiros, A.K.; Foltz, K.; Santanna, R.T.; Pires, L.M.; Kruse, M.L.; Lima, G.G.; Leiria, T.L. Clinical Differences between Subtypes of Atrial Fibrillation and Flutter: Cross-Sectional Registry of 407 Patients. Arq. Bras. Cardiol. 2015, 105, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Atar, D.; Berge, E.; Le Heuzey, J.Y.; Virdone, S.; Camm, A.J.; Steffel, J.; Gibbs, H.; Goldhaber, S.Z.; Goto, S.; Kayani, G.; et al. The association between patterns of atrial fibrillation, anticoagulation, and cardiovascular events. Europace 2020, 22, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Holthuis, E.; Smits, E.; Spentzouris, G.; Beier, D.; Enders, D.; Gini, R.; Bartolini, C.; Mazzaglia, G.; Penning-van Beest, F.; Herings, R. Increased Risk of Stroke Due to Non-adherence and Non-persistence with Direct Oral Anticoagulants (DOACs): Real-World Analyses Using a Nested Case-Control Study from The Netherlands, Italy and Germany. Drugs Real World Outcomes 2022, 9, 597–607. [Google Scholar] [CrossRef]
- Chen, J.H.; Hong, C.T.; Chung, C.C.; Kuan, Y.C.; Chan, L. Safety and efficacy of endovascular thrombectomy in acute ischemic stroke treated with anticoagulants: A systematic review and meta-analysis. Thromb. J. 2022, 20, 35. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, B. Impact of risk factors for recurrence after the first ischemic stroke in adults: A systematic review and meta-analysis. J. Clin. Neurosci. 2019, 60, 24–30. [Google Scholar] [CrossRef]

| Study ID | Author | Year | Country | Centres | Study Type | Reperfusion Type | AF (n) | Overall (n) | Age ± SD a | Male (%) a | Baseline NIHSS Score ± SD a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF | Non-AF | Overall | AF | Non-AF | Overall | AF | Non-AF | Overall | |||||||||
| 1 | Akbik et al. [64] | 2021 | International | 15 | Retrospective | EVT | 1517 | 4169 | 76 ± 11 | 65 ± 15 | - | 42.2 | 50.1 | - | 16 ± 6 | 15 ± 7 | - |
| 2 | Akbik et al. [28] | 2022 | International | 22 | Retrospective | BT | 1036 | 3140 | 76 ± 11 | 65 ± 15 | - | 46.0 | 52.0 | - | 16 ± 6 | 15 ± 7 | - |
| 3 | Al-Khaled et al. [46] | 2014 | Germany | 15 | Prospective | IVT | 387 | 1007 | - | - | 71.5 ± 12.2 | - | - | 49.6 | - | - | 11.6 ± 5.6 |
| 4 | Alobaida et al. [33] | 2023 | International | 27 | Retrospective | EVT | 1718 | 3106 | 73.6 ± 12.6 | 61.1 ± 14.8 | - | 47.1 | 57.1 | - | - | - | - |
| 4a | Alobaida et al. [33] | 2023 | International | 27 | Retrospective | BT | 549 | 889 | - | - | - | - | - | - | - | - | - |
| 5 | Awadh et al. [47] | 2010 | Scotland | 1 | Retrospective | IVT | 74 | 228 | 76 ± 10 | 66.4 ± 13.4 | - | 40.5 | 59.1 | - | 13.7 ± 8.3 | 13.8 ± 9.7 | - |
| 6 | Bavarsad Shahripour et al. [79] | 2022 | USA | 5 | Prospective | BT | 17 | 45 | 76.4 ± 11.3 | 65.4 ± 16.5 | - | 58.8 | 71.4 | - | - | - | - |
| 7 | Berkhemer et al. [65] | 2015 | The Netherlands | 16 | Prospective RCT | EVT | 66 | 233 | - | - | 65.4 ± 16.0 | - | - | 57.9 | 17.3 ± 5.2 | - | 17.3 ± 5.2 |
| 8 | Campbell et al. [80] | 2015 | Australia and New Zealand | 14 | Prospective RCT | BT | 12 | 35 | - | - | 68.6 ± 12.3 | - | - | 48.6 | 16.7 ± 5.4 | - | 16.7 ± 5.4 |
| 9 | Casetta et al. [66] | 2022 | Italy | - | Prospective | EVT | 1193 | 3422 | - | - | 70.6 | - | - | 47.4 | - | - | - |
| 10 | Chalos et al. [81] | 2019 | The Netherlands | - | Prospective | BT | 186 | 1144 | 76.3 ± 12.0 | 68 ± 15.6 | - | 46.8 | 54.8 | - | 16.3 ± 6.7 | 15 ± 5.9 | - |
| 11 | Churojana et al. [26] | 2018 | Thailand | 1 | Retrospective | EVT | 50 | 134 | 69.2 ± 12.9 | 60.2 ± 16 | - | 54.0 | 60.7 | - | 17.4 ± 5.5 | 17.1 ± 6.3 | - |
| 11a | Churojana et al. [26] | 2018 | Thailand | 1 | Retrospective | BT | 10 | 29 | - | - | - | - | - | - | - | - | - |
| 12 | Das et al. [48] | 2020 | India | 1 | Prospective | IVT | 6 | 60 | - | - | 63.9 | - | - | 56.7 | - | - | - |
| 13 | Dharmasaroja et al. [49] | 2012 | Thailand | 1 | Prospective | IVT | 46 | 194 | - | - | 64 ± 13 | - | - | 59.8 | - | - | 17.3 ± 27.6 |
| 14 | Fischer et al. [30] | 2022 | Europe and Canada | 48 | Prospective RCT | BT | 22 | 198 | - | - | 72.7 ± 11.9 | - | - | 49.8 | - | - | 16.3 ± 6.0 |
| 15 | Frank et al. [50] | 2012 | International | - | Retrospective | IVT | 639 | 3027 | 74.2 ± 9.5 | 65.7 ± 12.5 | 47.3 | 58.2 | - | - | - | - | |
| 16 | Fu et al. [27] | 2021 | Australia | 1 | Prospective | EVT | 171 | 349 | 77 ± 9.7 | 61 ± 31.4 | 48.5 | 58.4 | - | 17.7 ± 8.2 | 16.3 ± 9.0 | - | |
| 16a | Fu et al. [27] | 2021 | Australia | 1 | Prospective | BT | 53 | 132 | - | - | - | - | - | - | - | - | - |
| 17 | Goyal et al. [67] | 2015 | International | 22 | Prospective RCT | EVT | 61 | 165 | - | - | 70.7 ± 15.7 | - | - | - | - | - | 16.3 ± 5.2 |
| 18 | Huang et al. [68] | 2021 | China | - | Prospective | EVT | 123 | 245 | 73.3 ± 9.0 | 63 ± 12.8 | - | 43.9 | 68.9 | - | 16.3 ± 5.3 | 14.7 ± 4.5 | - |
| 18a | Huang et al. [68] | 2021 | China | - | Prospective | BT | 33 | 83 | - | - | - | - | - | - | - | - | - |
| 19 | Kimura et al. [51] | 2009 | Japan | - | Prospective | IVT | 44 | 85 | 77.2 ± 9 | 69.4 ± 12.5 | - | 61.4 | 70.7 | - | 17.3 ± 6.5 | 12.3 ± 7.5 | - |
| 20 | Kurmann et al. [52] | 2018 | Switzerland | 4 | Prospective | IVT | 586 | 1775 | - | - | 69.8 | - | - | 59.2 | - | - | 11.3 |
| 21 | Lasek-Bal et al. [69] | 2022 | Poland | 1 | Retrospective | EVT | 108 | 417 | 74.9 ± 9.2 | 66.8 ± 14.5 | - | 45.4 | 54.7 | - | 14.0 ± 5.4 | 12.2 ± 6.5 | - |
| 22 | LeCouffe et al. [82] | 2021 | Netherlands, Belgium and France | 20 | Prospective RCT | BT | 63 | 266 | - | - | 69 ± 11.9 | - | - | 54.1 | - | - | 15.3 ± 7.5 |
| 23 | Lee et al. [70] | 2018 | Korea | 3 | Retrospective | EVT | 354 | 720 | - | - | 67.5 | - | - | 55.1 | - | - | 16.3 ± 6.7 |
| 24 | Leker et al. [71] | 2020 | Israel | - | Retrospective | EVT | 109 | 230 | - | - | 69.3 ± 14.7 | 39.4 | 56.2 | - | - | - | 17.1 ± 6.6 |
| 24a | Leker et al. [71] | 2020 | Israel | - | Retrospective | BT | 24 | 56 | - | - | - | - | - | - | - | - | - |
| 25 | Lin et al. [72] | 2020 | Taiwan | 1 | Retrospective | EVT | 43 | 80 | 72.6 ± 9.5 | 70.9 ± 17.3 | - | 46.5 | 57.5 | - | 17.2 ± 5.1 | 17.9 ± 6.2 | - |
| 25a | Lin et al. [72] | 2020 | Taiwan | 1 | Retrospective | BT | 24 | 39 | - | - | - | - | - | - | - | - | - |
| 26 | Lin et al. [53] | 2022 | Taiwan | 30 | Prospective | IVT | 980 | 2351 | 71.7 ± 11.9 | 66.4 ± 13 | - | 58.2 | 66.7 | - | - | - | - |
| 27a | Loo et al. [73] | 2023 | Singapore, Germany, Italy, UK, China, Taiwan | 8 | Retrospective | BT | 182 | 451 | 73.2 ± 10.3 | 65.6 ± 14.1 | - | 40.7 | 63.2 | - | 18.3 ± 8.1 | 15.9 ± 7.6 | - |
| 27 | Loo et al. [73] | 2023 | Singapore, Germany, Italy, UK, China, Taiwan | 8 | Retrospective | EVT | 314 | 705 | 73.4 ± 10.5 | 65.3 ± 14.7 | - | 43.6 | 61.9 | - | 18.4 ± 8.3 | 16.5 ± 8 | - |
| 28 | Marko et al. [54] | 2020 | Austria | 38 | Retrospective | IVT | 5452 | 18,953 | - | - | 74.9 ± 13.5 | - | - | 52.8 | - | - | 9.3 ± 7.4 |
| 29 | Mehrpour et al. [24] | 2019 | Iran | 1 | Retrospective | IVT | 24 | 118 | - | - | 66.1 ± 13.4 | - | - | 66.1 | - | - | 11.1 ± 5.1 |
| 30 | Mitchell et al. [83] | 2022 | Australia, China, New Zealand, Vietnam | 25 | Prospective RCT | BT | 34 | 147 | - | - | 69.3 ± 14.2 | - | - | 59.9 | - | - | 15 ± 7.5 |
| 31 | Mujanovic et al. [74] | 2022 | Europe and Canada | 8 | Retrospective | EVT | 1347 | 2941 | 77 ± 11.1 | 69 ± 15.6 | - | 43.5 | 54.5 | - | 15.7 ± 6.7 | 14.3 ± 7.4 | - |
| 32 | Nogueira et al. [75] | 2015 | USA | 13 | Retrospective | EVT | 413 | 1122 | - | - | 67 ± 15 | - | - | 51.9 | - | - | 16.7 ± 5.2 |
| 33 | Padjen et al. [55] | 2013 | France and Serbia | - | Prospective | IVT | 155 | 734 | 75.3 ± 12.0 | 64 ± 17.8 | - | 41.9 | 55.6 | - | 13.3 ± 7.5 | 10.7 ± 7.4 | - |
| 34 | Sanak et al. [22] | 2010 | Czech Republic | 1 | Retrospective | IVT | 66 | 157 | 68.1 ± 8.2 | 66.5 ± 13.6 | - | 57.6 | 65.9 | - | 13.3 ± 5.4 | 11 ± 5.1 | - |
| 35 | Sandercock et al. [56] | 2012 | International | 156 | Prospective RCT | IVT | 473 | 1515 | - | - | - | - | - | 48.4 | - | - | - |
| 36 | Seet et al. [23] | 2011 | USA | 1 | Retrospective | IVT | 76 | 214 | 78.9 ± 9.9 | 71.5 ± 14.8 | - | 42.1 | 53.6 | - | 13 ± 4.5 | 12 ± 6.0 | - |
| 37 | Shon et al. [57] | 2016 | Korea | 4 | Prospective | IVT | 123 | 318 | - | - | - | - | - | 60.4 | - | - | 12.7 ± 6.7 |
| 38 | Smaal et al. [78] b | 2020 | International | - | Prospective RCT | EVT | 224 | 667 | 72.8 ± 10.1 | 63.1 ± 13.7 | - | 52.2 | 51.0 | - | 17.5 ± 4.8 | 16.5 ± 5.2 | - |
| 39 | Sung et al. [58] | 2013 | Taiwan | - | Retrospective | IVT | 72 | 143 | - | - | 68.3/64.6 | 58.3 | 64.8 | - | - | - | - |
| 40 | Tong et al. [59] | 2014 | USA | - | Retrospective | IVT | 1489 | 7193 | - | - | - | - | - | 49.5 | - | - | - |
| 41 | Tong et al. [25] | 2021 | China | 111 | Prospective | EVT | 550 | 1705 | 71 ± 10.4 | 62.3 ± 11.9 | - | 44.7 | 75.5 | - | 18 ± 5.9 | 15.7 ± 7.4 | - |
| 41a | Tong et al. [25] | 2021 | China | 111 | Prospective | BT | 145 | 513 | - | - | - | - | - | - | - | - | - |
| 42 | Vorasoot et al. [60] | 2020 | Thailand | 7 | Retrospective | IVT | 177 | 772 | - | - | 63 ± 13.2 | - | - | 54.4 | - | - | 2.5 ± 5.6 |
| 43 | Wu et al. [61] | 2022 | China | 8 | Retrospective | IVT | 242 | 630 | - | - | 72.4/61.8 | 50.0 | 69.6 | - | - | - | - |
| 44 | Yang et al. [84] | 2020 | China | 41 | Prospective RCT | BT | 149 | 329 | - | - | 68.7 ± 11.2 | - | - | 55.0 | - | - | 17.7 ± 6.0 |
| 45 | Yoshimura et al. [76] | 2018 | Japan | 46 | Prospective | EVT | 658 | 1278 | - | - | 74.7 ± 11.4 | - | - | 59.2 | - | - | 18 ± 7.4 |
| 46 | Zdraljevic et al. [77] | 2022 | Serbia | 1 | Prospective | EVT | 62 | 127 | 73.3 ± 9.5 | 59.8 ± 13.3 | - | 43.5 | 63.1 | - | 16.7 ± 5.8 | 15.5 ± 6.2 | - |
| 46a | Zdraljevic et al. [77] | 2022 | Serbia | 1 | Prospective | BT | 11 | 32 | - | - | - | - | - | 53.5 | - | - | - |
| 47 | Zhang et al. [62] | 2010 | China | - | Retrospective | IVT | 22 | 53 | 68.3 ± 8.8 | 60.7 ± 12.3 | - | 40.9 | 74.2 | - | 12 ± 7.1 | 9.1 ± 7.3 | - |
| 48 | Zhao et al. [63] | 2017 | China | - | Retrospective | IVT | 30 | 123 | - | - | 65.6 ± 11.8 | - | - | 62.6 | - | - | 7.7 ± 6 |
| 49 | Zi et al. [29] | 2021 | China | 33 | Prospective RCT | BT | 62 | 118 | - | - | 69.3 ± 13.5 | - | - | 55.9 | - | - | 16.3 ± 5.3 |
| Study ID | Author | Year | Diabetes, n (%) | Lipid Disorders, n (%) | Hypertension, n (%) | CAD, n (%) | Heart Failure, n (%) d | Previous Stroke/TIA, n (%) | Smoking, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Akbik et al. [64] | 2021 | 1174 (28.21) | 1647 (39.53) a | 3112 (74.65) | - | - | 383 (12.55) d | - |
| 2 | Akbik et al. [28] | 2022 | 784 (25.02) | 1255 (39.99) a | 2247 (71.56) | - | - | 320 (12.97) d | - |
| 3 | Al-Khaled et al. [46] | 2014 | 198 (19.66) | 477 (47.37) b | 795 (78.95) | - | - | 197 (19.56) d | - |
| 4 | Alobaida et al. [33] | 2023 | 945 (30.42) | 1550 (51.39) a | 2398 (77.21) | 870 (28.01) | 906 (29.17) | 304 (9.79) e | - |
| 4a | Alobaida et al. [33] | 2023 | - | - | - | - | - | - | - |
| 5 | Awadh et al. [47] | 2010 | 24 (10.53) | 48 (21.05) a | 151 (66.23) | - | - | 45 (19.74) f | 56 (24.56) i |
| 6 | Bavarsad Shahripour et al. [79] | 2022 | 5 (11.11) | - | 28 (62.22) | 8 (17.78) | 6 (13.33) | - | 4 (8.89) g |
| 7 | Berkhemer et al. [65] | 2015 | 34 (14.59) | 58 (24.89) a | 98 (42.06) | - | - | 29 (12.45) d | 65 (28.89) g |
| 8 | Campbell et al. [80] | 2015 | 2 (5.71) | - | 21 (60.00) | - | - | - | 12 (34.29) i |
| 9 | Casetta et al. [66] | 2022 | 519 (15.46) | 786 (23.41) c | 2099 (62.51) | - | - | - | 572 (17.04) h |
| 10 | Chalos et al. [81] | 2019 | 197 (17.06) | - | 562 (49.08) | - | - | 164 (14.21) d | - |
| 11 | Churojana et al. [26] | 2018 | - | - | - | - | - | - | - |
| 11a | Churojana et al. [26] | 2018 | - | - | - | - | - | - | - |
| 12 | Das et al. [48] | 2020 | 27 (45.00) | 36 (60.00) b | 44 (73.33) | 7 (11.67) | - | 9 (15.00) e | 15 (25.00) g |
| 13 | Dharmasaroja et al. [49] | 2012 | 50 (25.77) | 61 (31.44) a | 116 (59.79) | 28 (14.43) | - | 28 (14.43) d | - |
| 14 | Fischer et al. [30] | 2022 | - | 71 (36.60) b | 118 (58.42) | - | - | 20 (9.95) d, 14 (7.00) e | - |
| 15 | Frank et al. [50] | 2012 | 543 (17.94) | - | 1930 (63.76) | - | - | - | - |
| 16 | Fu et al. [27] | 2021 | 88 (25.21) | 205 (58.74) a | 250 (71.63) | - | 36 (10.32) | 73 (20.92) f | 68 (19.48) g |
| 16a | Fu et al. [27] | 2021 | - | - | - | - | - | - | - |
| 17 | Goyal et al. [67] | 2015 | 33 (20.00) | 58 (35.15) a | 105 (63.64) | 40 (24.24) | 24 (14.55) | 17 (10.30) d | 80 (48.48) h |
| 18 | Huang et al. [68] | 2021 | 37 (15.10) | 9 (3.67) a | 140 (57.14) | 45 (18.37) | - | 52 (21.22) d | 71 (28.98) i |
| 18a | Huang et al. [68] | 2021 | - | - | - | - | - | - | - |
| 19 | Kimura et al. [51] | 2009 | 17 (20.00) | 19 (22.35) a | 49 (57.65) | - | - | - | - |
| 20 | Kurmann et al. [52] | 2018 | 309 (16.66) | 910 (50.58) a | 1272 (68.39) | 370 (20.01) | - | - | 369 (19.79) g |
| 21 | Lasek-Bal et al. [69] | 2022 | 105 (25.18) | 168 (40.29) c | 315 (75.54) | 221 (54.17) | - | - | 115 (37.10) i |
| 22 | LeCouffe et al. [82] | 2021 | 50 (18.80) | 73 (27.44) b | 139 (52.45) | - | 15 (5.64) | 44 (16.54) d | 66 (25.38) h |
| 23 | Lee et al. [70] | 2018 | - | - | - | - | - | - | - |
| 24 | Leker et al. [71] | 2020 | 73 (31.74) | 111 (48.26) a | 157 (68.26) | - | - | 36 (15.65) d | 58 (25.22) i |
| 24a | Leker et al. [71] | 2020 | - | - | - | - | - | - | - |
| 25 | Lin et al. [72] | 2020 | 19 (22.89) | 34 (40.96) a | 53 (63.86) | 17 (20.48) | - | 18 (21.69) d | 21 (25.30) i |
| 25a | Lin et al. [72] | 2020 | - | - | - | - | - | - | - |
| 26 | Lin et al. [53] | 2022 | 755 (32.20) | 829 (35.26) a | 1679 (71.60) | 323 (13.77) | - | - | - |
| 27a | Loo et al. [73] | 2023 | 131 (29.05) | 188 (41.69) c | 326 (72.28) | 75 (16.63) | - | 57 (12.64) f | 52 (11.53) i |
| 27 | Loo et al. [73] | 2023 | 206 (29.22) | 283 (40.14) c | 509 (72.20) | 125 (17.73) | - | 115 (16.31) f | 81 (11.49) g |
| 28 | Marko et al. [54] | 2020 | 3957 (20.88) | 10,055 (53.05) b | 14,885 (78.54) | - | - | 3265 (17.23) d | 3099 (16.35) g |
| 29 | Mehrpour et al. [24] | 2019 | 41 (34.75) | 28 (23.73) c | 82 (69.49) | 55 (46.61) | - | 25 (21.19) d | 25 (21.19) i |
| 30 | Mitchell et al. [83] | 2022 | - | - | 89 (60.54) | - | - | 18 (12.24) f | - |
| 31 | Mujanovic et al. [74] | 2022 | 566 (19.54) | 1416 (49.13) c | 1997 (68.89) | - | - | 344 (13.89) d | 702 (24.98) i |
| 32 | Nogueira et al. [75] | 2015 | 265 (23.62) | - | 773 (68.80) | - | - | - | - |
| 33 | Padjen et al. [55] | 2013 | 122 (16.62) | 336 (45.78) b | 483 (65.80) | - | - | 78 (10.63) d | 203 (27.66) g |
| 34 | Sanak et al. [22] | 2010 | - | - | - | - | - | - | - |
| 35 | Sandercock et al. [56] | 2012 | - | - | - | - | - | - | - |
| 36 | Seet et al. [23] | 2011 | 28 (13.08) | 109 (50.93) a | 164 (76.64) | 79 (36.92) | - | 47 (21.96) f | 29 (13.55) g |
| 37 | Shon et al. [57] | 2016 | 94 (29.56) | 106 (33.33) c | 230 (72.33) | - | - | 54 (16.98) d | 77 (24.21) g |
| 38 | Smaal et al. [78] | 2020 | 114 (17.09) | - | 369 (55.32) | - | - | - | - |
| 39 | Sung et al. [58] | 2013 | 48 (33.57) | 83 (58.04) a | 112 (78.32) | - | - | 27 (18.88) d | 34 (23.78) g |
| 40 | Tong et al. [59] | 2014 | 1793 (24.93) | 3020 (41.99) c | 5757 (80.04) | - | 751 (10.44) | 1251 (17.39) d | 1537 (21.37) i |
| 41 | Tong et al. [25] | 2021 | 324 (19.00) | - | 1006 (59.00) | - | - | 337 (19.77) d | 706 (41.41) h |
| 41a | Tong et al. [25] | 2021 | - | - | - | - | - | - | - |
| 42 | Vorasoot et al. [60] | 2020 | 163 (21.11) | 137 (17.75) c | 378 (48.96) | 52 (6.74) | 11 (1.42) | 118 (15.28) d, 18 (2.33) e | 281 (36.40) i |
| 43 | Wu et al. [61] | 2022 | 106 (16.83) | 216 (34.29) a | 481 (76.35) | 34 (5.40) | - | 98 (15.56) d | 221 (35.08) g |
| 44 | Yang et al. [84] | 2020 | 65 (19.76) | 14 (4.26) b | 201 (61.09) | - | 17 (5.17) | 47 (14.29) d | 68 (20.67) i |
| 45 | Yoshimura et al. [76] | 2018 | 236 (18.47) | - | 739 (57.82) | - | - | 95 (7.43) d | 183 (14.32) g |
| 46 | Zdraljevic et al. [77] | 2022 | 22 (17.32) | 75 (59.06) a | 102 (80.31) | - | - | 19 (14.96) d | 32 (25.20) i |
| 46a | Zdraljevic et al. [77] | 2022 | - | - | - | - | - | - | - |
| 47 | Zhang et al. [62] | 2010 | 5 (9.43) | - | 26 (49.06) | - | - | - | - |
| 48 | Zhao et al. [63] | 2017 | 26 (21.14) | - | 83 (67.48) | 14 (11.38) | - | 40 (32.52) d | 49 (39.84) i |
| 49 | Zi et al. [29] | 2021 | 20 (16.95) | 22 (18.64) a | 74 (62.71) | 19 (16.10) | - | 19 (16.10) d | 29 (24.58) h |
| Study ID | Author | Reperfusion Therapy | Good Functional Outcome at 90 Days | sICH | 90-Day Mortality | sICH Definition | |||
|---|---|---|---|---|---|---|---|---|---|
| AF, n (%) | No AF, n (%) | AF, n (%) | No AF, n (%) | AF, n (%) | No AF, n (%) | ||||
| 1 | Akbik et al. [64] | EVT | 426 (31.14) | 1029 (42.28) | 89 (7.64) | 160 (7.28) | 354 (25.88) | 408 (16.76) | ECASS II |
| 2 | Akbik et al. [28] | BT | 295 (33.56) | 822 (46.13) | 91 (9.15) | 140 (7.02) | 222 (25.26) | 324 (18.18) | ECASS II |
| 3 | Al-Khaled et al. [46] | IVT | - | - | 29 (7.49) | 29 (4.68) | - | - | Any bleeding that was not detected on a previous CT scan and associated with an increase in NIHSS score of ≥4 |
| 5 | Awadh et al. [47] | IVT | - | - | 3 (4.05) | 7 (4.55) | - | - | Deterioration in NIHSS score of ≥4 within 72 h, and PH1 or PH2 present on CT |
| 10 | Chalos et al. [81] | BT | 52 (29.89) | 373 (42.78) | 11 (5.91) | 56 (5.85) | 65 (34.95) | 204 (21.29) | Heidelberg Bleeding Classification |
| 11 | Churojana et al. [26] | EVT | 19 (38.00) | 32 (38.10) | 6 (12.00) | 11 (13.10) | 10 (20.00) | 16 (19.05) | NR |
| 13 | Dharmasaroja et al. [49] | IVT | - | - | 6 (13.04) | 5 (3.42) | - | - | NINDS |
| 15 | Frank et al. [50] | IVT | 211 (33.02) | 1179 (49.37) | 17 (2.66) | 41 (1.72) | 139 (21.75) | 325 (13.61) | 24-h increase in NIHSS score by ≥4 or any stroke/ICH leading to death |
| 16 | Fu et al. [27] | EVT | 82 (47.95) | 85 (47.75) | 2 (1.17) | 7 (3.93) | 37 (21.64) | 32 (17.98) | SITS-MOST |
| 18 | Huang et al. [68] | EVT | 27 (38.57) | 31 (44.29) | 9 (12.86) | 5 (7.14) | 15 (21.43) | 14 (20.00) | ICH with a 24-h increase in NIHSS score of ≥4 |
| 21 | Lasek-Bal et al. [69] | EVT | 28 (25.93) | 89 (28.80) | 7 (6.48) | 15 (4.85) | 30 (27.78) | 72 (23.30) | ECASS II |
| 24 | Leker et al. [71] | EVT | 27 (24.77) | 51 (42.15) | 6 (5.50) | 5 (4.13) | 12 (11.01) | 23 (19.01) | ECASS III |
| 25 | Lin et al. [72] | EVT | 24 (55.81) | 7 (17.50) | 3 (6.98) | 4 (10.00) | 4 (9.30) | 6 (15.00) | SITS-MOST |
| 26 | Lin et al. [53] | IVT | 351 (39.35) | 574 (47.87) | 12 (1.22) | 14 (1.02) | 93 (10.43) | 106 (8.84) | SITS-MOST |
| 27 | Loo et al. [73] | EVT | 106 (34.30) | 146 (38.52) | 30 (9.55) | 51 (13.04) | 59 (19.09) | 63 (16.62) | SITS-MOST |
| 27a | Loo et al. [73] | BT | 63 (35.00) | 118 (45.21) | - | - | 34 (18.89) | 41 (15.71) | - |
| 29 | Mehrpour et al. [24] | IVT | 5 (20.83) | 55 (58.51) | - | - | - | - | - |
| 33 | Padjen et al. [55] | IVT | 74 (47.74) | 375 (64.77) | - | - | 34 (21.94) | 52 (8.98) | - |
| 34 | Sanak et al. [22] | IVT | 33 (50.00) | 66 (72.53) | 3 (4.55) | 0 (0.00) | - | - | ECASS II |
| 36 | Seet et al. [23] | IVT | 32 (42.11) | 77 (55.80) | 10 (13.16) | 7 (5.07) | - | - | Haemorrhagic transformation associated with an increase in NIHSS score of ≥4 |
| 38 | Smaal et al. [78] | EVT | 95 (42.41) | 213 (48.19) | 8 (3.57) | 20 (4.51) | 46 (20.54) | 58 (13.12) | Various |
| 39 | Sung et al. [58] | IVT | 34 (47.22) | 24 (33.80) | 6 (8.33) | 66 (9.86) | 4 (5.56) | 8 (11.27) | ECASS II |
| 40 | Tong et al. [59] | IVT | - | - | 98 (6.58) | 225 (3.94) | - | - | CT showing intracranial haemorrhage and medical records noting clinical deterioration due to haemorrhage |
| 41 | Tong et al. [25] | EVT | 160 (41.34) | 155 (40.16) | 36 (9.45) | 35 (9.07) | 63 (16.28) | 71 (18.39) | Heidelberg Bleeding Classification |
| 43 | Wu et al. [61] | IVT | 97 (40.08) | 279 (71.91) | - | - | 39 (16.12) | 22 (5.67) | - |
| 46 | Zdraljevic et al. [77] | EVT | 19 (30.65) | 35 (53.85) | 5 (8.06) | 4 (6.15) | 22 (35.48) | 11 (16.92) | SITS-MOST |
| 47 | Zhang et al. [62] | IVT | 9 (40.91) | 17 (54.84) | 4 (18.18) | 2 (6.45) | 4 (18.18) | 3 (9.68) | ECASS III |
| Outcome | Reperfusion Therapy | Effect Measure | Effect Measure (95% CI) | Test of ES = 0 | Tests of Overall Effect | Heterogeneity | Heterogeneity Variance Estimates | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chi-Squared | Cochran’s Q | p-Value | H (95% CI) | I2 (%) (95% CI) | tau2 | ||||||
| Prevalence | IVT | Prevalence | 0.31 (0.28 to 0.35) | p < 0.01 z = 30.02 | - | 738.91 | - | <0.01 | - | 97.29 | 0.03 |
| EVT | Prevalence | 0.42 (0.38 to 0.46) | p < 0.01 z = 30.94 | - | 606.22 | - | <0.01 | - | 97.20 | 0.03 | |
| BT | Prevalence | 0.36 (0.28 to 0.43) | p < 0.001 z = 14.86 | - | 630.87 | - | <0.01 | - | 97.31 | 0.10 | |
| 90-day mRS 0–2 | IVT | OR | 0.512 (0.376 to 0.696) | - | p < 0.001 z = −4.271 | - | 43.85 | <0.001 | 2.341 (1.000 to 3.897) | 81.8 (<0.1 to 93.4) | 0.1457 |
| EVT | OR | 0.826 (0.651 to 1.049) | - | p = 0.117 z = −1.568 | - | 38.44 | <0.001 | 1.961 (1.000 to 3.050) | 74.0 (<0.1 to 89.2) | 0.1009 | |
| sICH | IVT | OR | 1.690 (1.400 to 2.039) | - | p < 0.001 z = 5.473 | - | 8.14 | 0.520 | 0.951 (1.000 to 1.387) | <0.1 (<0.1 to 48.0) | <0.0001 |
| EVT | OR | 0.982 (0.815 to 1.184) | - | p = 0.851 z = −0.188 | - | 7.23 | 0.703 | 0.851 (1.000 to 1.254) | <0.1 (<0.1 to 36.4) | <0.0001 | |
| 90-day mortality | IVT | OR | 1.799 (1.218 to 2.657) | - | p = 0.003 z = 2.953 | - | 19.34 | 0.002 | 1.966 (1.000 to 3.432) | 74.1 (<0.1 to 91.5) | 0.1407 |
| EVT | OR | 1.236 (0.969 to 1.578) | - | p = 0.088 z = 1.706 | - | 26.14 | 0.004 | 1.617 (1.000 to 2.465) | 61.7 (<0.1 to 83.5) | 0.0857 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, J.; Bhaskar, S.M.M. Atrial Fibrillation and Reperfusion Therapy in Acute Ischaemic Stroke Patients: Prevalence and Outcomes—A Comprehensive Systematic Review and Meta-Analysis. Neurol. Int. 2023, 15, 1014-1043. https://doi.org/10.3390/neurolint15030065
Patel J, Bhaskar SMM. Atrial Fibrillation and Reperfusion Therapy in Acute Ischaemic Stroke Patients: Prevalence and Outcomes—A Comprehensive Systematic Review and Meta-Analysis. Neurology International. 2023; 15(3):1014-1043. https://doi.org/10.3390/neurolint15030065
Chicago/Turabian StylePatel, Jay, and Sonu M. M. Bhaskar. 2023. "Atrial Fibrillation and Reperfusion Therapy in Acute Ischaemic Stroke Patients: Prevalence and Outcomes—A Comprehensive Systematic Review and Meta-Analysis" Neurology International 15, no. 3: 1014-1043. https://doi.org/10.3390/neurolint15030065
APA StylePatel, J., & Bhaskar, S. M. M. (2023). Atrial Fibrillation and Reperfusion Therapy in Acute Ischaemic Stroke Patients: Prevalence and Outcomes—A Comprehensive Systematic Review and Meta-Analysis. Neurology International, 15(3), 1014-1043. https://doi.org/10.3390/neurolint15030065








