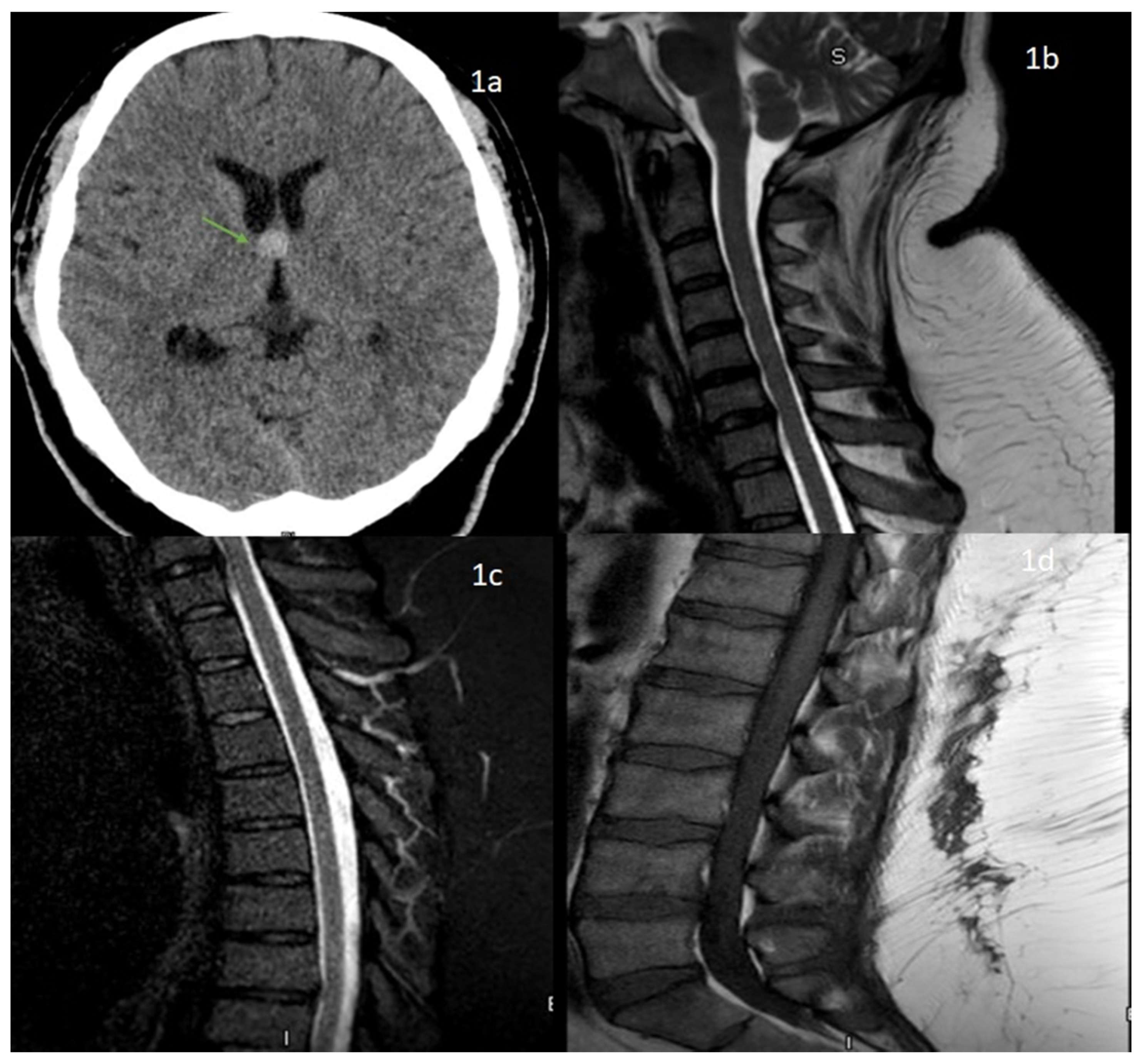
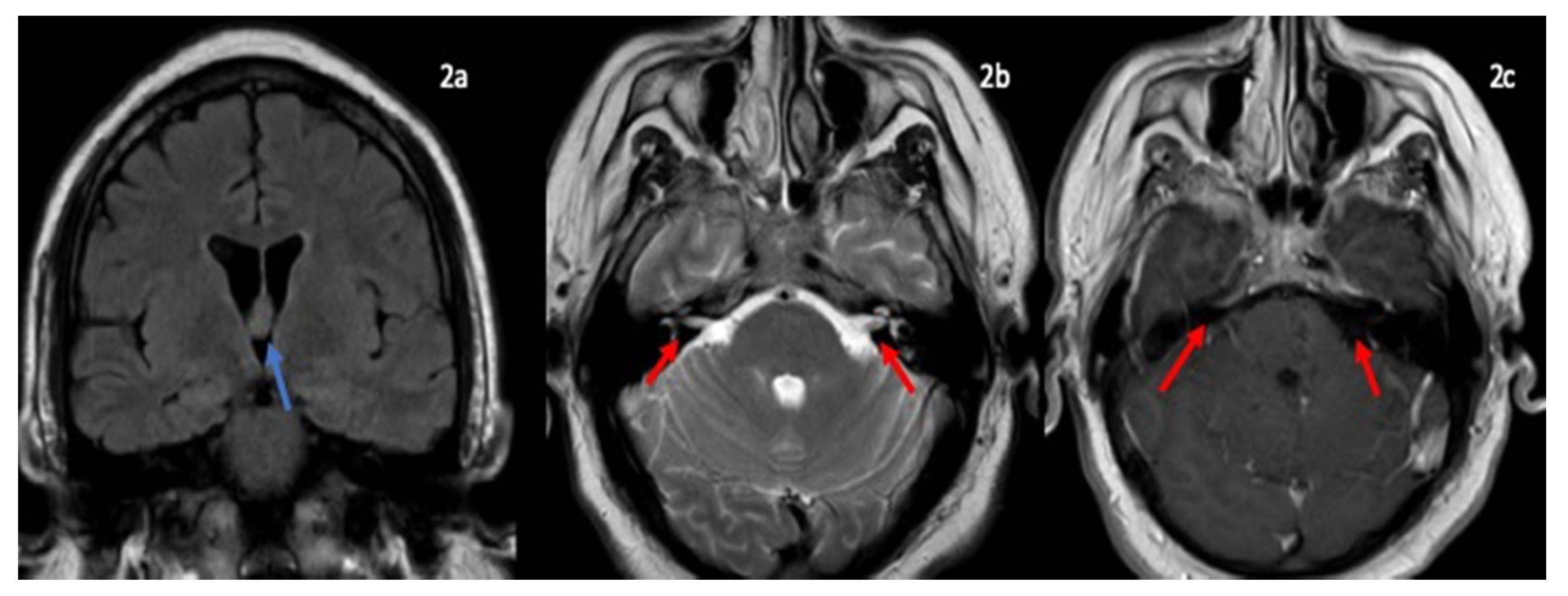
A Novel Case of Bifacial Diplegia Variant of Guillain-Barré Syndrome Following Janssen COVID-19 Vaccination
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Distress Syndrome coronavirus 2 |
| COVID-19 | Coronavirus infectious disease-2019 |
| nCov | Novel Coronavirus |
| AIDP | Acute inflammatory demyelinating polyneuropathy |
| AMSAN | Acute motor-sensory axonal neuropathy |
| AMAN | Acute motor axonal neuropathy |
| BFP | Bifacial weakness with paresthesias |
| MFS | Miller-Fisher syndrome |
| GBS | Guillain-Barré Syndrome |
| IVIG | Intravenous immunoglobulin |
| EMG | Electromyography |
| CSF | Cerebrospinal fluid |
| HIV | Human immunodeficiency virus |
| WHO | World Health Organization |
| MRC | Medical Research Council Scale for Muscle Strength |
References
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing COVID-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Burgos, R.M.; Badowski, M.E.; Drwiega, E.; Ghassemi, S.; Griffith, N.; Herald, F.; Johnson, M.; Smith, R.O.; Michienzi, S.M. The race to a COVID-19 vaccine: Opportunities and challenges in development and distribution. Drugs Context 2021, 10. [Google Scholar] [CrossRef]
- Learn More about COVID-19 Vaccines FDA. Available online: https://www.fda.gov/consumers/consumer-updates/learn-more-about-covid-19-vaccines-fda (accessed on 16 April 2021).
- CDC. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 16 April 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- CDC. Overview and Safety of Janssen COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html (accessed on 16 April 2021).
- Jacobs, B.C.; Rothbarth, P.H.; van der Meché, F.G.; Herbrink, P.; Schmitz, P.I.; de Klerk, M.A.; van Doorn, P.A. The spectrum of antecedent infections in Guillain-Barré syndrome: A case-control study. Neurology 1998, 51, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J.; Jacobs, B.C.; Van Doorn, P.A. Guillain-barre syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Schonberger, L.B.; Bregman, D.J.; Sullivan-Bolyai, J.Z.; Keenlyside, R.A.; Ziegler, D.W.; Retailliau, H.F.; Eddins, D.L.; Bryan, J.A. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am. J. Epidemiol. 1979, 110, 105–123. [Google Scholar] [CrossRef]
- Haber, P.; DeStefano, F.; Angulo, F.J.; Iskander, J.; Shadomy, S.V.; Weintraub, E.; Chen, R.T. Guillain-Barré syndrome following influenza vaccination. JAMA 2004, 292, 2478–2481. [Google Scholar] [CrossRef] [Green Version]
- Wakerley, B.R.; Yuki, N. Isolated facial diplegia in Guillain-Barré syndrome: Bifacial weakness with paresthesias. Muscle Nerve 2015, 52, 927–932. [Google Scholar] [CrossRef]
- Márquez Loza, A.M.; Holroyd, K.B.; Johnson, S.A.; Pilgrim, D.M.; Amato, A.A. Guillain-Barré Syndrome in the Placebo and Active Arms of a COVID-19 Vaccine Clinical Trial: Temporal Associations Do Not Imply Causality. Neurology 2021, 96, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Bayas, A.; Hindi, F.; Rizvi, Z.; Espinosa, P.S. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus 2021, 13, e13426. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Vaccine-preventable diseases, vaccines and Guillain-Barre’ syndrome. Vaccine 2019, 37, 5544–5550. [Google Scholar] [CrossRef] [PubMed]
- Vellozzi, C.; Iqbal, S.; Broder, K. Guillain-Barre syndrome, influenza, and influenza vaccination: The epidemiologic evidence. Clin. Infect. Dis. 2014, 58, 1149–1155. [Google Scholar] [CrossRef]
- Martín Arias, L.H.; Sanz, R.; Sáinz, M.; Treceño, C.; Carvajal, A. Guillain-Barré syndrome and influenza vaccines: A meta-analysis. Vaccine 2015, 33, 3773–3778. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- CDC. Understanding and Explaining Viral Vector COVID-19 Vaccines. Available online: https://www.cdc.gov/vaccines/covid-19/hcp/viral-vector-vaccine-basics.html (accessed on 16 April 2021).
- Repajic, M.; Lai, X.L.; Xu, P.; Liu, A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav. Immun. Health 2021, 13, 100217. [Google Scholar] [CrossRef]
- Cirillo, N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns. J. Oral Pathol. Med. 2021, 50, 424–427. [Google Scholar] [CrossRef]
- Bourdette, D.; Killestein, J. Quelling Public Fears about Guillain-Barre Syndrome and COVID-19 Vaccination. Neurology 2021, 96, 1021–1022. [Google Scholar] [CrossRef] [PubMed]
- Lunn, M.P.; Cornblath, D.R.; Jacobs, B.C.; Querol, L.; van Doorn, P.A.; Hughes, R.A.; Willison, H.J. COVID-19 vaccine and Guillain-Barré syndrome: Let’s not leap to associations. Brain 2021, 144, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Inaloo, S.; Katibeh, P. Guillain-barre syndrome presenting with bilateral facial nerve palsy. Iran. J. Child Neurol. 2014, 8, 70–72. [Google Scholar]
- Kumar, P.; Charaniya, R.; Bahl, A.; Ghosh, A.; Dixit, J. Facial Diplegia with Paresthesia: An Uncommon Variant of Guillain-Barre Syndrome. J. Clin. Diagn. Res. 2016, 10, OD01–OD02. [Google Scholar] [CrossRef] [PubMed]
- Janssen COVID-19 Vaccine (Johnson & Johnson) Center for Disease Control and Prevention. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/index.html (accessed on 16 April 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, A.; Hurlburt, G.; Podury, S.; Tandon, M.; Kingree, S.; Sriwastava, S. A Novel Case of Bifacial Diplegia Variant of Guillain-Barré Syndrome Following Janssen COVID-19 Vaccination. Neurol. Int. 2021, 13, 404-409. https://doi.org/10.3390/neurolint13030040
Prasad A, Hurlburt G, Podury S, Tandon M, Kingree S, Sriwastava S. A Novel Case of Bifacial Diplegia Variant of Guillain-Barré Syndrome Following Janssen COVID-19 Vaccination. Neurology International. 2021; 13(3):404-409. https://doi.org/10.3390/neurolint13030040
Chicago/Turabian StylePrasad, Apoorv, Gage Hurlburt, Sanjiti Podury, Medha Tandon, Seth Kingree, and Shitiz Sriwastava. 2021. "A Novel Case of Bifacial Diplegia Variant of Guillain-Barré Syndrome Following Janssen COVID-19 Vaccination" Neurology International 13, no. 3: 404-409. https://doi.org/10.3390/neurolint13030040
APA StylePrasad, A., Hurlburt, G., Podury, S., Tandon, M., Kingree, S., & Sriwastava, S. (2021). A Novel Case of Bifacial Diplegia Variant of Guillain-Barré Syndrome Following Janssen COVID-19 Vaccination. Neurology International, 13(3), 404-409. https://doi.org/10.3390/neurolint13030040





