Abstract
(1) Background: Parkinson’s disease (PD) is a relatively common and complex pathology, and some of its mechanisms remain to be elucidated. Change in host microbiota is related to the pathophysiology of numerous diseases. This systematic review aims to gather existing data on the occidental hemisphere, compare it, and search for any significant association between Parkinson’s disease and gut microbiota dysbiosis. (2) Methods: Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) and Meta-analyses Of Observational Studies in Epidemiology (MOOSE) protocols were used for this systematic review. PubMed was used as the database search engine. Of the 166 studies found, only 10 were used, as they met our inclusion criteria: case–control studies, studies that assessed the correlation of PD and gut microbiome, studies that took place in occidental regions, and studies that were performed on humans and were written in English. The Newcastle–Ottawa Scale was used as the assessment tool for overall risk of bias in this systematic review. (3) Results: The studies analyzed were divided into three geographic areas: Region 1: United States of America and Canada; Region 2: Germany, Ireland, and Finland; and Region 3: Italy; based on geographical similarities among these populations. The following statistically significant results were described in PD patients, compared with non-PD controls. In the first region, a significant increase in the following bacteria was seen: 1. Phylum: Actinobacteriota and its Genus: Bifidobacterium; 2. Phylum: Verrucomicrobiota and its Genus: Akkermansia; 3. Genus: Enterococcus, Hungatella, Lactobacillus, and Oscillospira of the Phylum: Firmicutes; 4. Family: Ruminococcaceae of Phylum: Firmicutes; 5. Phylum: Bacteroidetes and its Genus: Bacteroides; 6. Phylum: Proteobacteria. A significant decrease was described in the Family: Lachnospiraceae and its Genus: Blautia, Coprococcus, and Roseburia, which belong to the Phylum: Firmicutes. In the second region, a raised number of: 1. Phylum: Verrucomicrobiota, its Genus: Akkermansia, and its Species: Akkermansia muciniphila; 2. Family: Verrucomicrobiaceae of the Phylum: Verrucomicrobiota; 3. Genus: Lactobacillus and Roseburia of the Phylum: Firmicutes; 4. Family: Lactobacillaceae of the Phylum: Firmicutes; 5. Family: Barnesiellaceae of the Phylum: Bacteroidetes; 6. Genus: Bifidobacterium of the Phylum: Actinobacteriota; 7. Species: Bilophila wadsworthia of the Phylum: Thermodesulfobacteriota, was identified. Only one Genus: Prevotella of the Phylum: Bacteroidetes was decreased. In the third and last region, an augmented number of these bacteria were found: 1. Phylum: Verrucomicrobiota and its Genus: Akkermansia; 2. Family: Bifidobacteriaceae and Coriobacteriaceae of the Phylum: Actinobacteriota; 3. Phylum: Firmicutes and its Family: Christensenellaceae and Lactobacillaceae; 4. Family: Enterococcaceae and its Genus: Enterococcus, of the Phylum: Firmicutes; 5. Genus: Lactococcus and Oscillospira, of the Phylum: Firmicutes; 6. Phylum: Proteobacteria, its Family: Enterobacteriaceae, and the Genus: Citrobacter, Klebsiella, Salmonella, and Shigella; 7. Genus: ParaBacteroides of the Phylum: Bacteroidetes. In contrast, a significant decrease in 1. Phylum: Firmicutes, its Family: Lachnospiraceae, and its Genus: Roseburia and 2. Genus: Ruminococcus of the Phylum: Firmicutes, was described. (4) Conclusion: A significant gut dysbiosis, involving multiple bacterial taxa, was found in PD patients compared to healthy people in the occidental regions. However, more studies are needed to find the precise pathophysiologic involvement of other groups of pathogens, such as fungi and parasites, in the development and progression of PD.
1. Introduction
The gut microbiome is composed of microorganisms such as bacteria, viruses, and yeasts. Each individual has a unique microbiota that has developed since birth and continues molding within the first years of life [1]. Due to several environmental factors, it will thrive and achieve equilibrium during a lifetime. The microbiota plays an essential role in the symbiosis of the host, from digestion and absorption of nutrients to the protection against pathogens and education of the immune system [2,3].
Due to its influence on physiological processes, it has been proposed that any imbalance in this environment can have negative consequences in the host, leading to a dysregulation in pro-inflammatory and anti-inflammatory factors, causing disorders in distinct locations other than the gut, such as the central nervous system (CNS) [3]. The term “dysbiosis” refers to the disturbance of the bacterial species that make up the microbiota, and it has been the target of several studies in recent years.
It is well-known that the CNS has a selective immune defense driven by the blood–brain barrier (BBB). The BBB consists of the endothelial cells from the blood vessels that supply blood to the brain. These cells are interconnected with tight junctions, allowing a rigorous passage of cells, electrolytes, and nutrients from the systemic circulation into the brain [4]. However, other particles, such as metabolites (amino acids, essential vitamins) and bacterial products, can cross the BBB; this whole interaction forms the so-called Gut–Brain Axis (GBA). This complex is a two-way communication system that influences the immune, endocrine, and enteric nervous systems [5,6].
Due to this communication and early exposure, Socala et al. propose that the pathogenesis of certain neurological conditions has a root in this complex system, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, depression, and anxiety [7].
Parkinson’s disease (PD) is a chronic, neurodegenerative disorder characterized by motor symptoms, starting from mild tremors to difficulty walking due to stiffness; these motor symptoms are the Parkinsonian triad: rigidity, bradykinesia, and tremor. PD is also associated with other nonmotor symptoms such as cognitive impairment, depression, psychosis, sleep disturbances, and gastrointestinal disturbances in more than 80% of patients [8]. Gastrointestinal symptoms such as constipation, gastroparesis, and weight loss appear in the early stages of the disease [9], even before the development of motor symptoms. The pathogenesis of PD occurs due to damage to dopaminergic neurons in the substantia nigra pars compacta. The pathologic accumulation of Lewy bodies (mainly α-synuclein) in the presynaptic neurons is responsible for neuronal cell death [7], which causes the broad spectrum of manifestations in PD as represented in Figure 1.
The gut microbiota is one of the proposed mechanisms of signaling and inflammation in the CNS; these molecular changes induce a systemic response and further depositing of amyloid in the presynaptic space. Bacterial fermentation from dietary nutrients produces short-chain fatty acids (SCFAs); during absorption, some molecules can travel from the blood vessels and cross the BBB [10]. Some of these SCFAs are precursors of neurotransmitters such as GABA, serotonin, glutamate, adrenaline, noradrenaline, and dopamine [11]—the latter being a cornerstone in the pathophysiology of PD. However, some specific SCFAs, such as butyrate kinase, are produced by bacteria. These products impact the down-regulation of inflammation [12], and their absence could lead to a pro-inflammatory state.
There is increasing evidence for the involvement of neuroinflammatory molecules in the pathogenesis of PD [13]. Bacterial metabolites, such as Endotoxin, can trigger a local and systemic inflammatory response, increasing TNF-a, IL-8, and IL-6 levels, leading to dysregulated activation of glial cells and causing pathological α-Synuclein accumulation [14]. Li et al. found that the use of naturally occurring intraluminal antibiotics (some found in traditional Chinese medicine) can increase blood/fecal levodopa levels through changes in the intestinal flora, with an improvement of motor and cognitive symptoms, avoiding the need to increase standard treatment doses per os; they also found that an upgrade of the microbiome to a noninflammatory type led to a significant reduction in TNF-α, IL-6, and IL-8 serum levels, which could stop microglia activation and, therefore, theoretically stop substantia nigra degeneration [15].
Regardless of new evidence of these associations, and due to PD multifactorial pathogenesis, our team decided to further study this perplexing communication between the gut and the brain. In this systematic review, we aim to provide a thorough analysis of the impact of gut microbiota on the pathophysiology of PD.
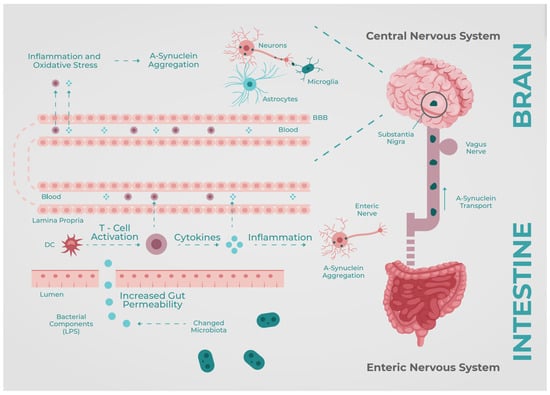
Figure 1.
A-synuclein Brain–Gut Axis. BBB: Blood–brain barrier, DC: Dendritic Cells, LPS: Lypopolysaccharides. Based on Perez-Pardo et al. [16].
2. Materials and Methods
2.1. Protocol
We carried out a systematic review using the PRISMA and MOOSE protocols (Figure 2). This systematic review was developed and reported following the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines which are evidence-based and consist of a minimum set of items focusing on the reporting of reviews evaluating various types of research. Before the formal screening of search results, the protocol for this study was registered in PROSPERO 2023 (link: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023421796; date accessed: 7 May 2023) under the registration number CRD42023421796.
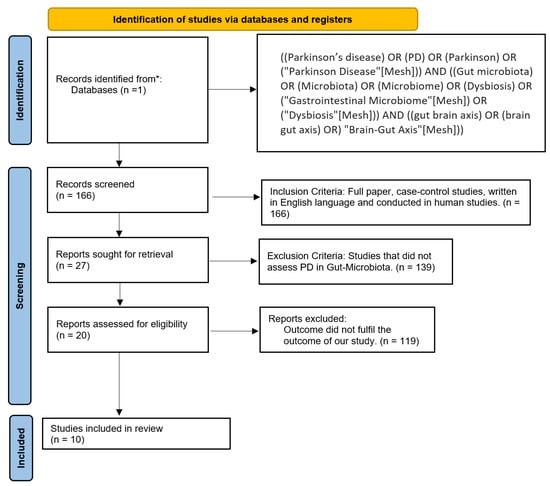
Figure 2.
PRISMA flow chart. * PubMed.
2.2. Eligibility Criteria and Study Selection
Inclusion criteria were clinical trials conducted on humans and written in English. Exclusion criteria included animal studies, studies that did not assess PD and gut microbiome, and articles that did not fulfill the aim of our research. As described in Figure 2, we only included articles about PD and its association with gut microbiome. After applying these filters, we discarded 156 studies and accepted 10 studies.
The purpose of this study is to review current evidence on the different species involved, since the microbiome is related to the sociodemographic characteristics of patients, and to address if there is a shared pathway despite these variables. We divided the studies into three groups because diet and habits are among the crucial factors influencing the microbiome. They differentiate qualitatively among the regions included but could have some similarities related to disease progression.
2.3. Database and Search Strategy
We used the PubMed database for this systematic meta-analysis review. We extrapolated the data between 12 April and 1 May 2023. We used an advanced search strategy with the following terms: “((Parkinson’s disease) OR (PD) OR (Parkinson) OR (“Parkinson Disease” [Mesh])) AND ((Gut microbiota) OR (Microbiota) OR (Microbiome) OR (Dysbiosis) OR (“Gastrointestinal Microbiome” [Mesh]) OR (“Dysbiosis” [Mesh])) AND ((gut brain axis) OR (brain gut axis) OR (“Brain-Gut Axis” [Mesh]))”.
2.4. Data Extraction and Analysis
From each paper we collected: author/year/country, methods, number of participants, and study design. We also extracted the main results, including the outcome measures and limitations of each observational/clinical trial. We analyzed the studies’ primary and secondary goals and the main conclusions from each study for further analysis divided into 3 subgroups.
Upon selection of the articles, we grouped them into three categories based on their region: Region 1: USA and Canada, Region 2: Germany, Ireland, and Finland, and Region 3: Italy, as described in Figure 3.
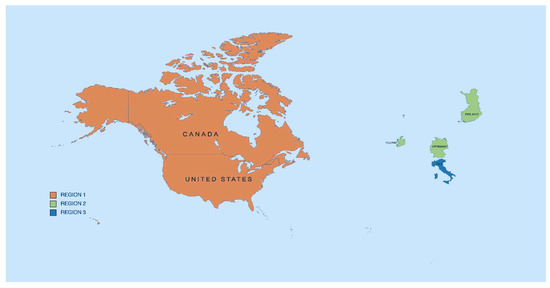
Figure 3.
Map: Region 1 (orange), Region 2 (green), and Region 3 (blue) [16].
2.5. Bias Analysis
The assessment of overall risk for bias in this systematic review was conducted with the Newcastle–Ottawa Scale, which divides the risk into three categories: high, moderate, and low; depending on its score, from 0 to 3, 4 to 6, and 7 to 9, respectively. With this tool, the systematic review has a moderate risk of overall bias, as demonstrated in Table 1.

Table 1.
Newcastle–Ottawa Scale.
3. Results
3.1. Region 1
Within Region 1 (Figure 3; Table 2 and Table 3), we found four articles. Keshavarzian et al. [17] (USA) investigated 38 PD patients and compared them to 34 controls. Their study showed an increase in Proteobacteria, Verrucomicrobiota, Bacteroidetes, Akkermansia, Oscillospira, and Bacteroides, and decreased Lachnospiraceae, Blautia, Coprococcus, and Roseburia in fecal samples. The authors mentioned that the characteristics between groups in terms of age, body mass index, previous medications, and duration of the disease were not considered and could have affected the results.

Table 2.
Region 1—USA and Canada.

Table 3.
Microbiota changes of PD patients VS controls in Region 1.
On the other hand, Hill-Burns et al. [18] (USA) accepted 327 patients divided into 197 cases and 130 controls. The fecal specimens showed a reduction in Lachnospiraceae, with an increase in Akkermansia, Lactobacillus, and Bifidobacterium compared to the controls. Variables such as PD medication, disease duration, and geographic location were cataloged as confounders and recognized as limitations for their study.
Lachnospiraceae was found to be reduced by Keshavarzian et al. [17] (USA) and Hill-Burns et al. [18] (USA), which is related to butyrate kinase production, an anti-inflammatory molecule; therefore, with a lower number of these bacteria, a pro-inflammatory setting would be present.
In the rural areas of California, Zhang et al. [20] (USA) recruited 170 patients, with 96 cases and 74 controls. The authors found that patients with PD had an increased biome of Proteobacteria, Verrucomicrobiota, Actinobacteriota, UBA1819 (Ruminococcaceae), DTU089 (Ruminococcaceae), Akkermansia, Enterococcus, and Hungatella. A compelling aspect of this research is that they classified the cases into motor-symptom subtypes (Postural Instability and Gait dysfunction [PIGD], Tremor Dominant [TD], and Indeterminate [IND]) and noticed that the PIGD motor subtype was more abundant in Verrucomicrobiota. In contrast, the other subtypes highlighted the presence of Proteobacteria. Their theory proposes that elevated lipopolysaccharides (LPS) in Gram-negative bacteria’s walls, such as Proteobacteria, Verrucomicrobiota, and Akkermansia, trigger the immune system. As data collection was at a single time, they stated that neither temporality nor causality can be determined. Additionally, they specified that their sample size, confounders control, and statistical power represented the limitations of this research.
The uniqueness between Hill-Burns et al. [18] (USA) and Zhang et al. [20] (USA) among their selection groups is that they used healthy controls (HC) living in the same household, and thus were comparable.
Most studies research bacteria; however, Appel-Cresswell et al. [19] (Canada) focused theirs on fungi. Through microbial DNA sequencing, 152 fecal samples were tested and contrasted with 95 cases versus 57 controls. The most common was Saccharomyces in 94% of all participants, followed by Candida spp. with 35%, and Cladosporium and Penicillium in last place with 23%.
3.2. Region 2
In Region 2 (Figure 2; Table 4 and Table 5), we selected four studies performed in countries on the northern side of Europe. Hertel et al. [25] (Ireland) investigated 60 subjects from the German longitudinal de novo Parkinson’s disease cohort; patients received dopaminergic modulators. The authors chose 30 PD patients and compared them to 30 healthy control volunteers. This investigation determined a rise of Akkermansia muciniphila and Bilophila wadsworthia within the cases. These two bacteria are associated with sulfur metabolism, whose byproducts are known to be related to local inflammation and neurotoxicity in the brain. Unlike the rest of the studies, they used the Virtual Metabolic Database to recognize bacteria and metabolites. Additionally, habits such as diet and exercise were considered, which could affect the conclusions.

Table 4.
Region 2—Germany, Ireland, and Finland.

Table 5.
Microbiota changes of PD patients VS controls in Region 2.
Aho et al. [26] (Finland) conducted a study with 128 subjects divided into 64 patients and 64 healthy participants. The bacteria they found most abundant in the case section were Bifidobacterium, Lactobacillus, and Roseburia. A secondary outcome of this research was the result of decreased levels of Prevotella in patients with a faster progression of the disease. This last finding led the researchers to conclude that reducing Prevotella in the intestinal lumen could influence the correlation to PD. The writers stated further investigation should be conducted with patients taking similar medication as PD patients (e.g., restless legs syndrome or de novo patients starting medication) as these drugs could influence the microbiome.
We selected two studies from Germany. Hopfner et al. [23] recruited 29 PD cases and 29 healthy volunteers. This investigation showed an increment in Lactobacillaceae and Barnesiellaceae. They did not state any possible mechanism through which these bacteria could influence the pathogenesis of this condition. The lack of information regarding diet habits, related factors, and cardiovascular comorbidities in the HC group raises the suspicion of possible confounders.
Heintz-Buschart et al. [24], with 99 patients and 76 controls, observed an increase in Akkermansia and Verrucomicrobiaceae in the samples of PD patients. The authors agreed with the current hypotheses that Akkermansia spp. is critical in the secretion of α-synuclein and future deposit in the CNS. Similar to Hopfner et al. [23], in this study diabetes and coronary artery disease were comorbidities present among the healthy participants, and the oral intake of medication could modify the microbiome.
In addition to the phenomenon in which bacteria could reach the CNS from the olfactory bulb, Heinz-Buschart et al. [24] have also included 76 nasal wash samples from PD patients and 78 HCs. However, the study showed no significant variation between both groups, showing that pathologic pathways may differ from the nasal and gut microbiota in PD patients. Nonetheless, the only significant difference was found in the Bacillaceae family, in a group of PD patients treated with L-dopa, suggesting that its variation might be attributed to the medication rather than the disease.
3.3. Region 3
In Region 3 (Figure 3; Table 6 and Table 7), we grouped two studies made in Italy mainly due to their specific Mediterranean diet. Piertrucci et al. [21] gathered 152 patients; the case group (N = 80) showed an elevation in the levels of Proteobacteria, Lactobacillaceae (phylum: Firmicutes), Enterobacteriaceae, Enterococcaceae, Citrobacter, Enterococcus, Lactococcus, Klebsiella, Salmonella, and Shigella, and a reduction in Lachnospiraceae (phylum: Firmicutes) and Roseburia in their fecal samples when compared to the control group (N = 72). The accumulation of LPS in the intestine was postulated again as a possible mechanism of inflammatory response. Additionally, this research found that the levels of phenylalanine, tryptophan, and tyrosine decreased in PD patients; nonetheless, they did not postulate any specific reason for this finding. Based on the premise that catechol-O-methyltransferase inhibitors (COMTi) alter the microbiota, eight patients taking this medication were extracted from the case group. They consisted of part of a subgroup and exhibited no change in the presence of Citrobacter, Enterococcus, Lactococcus, Klebsiella, Salmonella, Shigella, an unclassified Enterobacteriaceae, and Roseburia as to non-PD participants. There was a significant difference in age, sex, and weight loss between the two groups; these variables could be considered potential confounders.

Table 6.
Region 3—Italy.

Table 7.
Microbiota changes of PD patients VS controls in Region 3.
After collecting a cohort of 193 PD patients and 113 controls, Cereda et al.’s [22] research yielded an increase in Akkermansia, Proteobacteria, Enterobacteriaceae, Christensenellaceae, Lactobacillaceae, Coriobacteriaceae, Bifidobacteriaceae, ParaBacteroides, Oscillospira, and Verrucomicrobiota and a decrease in Lachnospiraceae, Roseburia, and Rominococcus in PD cases. As previously mentioned, Lachnospiraceae is associated with butyrate-related SCFA production; their metabolites preserve the integrity of the local immune system, hence the intestinal barrier. Moreover, the authors mentioned that Lactobacillaceae was elevated in the case group, and plays a role in α-synuclein secretion, a hallmark in the pathogenesis of PD. The writers tried to match the controls to the cases; however, the lack of biopsies from the sigmoid colon and metagenomic analyses were labeled as possible limitations.
4. Discussion
Parkinson’s disease is one of the most common neurodegenerative disorders. Its complex pathogenesis includes mitochondrial dysfunction, oxidative stress, intracellular protein accumulation, and abnormal protein degradation. Dysbiosis has been described in PD patients, but whether this happens as a cause or a consequence of disease progression remains unclear. There is growing evidence that the microbiome could be one of the most crucial factors contributing to disease progression and its clinical manifestations. Additionally, nonmotor symptoms (mainly gastrointestinal dysfunction) often precede by decades before their motor manifestations and diagnosis.
Our review attempts to address PD from a first principle thinking perspective, aiming at the true causes behind the pathophysiology of this disease and not only its clinical manifestations. Current treatments focus on the effects of substantia nigra degeneration, and a way to properly halt disease appearance and its progression remains elusive.
This systemic review suggests changes in the colonic microbiota in patients with Parkinson’s disease in the three regions of our study.
There are alterations in the microbiota of patients with Parkinson’s disease; some bacteria are more common than others, depending on their geographic region. Both USA and Italy regions have an increase in Proteobacteria and a decrease in Lachnospiraceae in their fecal samples, while in the northern side of Europe Lactobacillaceae and Akkermansia are the most prevalent; in the case of Finland, they reported a reduction in Prevotella. This may be mainly due to the similarities in the diet (probably Mediterranean/European) of the last two regions; however, other confounders could influence the result, such as genetics, medication utilization, and lifestyle. In Germany, the nasal microbiota was analyzed in patients with PD, since it is proposed that the toxin from the bacteria can reach the CNS from the olfactory bulb, which is also affected in patients with PD due to the aggregation of the α -synuclein. Therefore, hyposmia is also characteristic in these patients. However, there was no significant difference in the nasal microbiota.
Since the decrease in butyrate-related SCFA production and the increase in LPS are hypotheses that need to be confirmed, it is of the utmost importance to further investigate the mechanisms by which bacteria influence the pathology of Parkinson’s disease.
The limitations of this review were the limited number of case–control studies and the lack of data in some areas, such as Latin America. The long disease progression makes it harder to properly design cohort studies and clinical trials, especially in low-income countries. Diet is usually self-reported, increasing the chance of recall bias.
5. Conclusions
A clear association was found between PD and gut dysbiosis when comparing patients in the occidental region, with more similarities between European countries and the Americas. Due to its insidious onset regarding nonmotor symptoms, the screening and caption of PD patients for clinical trials are limited. Latin America is a relegated area in terms of research.
Author Contributions
Conceptualization, A.C.P. and J.A.M.; methodology, J.A.M.; validation, D.V.R., A.C.T. and M.G.-M.; formal analysis, M.A.C., S.C.C. and D.F.M.; investigation, A.C.P., S.C.C., A.C.T., J.A.V., E.N.O. and M.A.C.; resources, D.V.R.; writing—original draft preparation, A.C.P., J.A.M. and M.G.-M.; writing—review and editing, S.C.C., J.A.V. and E.N.O.; supervision, J.A.M.; project administration, A.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| COMTi | Catechol-O-methyltransferase inhibitors |
| GBA | Gut–Brain Axis |
| HC | Healthy controls |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IND | Indeterminate |
| LPS | Lipopolysaccharides |
| MOOSE | Meta-analysis of observational studies in epidemiology |
| PD | Parkinson’s disease |
| PIGD | Postural Instability and Gait dysfunction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SCFAs | Short-chain fatty acids |
References
- Adlerberth, I. Factors Influencing the Establishment of the Intestinal Microbiota in Infancy. In Nestlé Nutrition Workshop Series: Pediatric Program; Bier, D.M., German, J.B., Lönnerdal, B., Eds.; KARGER: Basel, Switzerland, 2008; Volume 62, pp. 13–33. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The Gut Microbiota—Masters of Host Development and Physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Julian, R.Y.; Hardesty, D.A.; Lonser, R.R. Cerebral Edema. In Youmans & Winn Neurological Surgery; Winn, H.R., Ed.; Elsevier: Philadelphia, PA, USA, 2023; pp. 473.e1–473.e13. [Google Scholar]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-Analysis of Gut Microbiome Studies Identifies Disease-Specific and Shared Responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Fasano, A.; Visanji, N.P.; Liu, L.W.C.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory Effects and Targets of the SCFAs and Gasotransmitters Produced by the Human Symbiotic Microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted Distribution of the Butyrate Kinase Pathway among Butyrate-Producing Bacteria from the Human Colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Van IJzendoorn, S.C.D.; Derkinderen, P. The Intestinal Barrier in Parkinson’s Disease: Current State of Knowledge. J. Park. Dis. 2019, 9, S323–S329. [Google Scholar] [CrossRef]
- Fayyad, M.; Salim, S.; Majbour, N.; Erskine, D.; Stoops, E.; Mollenhauer, B.; El-Agnaf, O.M.A. Parkinson’s Disease Biomarkers Based on A-synuclein. J. Neurochem. 2019, 150, 626–636. [Google Scholar] [CrossRef]
- Li, J.; Meng, P.; Zhang, J.; He, M. Effect of Berberine Hydrochloride on the Diversity of Intestinal Flora in Parkinson’s Disease Patients. Contrast Media Mol. Imaging 2022, 2022, 8381870. [Google Scholar] [CrossRef]
- MapChart. Available online: https://www.mapchart.net/ (accessed on 17 May 2023).
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic Bacterial Composition in Parkinson’s Disease: Colonic Microbiota in Parkinson’s Disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome: PD, Medications, and Gut Microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, M.S.; Sundvick, K.; Golz, E.; Yu, A.C.; Boutin, R.C.T.; Kliger, D.; Finlay, B.B.; Appel-Cresswell, S. The Gut Mycobiome in Parkinson’s Disease. J. Park. Dis. 2021, 11, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Paul, K.C.; Jacobs, J.P.; Chou, H.-C. (Lori); Duarte Folle, A.; Del Rosario, I.; Yu, Y.; Bronstein, J.M.; Keener, A.M.; Ritz, B. Parkinson’s Disease and the Gut Microbiome in Rural California. J. Park. Dis. 2022, 12, 2441–2452. [Google Scholar] [CrossRef]
- Pietrucci, D.; Cerroni, R.; Unida, V.; Farcomeni, A.; Pierantozzi, M.; Mercuri, N.B.; Biocca, S.; Stefani, A.; Desideri, A. Dysbiosis of Gut Microbiota in a Selected Population of Parkinson’s Patients. Park. Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling Gut Microbiota in Parkinson’s Disease and Atypical Parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Künstner, A.; Müller, S.H.; Künzel, S.; Zeuner, K.E.; Margraf, N.G.; Deuschl, G.; Baines, J.F.; Kuhlenbäumer, G. Gut Microbiota in Parkinson Disease in a Northern German Cohort. Brain Res. 2017, 1667, 41–45. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The Nasal and Gut Microbiome in Parkinson’s Disease and Idiopathic Rapid Eye Movement Sleep Behavior Disorder: Nose and Gut Microbiome in PD and IRBD. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Wareham, N.J.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Gut Microbiota in Parkinson’s Disease: Temporal Stability and Relations to Disease Progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).