Copeptin Implementation on Stroke Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
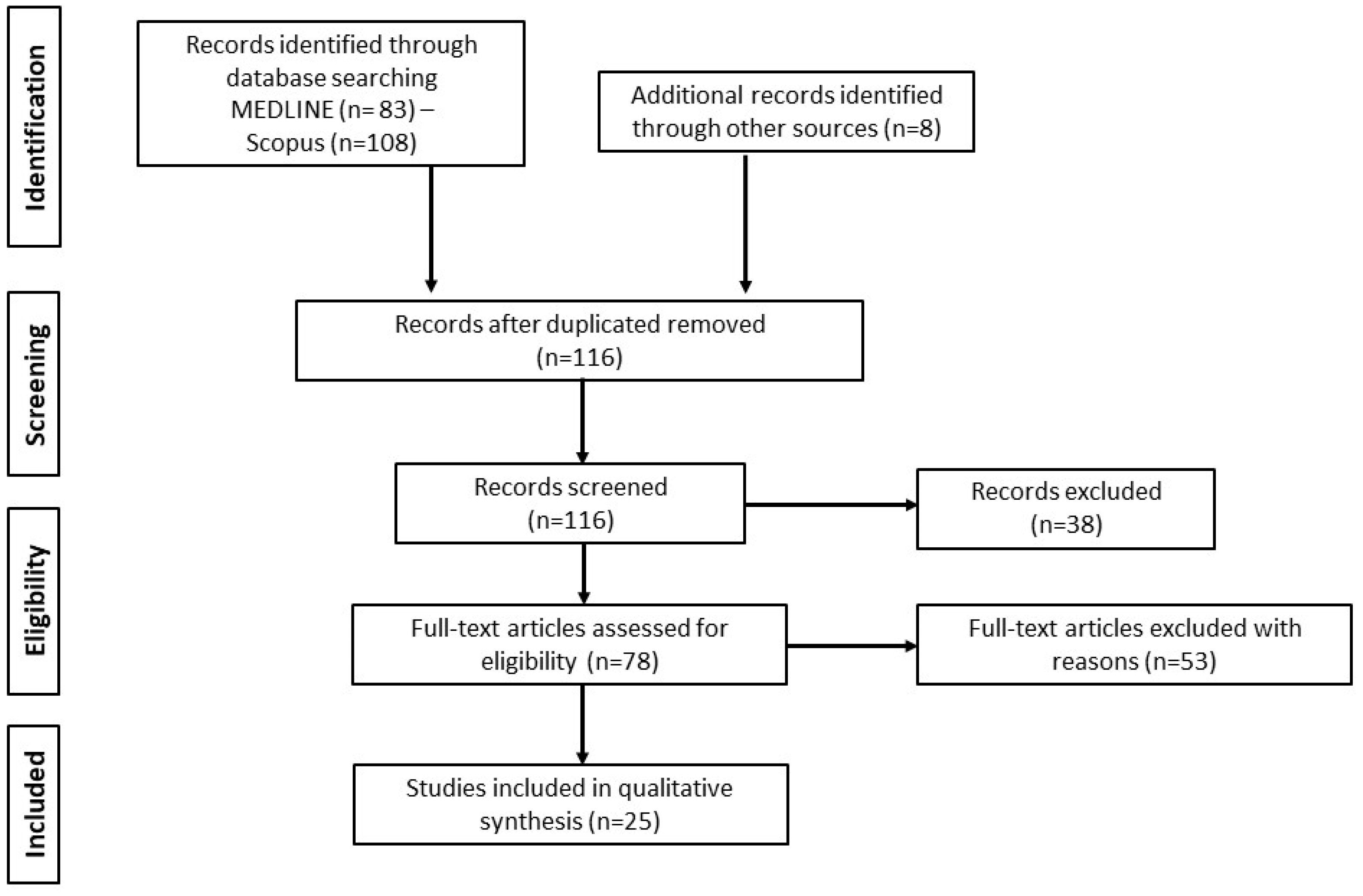
3.1. Database Searches
3.2. Study Characteristics
3.3. Stroke Patient Groups and Demographic Profile
3.4. Reference Groups
3.5. Time of Blood Sampling
3.6. Scales of Stroke Severity and Prognosis/Clinical Outcome
| Authors, Year of Publication | Type of Study | Number of Participants /Mean or Median Age | Time of Copeptin Measurement | Follow-Up Time | Assessment Stroke Scales | Cutoff Values; (Specificity); [Sensitivity] | Main Results | |
|---|---|---|---|---|---|---|---|---|
| Ischemic Stroke (IS) | ||||||||
| 1. | DeMarchis et al., 2013 [27] | Longitudinal | 783 patients/median age: 71 (60.5–80) | Within 24 h from symptom onset | 3 months | NIHSS (on admission) mRS (at 3 months) | Copeptin may act as an independent predictor of both unfavorable functional outcome and mortality at three months following stroke, as well as accurately forecast the development of in-hospital complications, providing additional valuable prognostic information | |
| 2. | Perovic et al., 2017 [28] | Longitudinal | 109 patients/median age: 78 (69–84), 63 controls/median age: 75 (70–77) | Within 24 h frοm symptom onset | At discharge (median in-hospital stay 10 days) | mNIHSS (on admission) BI (at discharge) | Copeptin concentrations early after stroke onset were negatively correlated with functional outcome at discharge | |
| 3. | Tu et al., 2017 [34] | Longitudinal | 4215 patients | Within 48 h from symptom onset | 1 month, 6 months, 1 year | NIHSS (on admission) | Elevated plasma copeptin levels were strongly associated with the group of non-survivors, supporting the utility of copeptin as an independent indicator of stroke-related mortality | |
| 4. | Wang et al., 2016 [37] | Longitudinal | 247 patients/median age: 65 (54–77) | Within 48 h from symptom onset | 3 months | NIHSS (on admission) mRS (at 3 months) | For unfavorable functional outcome: 15.4 pmol/L; (84.6%); [62.8%] | Baseline copeptin levels were found to be strongly correlated not only with unfavorable functional outcome but also with mortality, independently of NIHSS and other known risk factors in IS patients diagnosed with type 2 diabetes mellitus |
| 5. | Zhang et al., 2013 [35] | Longitudinal | 245 patients/mean age: 72 ± 11, 100 controls | Within 72 h from symptom onset | 1 year | NIHSS (on admission) mRS (at 1 year) | For 1 year mortality: 12.55 pM | Significantly higher copeptin levels on admission were detected among patients with poor functional outcomes and non-survivors following an IS. Copeptin evaluation may increase the prognostic ability of the established clinical score |
| 6. | Dong et al., 2013 [29] | Longitudinal | 125 patients/median age: 69 (61–85), 100 controls | Within 48 h from symptom onset | 3 months | NIHSS (on admission) mRS (at 3 months) | Elevated baseline copeptin concentrations were coupled with increased severity of stroke and were accompanied by both an unfavorable functional outcome and higher mortality risk at 3 months poststroke | |
| 7. | Hotter et al., 2020 [38] | Longitudinal | 573 patients/mean age: 72.1 ± 12.2 | Within the first 4 days of admission | 3 months | NIHSS (on admission) mRS (at 3 months) | For SAP: 6.2 μg/L; (30%); [96%] | Copeptin has the potential to independently predict the development of pneumonia during hospitalization, as well as reliably provide an estimation of the functional outcome at 3- months poststroke. However, the added prognostic value of copeptin was found to be limited, while no correlation was demonstrated between plasma copeptin level and mortality |
| 8. | Spagnolello et al., 2019 [30] | Longitudinal | 34 patients/mean age: 70.5 ± 16.8 | at baseline at 24 h between third and fifth day from admission | 1 year | NIHSS (on admission) mRS (at 1 year) | Plasma copeptin levels at 24 h were strongly correlated with poor outcome and mortality at 1-year poststroke, potentially related to brain edema or hemorrhagic transformation. The copeptin’s decremental course within 24 h poststroke was found significantly steeper in patients undergoing combined recanalization strategies | |
| 9. | Wang et al., 2014 [36] | Longitudinal | 285 patients/median age: 68 (60–79), 100 controls/median age: 68 9 (60–79) | On the first day of admission | 1 year | NIHSS (on admission) mRS (at 1 year) | For mortality: 20.5 pmol/L; (84.5%); [90.7%] | Copeptin measurement might add valuable predictive information beyond stroke severity and reliably forecast 1- year mortality in patients presenting with IS |
| 10. | Tu et al., 2013 [31] | Longitudinal | 189 patients/median age: 66 (58–75), 200 controls | Within 48 h from symptom onset | 3 months | NIHSS (on admission) mRS (at 3 months) | Early measurement of plasma copeptin levels may serve as an independent prognostic outcome predictor with the greatest prognostic potential among the biomarkers under research. A biomarker panel including copeptin might accurately predict unfavorable outcome at 90 days poststroke | |
| 11. | Hotter et al., 2019 [33] | Longitudinal | 91 patients/mean age: 68.0 ± 10.5 | Within the first 4 days of admission | 3 months | NIHSS (on admission) mRS (at 3 months) | Copeptin evaluation was significantly associated with functional outcome at 90 days poststroke, thus ultrasensitive copeptin may add useful prognostic information after stroke | |
| 12. | Oraby et al., 2021 [32] | Longitudinal | 45 patients/mean age: 55.2 ± 13.8, 45 controls/mean age: 51.13 ± 13.4 | Within 24 h from symptom onset | 3 months | NIHSS (on admission) mRS (at 3 months) | For unfavorable outcome: 125.30 pg/mL; (84.4%); [62.2%] | Elevated copeptin levels were highly correlated with a more severe stroke, as well as poor short-term functional outcome at 3 months. Lower copeptin concentrations were found in the group of patients undergoing thrombolytic therapies |
| Transient ischemic attack (TIA) | ||||||||
| 13. | Pedersen et al., 2019 [42] | Longitudinal | 114 patients/median age: 66.3 (54.5–71.9) | Within 24 h from symptom onset | Median cardiac monitoring time: 2.2 years | N/A | Copeptin was of limited value in forecasting AF among TIA patients | |
| 14. | De Marchis et al., 2014 [41] | Longitudinal | 302 patients/median age: 69 (59–78) | Within 24 h from symptom onset | 3 months | N/A | For stroke after TIA: 1.88 pmol/L; (12%); [100%] 53.50 pmol/L; (90%); [27%] | Plasma baseline copeptin levels were strongly correlated with recurrent stroke but not TIA within 3 months after the index TIA. Copeptin assessment seems to improve the discriminatory accuracy of ABCD2 score |
| 15. | Purroy et al., 2016 [40] | Longitudinal | 237 patients | Within 24 h from symptom onset | 7 days, 3 months | mRS (at baseline) | For stroke recurrence: 13.8 pmol/L had a great negative prognostic value (97.4%). Prognostic accuracy was 66.7%. | Abnormally high copeptin concentrations 24 h after TIA symptom onset appears to be indicative of recurrent stroke at 7 days follow-up, but not at 3 months |
| 16. | Griesenegger et al., 2015 [39] | Longitudinal | 1076 patients/median age: 75 (66–83), 401 controls | within 5 days from symptom onset at 1 year | Median follow up time: 5, 7 years | N/A | In patients with TIA and ischemic stroke, copeptin was highly predictive of recurrent vascular events and death, especially after TIA or stroke of cardioembolic source | |
| Intracerebral hemorrhage (ICH) | ||||||||
| 17. | Yu et al., 2014 [43] | Longitudinal | 118 patients/mean age: 64.1 ± 9.1, 118 controls/mean age: 62.3 ± 7.8 | Within 6 h from symptom onset | 6 months | NIHSS (on admission) mRS (at 6 months) | For mortality: 2518.2 pg/mL; (74.1%); [78.4%] For unfavorable outcome: 2369.1 pg/mL; (82.0%); [70.6%] | Significantly higher copeptin concentrations were found on admission among non-survivors and patients with poor functional outcome within 6 months following ICH. Only copeptin has the potential to improve the predictive performance of NIHSS scale |
| 18. | Zhang et al., 2013 [45] | Longitudinal | 120 patients/mean age: 60 ± 14, 60 controls | On admission | 3 months | ICH Score (on admission) mRS (at 3 months) | Elevated copeptin concentrations were observed among ICH patients with impaired nerve function and unfavorable functional outcome at 90 days following hemorrhage | |
| 19. | Zhang et al., 2012 [44] | Longitudinal | 89 patients/mean age: 64.5 ± 10.9, 50 controls | On admission | 1 year | NIHSS (on admission) mRS (at 1 year) | For mortality: >23.8 pmol/L; (70.6%); [81.6%] For unfavourable outcome: >23.5 pmol/L; (87.9%); [76.8%] For END: >26.3 pmol/L; (73.1%); [81.8] | Increased plasma copeptin level may serve as an independent prognostic marker of 1- year mortality, 1-year unfavorable outcome, and early neurological deterioration after ICH, but it does not improve significantly the predictive value of NIHSS score |
| 20. | Yang et al., 2021 [47] | Longitudinal | 156 patients/mean age: 45.06 ± 9.78 | Within 24 h of admission | 3 months | MICH score (on admission) mRS (at 3 months) | Baseline plasma copeptin levels were markedly higher within the non- survivors group accompanied by the copeptin concentrations among the ICH patients with poor functional outcome at 3-month follow up | |
| 21. | Wei et al., 2014 [46] | Longitudinal | 271 patients/median age: 69 (59–81), 200 healthy controls/ median age: 69 (58–80) | Within 48 h from symptom onset | 3 months | ICH score (on admission) mRS (at 3 months) | Increased copeptin levels were found within ICH population with poor prognosis and non- survivors, suggesting the role of copeptin as an independent marker of functional outcome and death at 3-month follow- up | |
| Subarachnoid hemorrhage (SAH) | ||||||||
| 22. | Fung et al., 2013 [48] | Longitudinal | 18 patients/median age: 57 (48–67) | On admission | 6 months | WFNS (on admission) mRS (at 6 months) | Circulating copeptin levels were found to be strongly correlated with SAH severity, as assessed by the WFNS scale. Copeptin seems to have an interesting prognostic potential regarding functional outcomes at 6 months, as it tended to be higher among patients with poor prognosis | |
| 23. | Zuo et al., 2019 [50] | Longitudinal | 243 patients/median age: 58 (49–69) | Within 48 h from symptom onset | 3 months | WFNS (on admission) Glasgow outcome scale (at 3 months) | For poor outcome: 24.0 pmol/L; (69.6%); [70.5%] | Copeptin evaluation may serve as an independent marker of short-term prognosis after SAH, with elevated copeptin concentrations being detected among non-survivors and SAH patients with poor functional outcome at 3 months. The prognostic accuracy was in the range of WFNS scale |
| 24. | Rhim et al., 2021 [51] | Longitudinal | 86 patients | Consecutive measurements every 2 days from day 1 until day 13 | 13 days | N/A | Elevated copeptin concentrations stand for a significant risk factor for delayed cerebral ischemia (DCI) occurrence throughout SAH clinical course, enabling a better risk stratification for SAH patients | |
| 25. | Zheng et al., 2017 [49] | Longitudinal | 105 patients/median age: 52 (37–60) | On admission | 6 months | WFNS (on admission) Glasgow Outcome Scale (at 6 months) | Copeptin levels were associated with WFNS scale scores, reflecting SAH severity. SAH patients with an unfavorable 6-month clinical outcome, as well as patients developing symptomatic cerebral vasospasm carried higher copeptin levels on admission | |
4. Discussion
4.1. Ischemic Stroke
4.2. Transient Ischemic Attack
4.3. Intracerebral Hemorrhage
4.4. Subarachnoid Hemorrhage
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.; Mensah, G.; Johnson, C.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2019, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.; Donnan, G.; Hennerici, M. Classification of stroke subtypes. Cerebrovasc. Dis. 2009, 27, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.J.; Singh, S.; Lees, K.R.; Bath, P.M.; Myint, P.K. VISTA Collaborators. Validating and comparing stroke prognosis scales. Neurology 2017, 89, 997–1002. [Google Scholar] [CrossRef]
- Karatzetzou, S.; Tsiptsios, D.; Terzoudi, A.; Aggeloussis, N.; Vadikolias, K. Transcranial magnetic stimulation implementation on stroke prognosis. Neurol. Sci. 2022, 43, 873–888. [Google Scholar] [CrossRef]
- Saposnik, G.; Guzik, A.; Reeves, M.; Ovbiagele, B.; Johnston, S. Stroke Prognostication using Age and NIH Stroke Scale: SPAN-100. Neurology 2013, 80, 21–28. [Google Scholar] [CrossRef]
- Wardlaw, J.; Brazzelli, M.; Miranda, H.; Schuler, K.; Sandercock, P.A.G.; Dennis, M.S. ABCD2 score and risk of stroke after transient ischaemic attack and minor stroke. In An Assessment of the Cost-Effectiveness of Magnetic Resonance, Including Diffusion-Weighted Imaging, in Patients with Transient Ischemic Attack and Minor Stroke: A Systematic Review, Meta-Analysis and Economic Evaluation; NIHR Journals Library, Health Technology Assessment: Southampton, UK, 2014; p. 18. [Google Scholar]
- Witsch, J.; Siegerink, B.; Nolte, C.; Sprügel, M. Prognostication after intracerebral hemorrhage: A review. Neurol. Res. Pract. 2021, 3, 22. [Google Scholar] [CrossRef]
- Hunt, W.; Hess, R. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J. Neurosurg. 1968, 28, 14–20. [Google Scholar] [CrossRef]
- Drake, C. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage scale. J. Neurosurg. 1988, 68, 985–986. [Google Scholar] [CrossRef]
- Counsell, C.; Dennis, M.; McDowall, M. Predicting functional outcome in acute stroke: Comparison of a simple six variable model with other predictive systems and informal clinical prediction. J. Neurol. Neurosurg. Psychiatry 2004, 75, 401–405. [Google Scholar] [CrossRef]
- Christidi, F.; Tsiptsios, D.; Sousanidou, A.; Karamanidis, S.; Kitmeridou, S.; Karatzetzou, S.; Aitsidou, S.; Tsamakis, K.; Psatha, E.A.; Karavasilis, E.; et al. The Clinical Utility of Leukoaraiosis as a Prognostic Indicator in Ischemic Stroke Patients. Neurol. Int. 2022, 14, 952–980. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.D.; Rost, N.; Leung, T.; Montaner, J.; Muir, K.W.; Brown, S.; Arenillas, J.F.; Feldmann, E.; Liebeskind, D.S. Principles of precision medicine in stroke. J. Neurol. Neurosurg. Psychiatry 2017, 88, 54–61. [Google Scholar] [CrossRef]
- Jauch, E.; Barreto, A.; Broderick, J.; Char, D.; Cucchiara, B.L.; Devlin, T.G. Biomarkers of Acute Stroke Etiology (BASE) study methodology. Transl. Stroke Res. 2017, 8, 424–428. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration: Silver Spring, MD, USA, 2016. [Google Scholar]
- Gkantzios, A.; Tsiptsios, D.; Karatzetzou, S.; Kitmeridou, S.; Karapepera, V.; Giannakou, E.; Vlotinou, P.; Aggelousis, N.; Vadikolias, K. Stroke and Emerging Blood Biomarkers: A Clinical Prospective. Neurol. Int. 2022, 14, 784–803. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Qualifying Biomarkers. In Emerging Safety Science: Workshop Summary; National Academies Press: Washington, DC, USA, 2008; pp. 65–73. [Google Scholar]
- Soldozy, S.; Yağmurlu, K.; Norat, P.; Elsarrag, M.; Costello, J.; Farzad, F.; Sokolowski, J.D.; Sharifi, K.A.; Elarjani, T.; Burks, J.; et al. Biomarkers Predictive of Long-Term Outcome After Ischemic Stroke: A Meta-Analysis. World Neurosurg. 2021, 163, e1–e42. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, K.; Schmidt, R.; Mossner, R.; Daffertshofer, M.; Hennerici, M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke 1994, 25, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Sharman, A.; Low, J. Vasopressin and its role in critical care. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 134–137. [Google Scholar] [CrossRef]
- Bankir, L.; Bichet, D.; Morgenthaler, N. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef]
- Katan, M.; Christ-Crain, M. The stress hormone copeptin: A new prognostic biomarker in acute illness. Swiss Med. Wkly 2010, 140, w13101. [Google Scholar] [CrossRef]
- Struck, J.; Morgenthaler, N.; Bergmann, A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides 2005, 26, 2500–2504. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Fluri, F.; Morgenthaler, N.G.; Schuetz, P.; Zweifel, C.; Bingisser, R.; Muller, K.; Meckel, S.; Gass, A.; Kappos, L.; et al. Copeptin: A novel, independent prognostic marker in patients with ischemic stroke. Ann. Neurol. 2009, 66, 799–808. [Google Scholar] [CrossRef]
- Xu, Q.; Tian, Y.; Peng, H.; Li, H. Copeptin as a biomarker for prediction of prognosis of acute ischemic stroke and transient ischemic attack: A meta-analysis. Hypertens. Res. 2016, 40, 465–471. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, G.; Katan, M.; Weck, A.; Fluri, F.; Foerch, C.; Findling, O.; Schuetz, P.; Buhl, D.; El-Koussy, M.; Gensicke, H.; et al. Copeptin adds prognostic information after ischemic stroke: Results from the CoRisk study. Neurology 2013, 80, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Perovic, E.; Mrdjen, A.; Harapin, M.; Tesija Kuna, A.; Simundic, A.M. Diagnostic and prognostic role of resistin and copeptin in acute ischemic stroke. Top Stroke Rehabil. 2017, 24, 614618. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tao, D.; Wang, Y.; Cao, H.; Xu, Y.-S.; Wang, Q.-Y. Plasma copeptin levels in Chinese patients with acute ischemic stroke: A preliminary study. Neurol. Sci. 2013, 34, 1591–1595. [Google Scholar] [CrossRef]
- Spagnolello, O.; De Michele, M.; Lorenzano, S.; Cerulli Irelli, E.; Naitana, F.; Falcou, A.; Letteri, F.; Bachetoni, A.; Collepardo, D.; Bertazzoni, G.; et al. Copeptin Kinetics in Acute Ischemic Stroke May Differ According to Revascularization Strategies: Pilot Data. Stroke 2019, 50, 3632–3635. [Google Scholar] [CrossRef]
- Tu, W.; Dong, X.; Zhao, S.; Yang, D.; Chen, H. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J. Neuroendocrinol. 2013, 25, 771–778. [Google Scholar] [CrossRef]
- Oraby, M.; Soliman, R.; Elkareem, R.; Mohammed, A. Copeptin: A potential blood biomarker for acute ischemic stroke. Egypt J. Neurol. Psychiatry Neurosurg. 2021, 57, 140. [Google Scholar] [CrossRef]
- Hotter, B.; Hoffmann, S.; Ulm, L.; Meisel, C.; Fiebach, J.B.; Meisel, A. IL-6 Plasma Levels Correlate With Cerebral Perfusion Deficits and Infarct Sizes in Stroke Patients Without Associated Infections. Front. Neurol. 2019, 10, 83. [Google Scholar] [CrossRef]
- Tu, W.; Ma, G.; Ni, Y.; Hu, X.-S.; Luo, D.-Z.; Zeng, X.-W.; Liu, Q.; Xu, T.; Yu, L.; Wu, B. Copeptin and NT-proBNP for prediction of all-cause and cardiovascular death in ischemic stroke. Neurology 2017, 88, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, C.; Zhang, Y.; Zhao, L.-B.; Fu, H.-J.; Feng, J.-C. Plasma copeptin and long-term outcomes in acute ischemic stroke. Acta Neurol. Scand. 2013, 128, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zhang, Y.; Li, Q.; Guo, S.-X.; Ji, S.-B. Plasma levels of copeptin predict 1-year mortality in patients with acute ischemic stroke. Neuroreport 2014, 25, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, M.; Lu, S.; Tian, Z. Plasma copeptin and functional outcome in patients with ischemic stroke and type 2 diabetes. J. Diabetes Complicat. 2016, 30, 1532–1536. [Google Scholar] [CrossRef]
- Hotter, B.; Hoffmann, S.; Ulm, L.; Montaner, J.; Bustamante, A.; Meisel, C.; Meisel, A. Inflammatory and stress markers predicting pneumonia, outcome, and etiology in patients with stroke: Biomarkers for predicting pneumonia, functional outcome, and death after stroke. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e692. [Google Scholar] [CrossRef]
- Greisenegger, S.; Segal, H.; Burgess, A.; Poole, D.; Mehta, Z.; Rothwell, P.M. Copeptin and long-term risk of recurrent vascular events after transient ischemic attack and ischemic stroke: Population-based study. Stroke 2015, 46, 3117–3123. [Google Scholar] [CrossRef]
- Purroy, F.; Suárez-Luis, I.; Cambray, S.; Farré, J.; Benabdelhak, I.; Mauri-Capdevila, G.; Sanahuja, J.; Quílez, A.; Begué, R.; Gil, M.I.; et al. The determination of copeptin levels helps management decisions among transient ischaemic attack patients. Acta Neurol. Scand. 2016, 134, 140–147. [Google Scholar] [CrossRef]
- De Marchis, G.; Weck, A.; Audebert, H.; Benik, S.; Foerch, C.; Buhl, D.; Schuetz, P.; Jung, S.; Seiler, M.; Morgenthaler, N.G.; et al. Copeptin for the prediction of recurrent cerebrovascular events after transient ischemic attack: Results from the CoRisk study. Stroke 2014, 45, 2918–2923. [Google Scholar] [CrossRef]
- Pedersen, K.; Madsen, C.; Sandgaard, N.; Diederichsen, A.C.P.; Bak, S.; Nybo, M.; Brandes, A. Predictive Markers of Atrial Fibrillation in Patients with Transient Ischemic Attack. J. Stroke Cerebrovasc. Dis. 2020, 29, 104643. [Google Scholar] [CrossRef]
- Yu, W.; Wang, W.; Dong, X.; Du, Q.; Yang, D.-B.; Shen, Y.-F.; Wang, H.; Zhang, Z.-Y.; Zhang, Q.; Zhu, Q.; et al. Prognostic significance of plasma copeptin detection compared with multiple biomarkers in intracerebral hemorrhage. Clin. Chim. Acta 2014, 33, 174–178. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Huang, L.; Ye, H. Copeptin is associated with one-year mortality and functional outcome in patients with acute spontaneous basal ganglia hemorrhage. Peptides 2012, 33, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Li, J.; Li, X.; Li, H. The prognostic value of copeptin for acute intracerebral hemorrhage patients. Exp. Ther. Med. 2013, 5, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Ou, Y.; Li, X.; Li, H. The 90-day prognostic value of copeptin in acute intracerebral hemorrhage. Neurol. Sci. 2014, 35, 1673–1679. [Google Scholar] [CrossRef]
- Yang, G.; Li, Z.; Zhang, P.; Sun, K.; Xu, X.; Cheng, X.; Li, N.; He, X. The evaluation value of serum GFAP, CRP, and copeptin combined with mich score for the prognosis of patients with spontaneous cerebral hemorrhage. Acta Med. Mediterr. 2021, 37, 1611–1615. [Google Scholar] [CrossRef]
- Fung, C.; De Marchis, G.; Katan, M.; Seiler, M.; Arnold, M.; Gralla, J.; Raabe, A.; Beck, J. Copeptin as a marker for severity and prognosis of aneurysmal subarachnoid hemorrhage. PLoS ONE 2013, 8, e53191. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, X.; Du, Q.; Wang, H.; Yang, D.-B.; Zhu, Q.; Che, Z.-H.; Shen, Y.-F.; Jiang, L.; Hu, W.; et al. Comparison of plasma copeptin and multiple biomarkers for assessing prognosis of patients with aneurysmal subarachnoid hemorrhage. Clin. Chim. Acta 2017, 475, 64–69. [Google Scholar] [CrossRef]
- Zuo, Z.; Ji, X. Prognostic value of copeptin in patients with aneurysmal subarachnoid hemorrhage. J. Neuroimmunol. 2019, 330, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.; Youn, D.; Kim, B.; Kim, Y.; Kim, S.; Kim, H.; Jeon, J. The Role of Consecutive Plasma Copeptin Levels in the Screening of Delayed Cerebral Ischemia in Poor-Grade Subarachnoid Hemorrhage. Life 2021, 11, 274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatzetzou, S.; Tsiptsios, D.; Sousanidou, A.; Fotiadou, S.; Christidi, F.; Kokkotis, C.; Gkantzios, A.; Stefas, E.; Vlotinou, P.; Kaltsatou, A.; et al. Copeptin Implementation on Stroke Prognosis. Neurol. Int. 2023, 15, 83-99. https://doi.org/10.3390/neurolint15010008
Karatzetzou S, Tsiptsios D, Sousanidou A, Fotiadou S, Christidi F, Kokkotis C, Gkantzios A, Stefas E, Vlotinou P, Kaltsatou A, et al. Copeptin Implementation on Stroke Prognosis. Neurology International. 2023; 15(1):83-99. https://doi.org/10.3390/neurolint15010008
Chicago/Turabian StyleKaratzetzou, Stella, Dimitrios Tsiptsios, Anastasia Sousanidou, Styliani Fotiadou, Foteini Christidi, Christos Kokkotis, Aimilios Gkantzios, Eleftherios Stefas, Pinelopi Vlotinou, Antonia Kaltsatou, and et al. 2023. "Copeptin Implementation on Stroke Prognosis" Neurology International 15, no. 1: 83-99. https://doi.org/10.3390/neurolint15010008
APA StyleKaratzetzou, S., Tsiptsios, D., Sousanidou, A., Fotiadou, S., Christidi, F., Kokkotis, C., Gkantzios, A., Stefas, E., Vlotinou, P., Kaltsatou, A., Aggelousis, N., & Vadikolias, K. (2023). Copeptin Implementation on Stroke Prognosis. Neurology International, 15(1), 83-99. https://doi.org/10.3390/neurolint15010008










