Depression Severity Is Different in Dysosmic Patients Who Have Experienced Traumatic Brain Injury Compared with Those Who Have Not
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. TBI Experience
2.4. Olfactory Testing
2.5. Questionnaires and Neuropsychological Tests
2.5.1. Cognitive Testing
2.5.2. Affective Testing
2.6. Ethical Issues
2.7. Data Analysis
3. Results
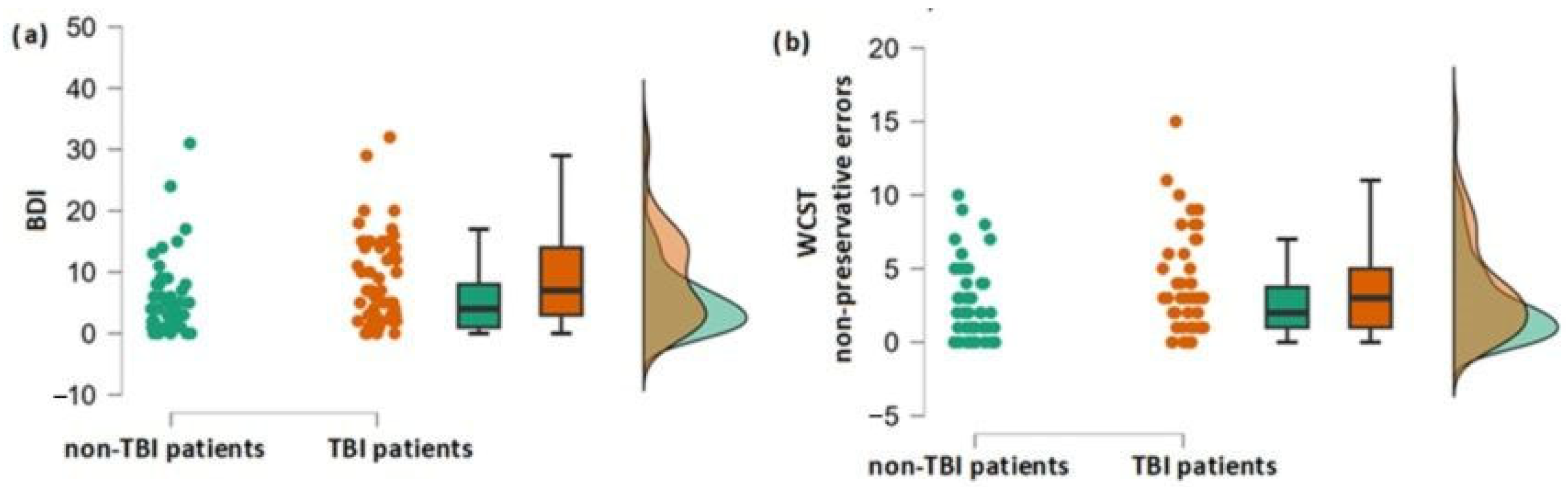
3.1. Differences in Olfactory, Cognitive and Depression Scores between the Group of Patients with and without TBI Experience
3.2. Association between TBI and Cognitive and Affective Performance
3.3. Correlation between the Duration since TBI Occurrence and Depression Severity
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Draper, K.; Ponsford, J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology 2008, 22, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, T.; Steffens, D.C.; Phillips, C.; Helms, M.J.; Havlik, R.J.; Breitner, J.C.; Guralnik, J.M.; Plassman, B.L. Head injury in early adulthood and the lifetime risk of depression. Arch. Gen. Psychiatry 2002, 59, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Feltrin, F.S.; Zaninotto, A.L.; Guirado, V.; Macruz, F.; Sakuno, D.; Dalaqua, M.; Magalhães, L.G.A.; Paiva, W.S.; De Andrade, A.F.; Otaduy, M.C.G.; et al. Longitudinal changes in brain volumetry and cognitive functions after moderate and severe diffuse axonal injury. Brain Inj. 2018, 32, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Goleburn, C.R.; Golden, C.J. Traumatic brain injury outcome in older adults: A critical review of the literature. J. Clin. Geropsychol. 2001, 7, 161–187. [Google Scholar] [CrossRef]
- Schretlen, D.J.; Shapiro, A.M. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int. Rev. Psychiatr. 2003, 15, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Bonnelle, V.; Leech, R.; Kinnunen, K.M.; Ham, T.E.; Beckmann, C.F.; De Boissezon, X.; Greenwood, R.J.; Sharp, D.J. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 2011, 31, 13442–13451. [Google Scholar] [CrossRef]
- Fortin, S.; Godbout, L.; Braun, C.M. Cognitive structure of executive deficits in frontally lesioned head trauma patients performing activities of daily living. Cortex 2003, 39, 273–291. [Google Scholar] [CrossRef]
- Ellis, C.; Peach, R.K. Sentence planning following traumatic brain injury. NeuroRehabilitation 2009, 24, 255–266. [Google Scholar] [CrossRef]
- Shum, D.H.; Harris, D.; O’Gorman, J.G. Effects of severe traumatic brain injury on visual memory. J. Clin. Exp. Neuropsychol. 2000, 22, 25–39. [Google Scholar] [CrossRef]
- Rao, V.; Lyketsos, C.G. Psychiatric aspects of traumatic brain injury. Psychiatr. Clin. 2002, 25, 43–69. [Google Scholar] [CrossRef]
- Fakhoury, M.; Shakkour, Z.; Kobeissy, F.; Lawand, N. Depression following traumatic brain injury: A comprehensive overview. Rev. Neurosci. 2021, 32, 289–303. [Google Scholar] [CrossRef]
- Koponen, S.; Taiminen, T.; Portin, R.; Himanen, L.; Isoniemi, H.; Heinonen, H.; Hinkka, S.; Tenovuo, O. Axis I and II psychiatric disorders after traumatic brain injury: A 30-year follow-up study. Am. J. Psychiatr. 2002, 159, 1315–1321. [Google Scholar] [CrossRef]
- Jorge, R.E.; Robinson, R.G.; Starkstein, S.E.; Arndt, S.V.; Forrester, A.W.; Geisler, F.H. Secondary mania following traumatic brain injury. Am. J. Psychiatr. 1993, 150, 916. [Google Scholar] [CrossRef]
- Jorge, R.E.; Robinson, R.G.; Moser, D.; Tateno, A.; Crespo-Facorro, B.; Arndt, S. Major depression following traumatic brain injury. Arch. Gen. Psychiatry 2004, 61, 42–50. [Google Scholar] [CrossRef]
- Alway, Y.; McKay, A.; Gould, K.R.; Johnston, L.; Ponsford, J. Factors associated with posttraumatic stress disorder following moderate to severe traumatic brain injury: A prospective study. Depress. Anxiety 2016, 33, 19–26. [Google Scholar] [CrossRef]
- Hibbard, M.R.; Uysal, S.; Kepler, K.; Bogdany, J.; Silver, J. Axis I psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 1998, 13, 24–39. [Google Scholar] [CrossRef]
- Doty, R.L.; Yousem, D.M.; Pham, L.T.; Kreshak, A.A.; Geckle, R.; Lee, W.W. Olfactory dysfunction in patients with head trauma. Arch. Neurol. 1997, 54, 1131–1140. [Google Scholar] [CrossRef]
- Han, P.; Winkler, N.; Hummel, C.; Hähner, A.; Gerber, J.; Hummel, T. Alterations of brain gray matter density and olfactory bulb volume in patients with olfactory loss after traumatic brain injury. J. Neurotrauma 2018, 35, 2632–2640. [Google Scholar] [CrossRef]
- Schneider, A.L.; Gottesman, R.F.; Mosley, T.H.; Shrestha, S.; Rowan, N.R.; Sharrett, A.R.; Chen, H.; Kamath, V. Associations of prior head injury with olfaction in older adults: Results from the atherosclerosis risk in communities (ARIC) study. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 840–848. [Google Scholar] [CrossRef]
- Zigrand, C.; Jobin, B.; Lecuyer Giguère, F.; Giguère, J.F.; Boller, B.; Frasnelli, J. Olfactory perception in patients with a mild traumatic brain injury: A longitudinal study. Brain Inj. 2022, 8, 1–6. [Google Scholar] [CrossRef]
- Coelho, D.H.; Costanzo, R.M. Posttraumatic olfactory dysfunction. Auris Nasus Larynx 2016, 43, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Haxel, B.R.; Grant, L.; Mackay-Sim, A. Olfactory dysfunction after head injury. J. Head Trauma Rehabil. 2008, 23, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Christianson, T.J.; Kremers, W.K.; Mielke, M.M.; Machulda, M.M.; Vassilaki, M.; Alhurani, R.E.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016, 73, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Hummel, T. Olfaction as a marker for depression. J. Neurol. 2017, 264, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Deems, D.A.; Doty, R.L.; Settle, R.G.; Moore-Gillon, V.; Shaman, P.; Mester, A.F.; Kimmelman, C.P.; Brightman, V.J.; Snow, J.B. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 519–528. [Google Scholar] [CrossRef]
- Hummel, T.; Nordin, S. Olfactory disorders and their consequences for quality of life. Acta Oto-Laryngol. 2005, 125, 116–121. [Google Scholar] [CrossRef]
- Fang, T.C.; Chang, M.H.; Yang, C.P.; Chen, Y.H.; Lin, C.H. The association of olfactory dysfunction with depression, cognition, and disease severity in Parkinson’s Disease. Front. Neurol. 2021, 12, 779712. [Google Scholar] [CrossRef]
- Pirker-Kees, A.; Platho-Elwischger, K.; Hafner, S.; Redlich, K.; Baumgartner, C. Hyposmia is associated with reduced cognitive function in COVID-19: First preliminary results. Dement. Geriatr. Cogn. Disord. 2021, 50, 68–73. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 1997, 18, 351–357. [Google Scholar] [CrossRef]
- Kovács, T.; Cairns, N.J.; Lantos, P.L. Olfactory centres in Alzheimer’s disease: Olfactory bulb is involved in early Braak’s stages. Neuroreport 2001, 12, 285–288. [Google Scholar] [CrossRef]
- Price, J.L.; Slotnick, B.M.; Revial, M.F. Olfactory projections to the hypothalamus. J. Comp. Neurol. 1991, 306, 447–461. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ sticks’: Olfactory performance assessed by the combined testing of odour identification, odour discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef]
- Hummel, T.; Welge-Luessen, A. Erfassung des Riech-und Schmeckvermögens. In Riech-und Schmeckstörungen; Thieme: Stuttgart, Germany, 2009; pp. 43–59. [Google Scholar]
- Grant, D.A.; Berg, E.A. Wisconsin Card Sorting Test; Psychological Assessment Resources: Odessa, Ukraine, 1993. [Google Scholar]
- Barceló, F. Does the Wisconsin card sorting test measure prefontral function? Span. J. Psychol. 2001, 4, 79–100. [Google Scholar] [CrossRef]
- Miles, S.; Howlett, C.A.; Berryman, C.; Nedeljkovic, M.; Moseley, G.L.; Phillipou, A. Considerations for using the Wisconsin Card Sorting Test to assess cognitive flexibility. Behav. Res. Methods 2021, 53, 2083–2091. [Google Scholar] [CrossRef]
- Benton, A.L.; Hamsher, D.S.; Sivan, A.B. Controlled Oral Word Association Test; AJA Associates: Iowa City, IA, USA, 1994. [Google Scholar]
- Strauss, E.; Sherman, E.M.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary; American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Brickenkamp, R. Test d2: Aufmerksamkeits-Belastungs-Test; Hogrefe: Gottingen, Germany, 1962. [Google Scholar]
- Vanhelst, J.; Béghin, L.; Duhamel, A.; Manios, Y.; Molnar, D.; De Henauw, S.; Moreno, L.A.; Ortega, F.B.; Sjöström, M.; Widhalm, K.; et al. Physical activity is associated with attention capacity in adolescents. J. Pediatr. 2016, 168, 126–131. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Jackson-Koku, G. Beck depression inventory. Occup. Med. 2016, 66, 174–175. [Google Scholar] [CrossRef]
- Berry, D.A. Bayesian clinical trials. Nat. Rev. Drug Discov. 2006, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bittl, J.A.; He, Y. Bayesian analysis: A practical approach to interpret clinical trials and create clinical practice guidelines. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003563. [Google Scholar] [CrossRef] [PubMed]
- Dienes, Z. Using Bayes to get the most out of non-significant results. Front. Psychol. 2014, 5, 781. [Google Scholar] [CrossRef] [PubMed]
- Domurat, A.; Białek, M. Dowodzenie hipotez za pomocą czynnika bayesowskiego (bayes factor): Przykłady użycia w badaniach empirycznych. Decyzje 2016, 26, 109–141. [Google Scholar] [CrossRef]
- Green, A.; Felmingham, K.; Baguley, I.J.; Slewa-Younan, S.; Simpson, S. The clinical utility of the Beck Depression Inventory after traumatic brain injury. Brain Inj. 2001, 15, 1021–1028. [Google Scholar] [CrossRef]
- Homaifar, B.Y.; Brenner, L.A.; Gutierrez, P.; Harwood, J.F.; Thompson, C.; Filley, C.M.; Kelly, J.P.; Adler, L.E. Sensitivity and specificity of the Beck Depression Inventory-II in persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 2009, 90, 652–656. [Google Scholar] [CrossRef]
- Oliveri, L.N.; Awerbuch, A.W.; Jarskog, L.F.; Penn, D.L.; Pinkham, A.; Harvey, P.D. Depression predicts self assessment of social function in both patients with schizophrenia and healthy people. Psychiatry Res. 2020, 284, 112681. [Google Scholar] [CrossRef]
- Kamrava, S.K.; Tavakol, Z.; Talebi, A.; Farhadi, M.; Jalessi, M.; Hosseini, S.F.; Amini, E.; Ben Chen, B.; Hummel, T.; Alizadeh, R. A study of depression, partnership and sexual satisfaction in patients with post-traumatic olfactory disorders. Sci. Rep. 2021, 11, 20218. [Google Scholar] [CrossRef]
- Kreutzer, J.S.; Seel, R.T.; Gourley, E. The prevalence and symptom rates of depression after traumatic brain injury: A comprehensive examination. Brain Inj. 2001, 15, 563–576. [Google Scholar] [CrossRef]
- Dikmen, S.S.; Ross, B.L.; Machamer, J.E.; Temkin, N.R. One year psychosocial outcome in head injury. J. Int. Neuropsychol. Soc. 1995, 1, 67–77. [Google Scholar] [CrossRef]
- Lehtonen, S.; Stringer, A.Y.; Millis, S.; Boake, C.; Englander, J.; Hart, T.; High, W.; Macciocchi, S.; Meythaler, J.; Novack, T.; et al. Neuropsychological outcome and community re-integration following traumatic brain injury: The impact of frontal and non-frontal lesions. Brain Inj. 2005, 19, 239–256. [Google Scholar] [CrossRef]
- Novack, T.A.; Bush, B.A.; Meythaler, J.M.; Canupp, K. Outcome after traumatic brain injury: Pathway analysis of contributions from premorbid, injury severity, and recovery variables. Arch. Phys. Med. Rehabil. 2001, 82, 300–305. [Google Scholar] [CrossRef]
- Oddy, M.; Coughlan, T.; Tyerman, A.; Jenkins, D. Social adjustment after closed head injury: A further follow-up seven years after injury. J. Neurol. Neurosurg. Psychiatr. 1985, 48, 564–568. [Google Scholar] [CrossRef]
- Olver, J.H.; Ponsford, J.L.; Curran, C.A. Outcome following traumatic brain injury: A comparison between 2 and 5 years after injury. Brain Inj. 1996, 10, 841–848. [Google Scholar] [CrossRef]
- Ahmed, S.; Venigalla, H.; Mekala, H.M.; Dar, S.; Hassan, M.; Ayub, S. Traumatic brain injury and neuropsychiatric complications. Indian J. Psychol. Med. 2017, 39, 114–121. [Google Scholar] [CrossRef]
- Hodge, S.D., Jr.; Hubbard, J.E. Depression: The often overlooked sequela of head trauma. Clevland State Law Rev. 2017, 66, 31. [Google Scholar]
- Karol, R.L.; Micka, R.G.; Kuskowski, M. Physical, emotional, and sexual abuse among pain patients and health care providers: Implications for psychologists in multidisciplinary pain treatment centers. Prof. Psychol. Res. Pract. 1992, 23, 480–485. [Google Scholar] [CrossRef]
- Howell, J.; Costanzo, R.M.; Reiter, E.R. Head trauma and olfactory function. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 39–45. [Google Scholar] [CrossRef]
- Ilkiw, J.L.; Kmita, L.C.; Targa, A.D.S.; Noseda, A.C.D.; Rodrigues, L.S.; Dorieux, F.W.C.; Fagotti, J.; dos Santos, P.; Lima, M.M.S. Dopaminergic lesion in the olfactory bulb restores olfaction and induces depressive-like behaviors in a 6-OHDA model of Parkinson’s disease. Mol. Neurobiol. 2019, 56, 1082–1095. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi-Kwon, S. Poststroke depression and emotional incontinence: Correlation with lesion location. Neurology 2000, 54, 1805–1810. [Google Scholar] [CrossRef]
- Taylor, W.D.; Macfall, J.R.; Payne, M.E.; McQuoid, D.R.; Steffens, D.C.; Provenzale, J.M.; Krishnan, K.R.R. Orbitofrontal cortex volume in late life depression: Influence of hyperintense lesions and genetic polymorphisms. Psychol. Med. 2007, 37, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, B.; Graux, J.; El Hage, W.; Hommet, C.; Camus, V.; Belzung, C. Olfaction: A potential cognitive marker of psychiatric disorders. Neurosci. Biobehav. Rev. 2008, 32, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Sabiniewicz, A.; Hoffmann, L.; Haehner, A.; Hummel, T. Symptoms of depression change with olfactory function. Sci. Rep. 2022, 12, 5656. [Google Scholar] [CrossRef] [PubMed]
- Caeyenberghs, K.; Leemans, A.; Heitger, M.H.; Leunissen, I.; Dhollander, T.; Sunaert, S.; Dupont, P.; Swinnen, S.P. Graph analysis of functional brain networks for cognitive control of action in traumatic brain injury. Brain 2012, 135, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.; Kraus, M.F. The frontal lobes and traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 1994, 6, 443–454. [Google Scholar]
- Godefroy, O. Frontal syndrome and disorders of executive functions. J. Neurol. 2003, 250, 1–6. [Google Scholar] [CrossRef]
- Owens, J.A.; Spitz, G.; Ponsford, J.L.; Dymowski, A.R.; Willmott, C. An investigation of white matter integrity and attention deficits following traumatic brain injury. Brain Inj. 2018, 32, 776–783. [Google Scholar] [CrossRef]
- Barceló, F.; Knight, R.T. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia 2002, 40, 349–356. [Google Scholar] [CrossRef]
- Windon, M.J.; Kim, S.J.; Oh, E.S.; Lin, S.Y. Predictive value of olfactory impairment for cognitive decline among cognitively normal adults. Laryngoscope 2020, 130, 840–847. [Google Scholar] [CrossRef]
- Wang, Q.; Ben Chen, B.; Zhong, X.; Zhou, H.; Zhang, M.; Mai, N.; Wu, Z.; Huang, X.; Haehner, A.; Chen, X.; et al. Olfactory dysfunction is already present with subjective cognitive decline and deepens with disease severity in the Alzheimer’s disease spectrum. J. Alzheimer’s Dis. 2021, 79, 585–595. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Marshall, S.W.; Bailes, J. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sport. Exerc. 2007, 39, 903–909. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness: A practical scale. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Fraser, E.E.; Downing, M.G.; Biernacki, K.; McKenzie, D.P.; Ponsford, J.L. Cognitive reserve and age predict cognitive recovery after mild to severe traumatic brain injury. J. Neurotrauma 2019, 36, 2753–2761. [Google Scholar] [CrossRef]
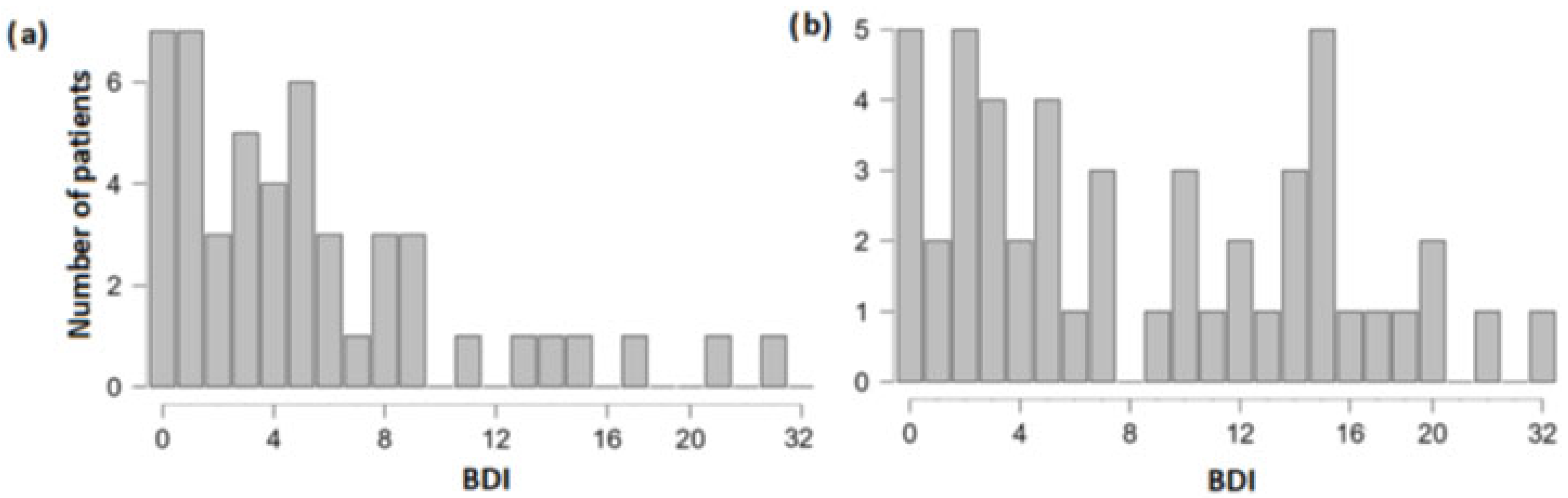
| Women | Men | Total | |
|---|---|---|---|
| Number of patients | 38 | 63 | 101 |
| Age (M ± SD) in years | 51.7 ± 15.3 | 52.6 ± 4.8 | 52.3 ± 14.9 |
| Group of TBI patients (n = 51) | Control group (n = 50) | ||
| Men % | 65% | 60% | |
| Age (M ± SD) | 51.9 ± 14.9 | 52.6 ± 15.1 | |
| Anosmic patients % | 51% | 44% | |
| Cause of olfactory loss | |||
| Traumatic brain injury | N = 51 | - | |
| Chronic rhinosinusitis | - | N = 11 | |
| Congenital anosmia | - | N = 3 | |
| Postinfectious olfactory loss | - | N = 13 | |
| Idiopathic olfactory dysfunction | - | N = 21 | |
| Parkinson disease | - | N = 2 | |
| Type of TBI | unspecific (33%); occipital (26%); frontal (18%); polytrauma (2%); no information available (22%) | - | |
| Cause of TBI | accident (29%); fall (24%); fight (2%); other (12%); no information available (33%) | - | |
| Test Name | TBI Patients | Control Patients | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Olfactory tests | ||||
| T | 2.6 | 2.6 | 3 | 2.9 |
| D | 8 | 2.7 | 7.9 | 3.6 |
| I | 6 | 3 | 7 | 4 |
| TDI | 16.7 | 7.1 | 17.9 | 8.3 |
| Cognitive tests | ||||
| d2II TN | 148.6 | 29 | 144.1 | 42 |
| d2II O | 22.2 | 20.7 | 17.7 | 19.8 |
| d2II C | 5.5 | 15.2 | 2.8 | 3 |
| d2II CP | 120.8 | 39.2 | 123.6 | 129.4 |
| WCST PE | 7.3 | 3.2 | 7.5 | 2.3 |
| WCST NPE | 3.6 | 3.4 | 2.5 | 2.5 |
| COWA T | 60.8 | 15.2 | 66.4 | 15.1 |
| TMT A | 35.6 | 20.6 | 32.4 | 12.7 |
| Affective test | ||||
| BDI | 8.9 | 7.5 | 5.6 | 6.2 |
| Outcome | Cohen’s d | t | p | df |
|---|---|---|---|---|
| BDI | −0.47 | 2.3 * | 0.011 | 96 |
| COWA TW | 0.37 | 1.8 | 0.958 | 89 |
| WCST PE | 0.05 | 0.3 | 0.599 | 98 |
| WCST NPE | 0.38 | 1.9 | 0.029 | 98 |
| TMT A | −0.189 | 1 | 0.173 | 99 |
| D2 TN | −0.12 | 0.6 | 0.268 | 99 |
| D2 O | −0.22 | 1.1 | 0.132 | 99 |
| D2 C | −0.24 | 1.2 | 0.112 | 99 |
| D2 CP | 0.07 | 0.4 | 0.639 | 99 |
| TDI | 0.15 | 0.8 | 0.421 | 99 |
| T | 0.16 | 0.9 | 0.828 | 99 |
| D | −0.04 | 0.2 | 0.19 | 99 |
| I | 0.26 | 1.3 | 0.453 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabiniewicz, A.; Lindner, K.-K.; Haehner, A.; Hummel, T. Depression Severity Is Different in Dysosmic Patients Who Have Experienced Traumatic Brain Injury Compared with Those Who Have Not. Neurol. Int. 2023, 15, 638-648. https://doi.org/10.3390/neurolint15020040
Sabiniewicz A, Lindner K-K, Haehner A, Hummel T. Depression Severity Is Different in Dysosmic Patients Who Have Experienced Traumatic Brain Injury Compared with Those Who Have Not. Neurology International. 2023; 15(2):638-648. https://doi.org/10.3390/neurolint15020040
Chicago/Turabian StyleSabiniewicz, Agnieszka, Kyri-Kristin Lindner, Antje Haehner, and Thomas Hummel. 2023. "Depression Severity Is Different in Dysosmic Patients Who Have Experienced Traumatic Brain Injury Compared with Those Who Have Not" Neurology International 15, no. 2: 638-648. https://doi.org/10.3390/neurolint15020040
APA StyleSabiniewicz, A., Lindner, K.-K., Haehner, A., & Hummel, T. (2023). Depression Severity Is Different in Dysosmic Patients Who Have Experienced Traumatic Brain Injury Compared with Those Who Have Not. Neurology International, 15(2), 638-648. https://doi.org/10.3390/neurolint15020040






