Surgical Management for Dystonia: Efficacy of Deep Brain Stimulation in the Long Term
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Global Dystonia Severity Scale (GDS) and Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) Evaluation
2.3. Neuropsychological and Psychiatric Evaluations
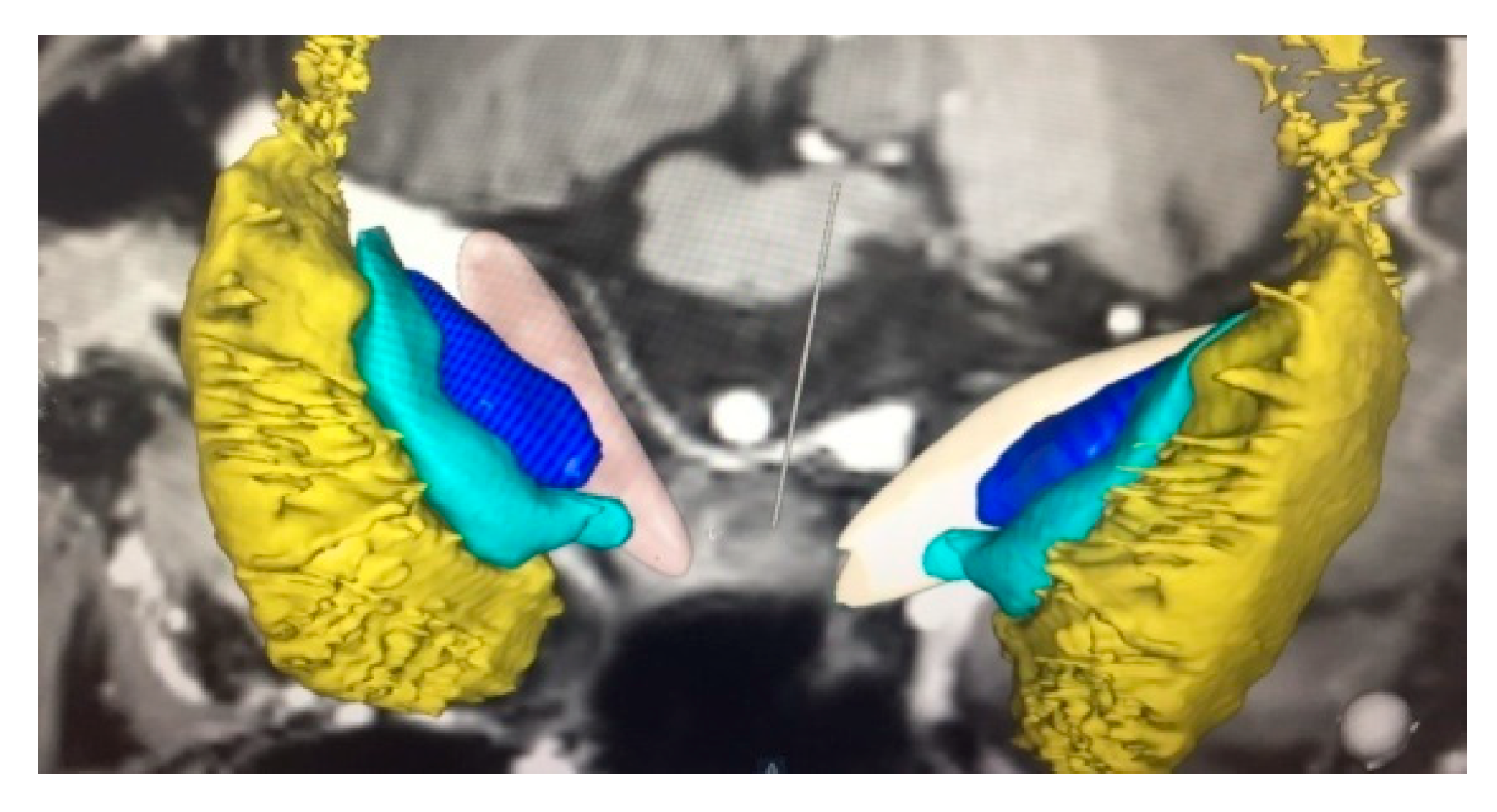
2.4. Operative Technique
2.5. Post-Operative DBS Programming
2.6. Statistical Analysis
3. Results
3.1. Post-Operative 2014 Batch of 10 Patients’ Improvement (3 Months to 84 Months Post-DBS)
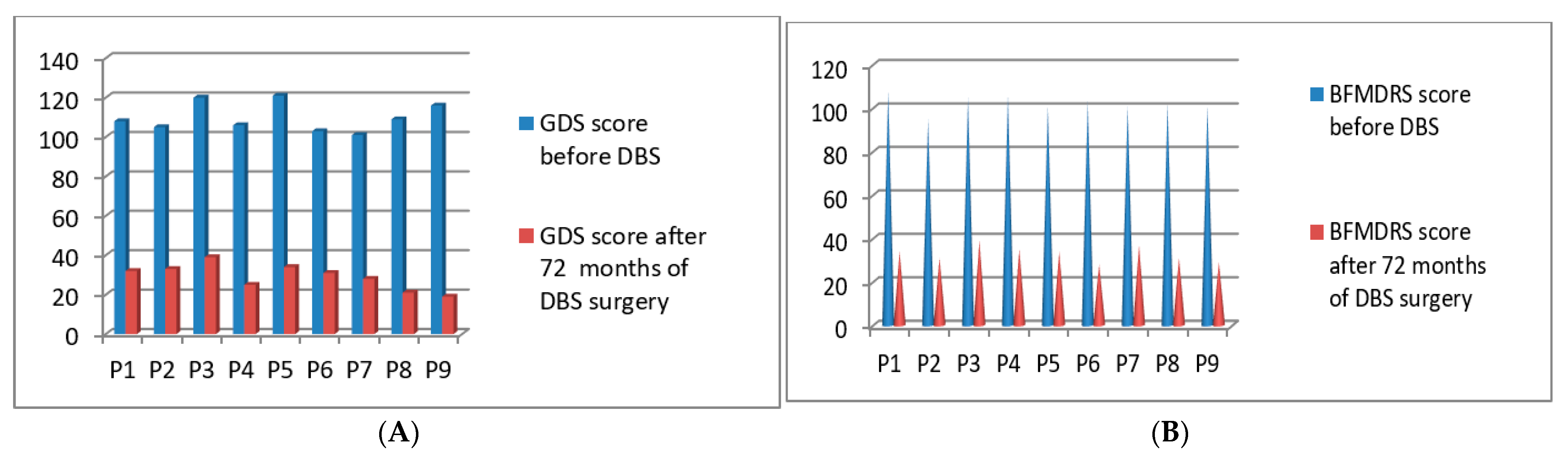
3.2. Post-Operative 2015 Batch of 9 Patients’ Improvement (3 Months to 72 Months Post-DBS)
3.3. Post-Operative 2016 Batch of 11 Patients’ Improvement (3 Months to 60 Months Post-DBS)
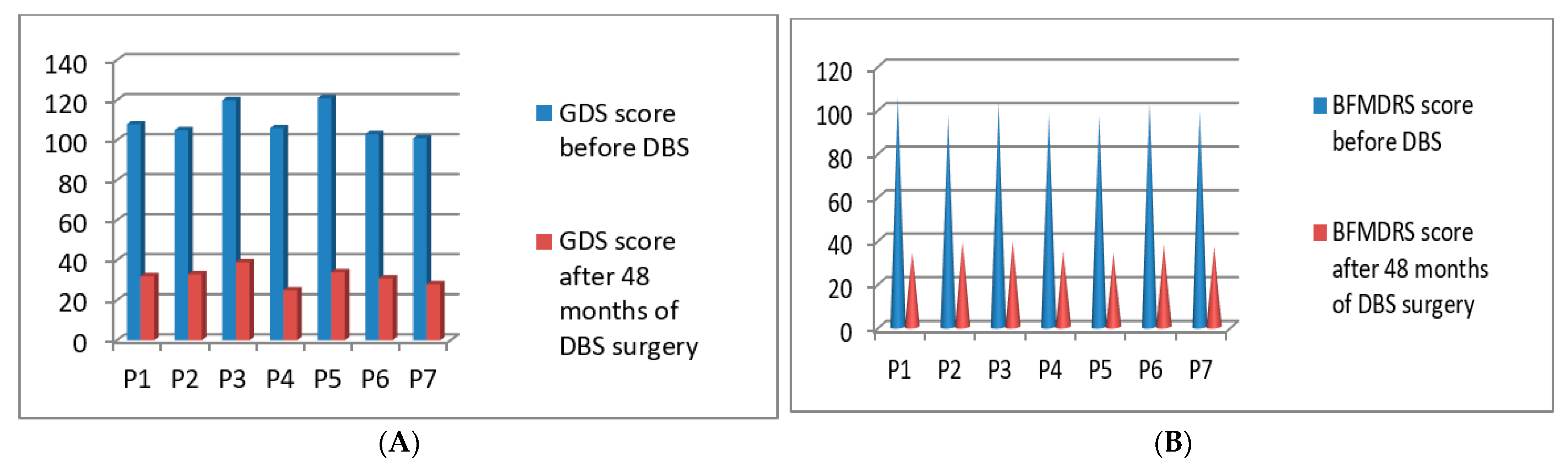
3.4. Post-Operative 2017 Batch of 7 Patients’ Improvement (3 Months to 48 Months Post-DBS)
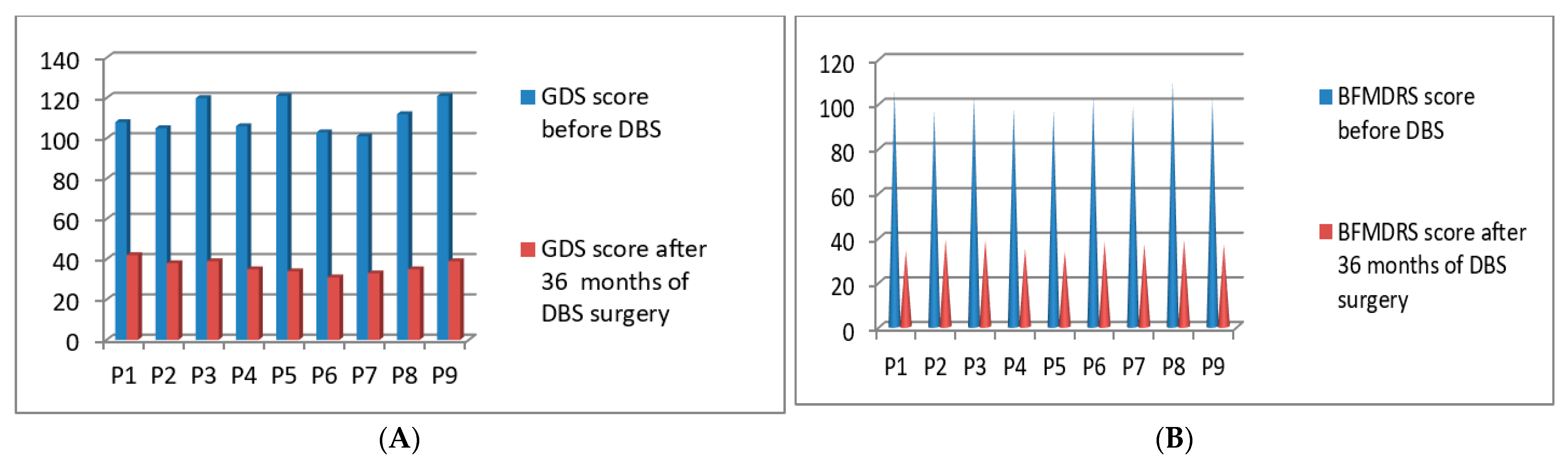
3.5. Post-Operative 2018 Batch of 9 Patients’ Improvement (3 Months to 36 Months Post-DBS)
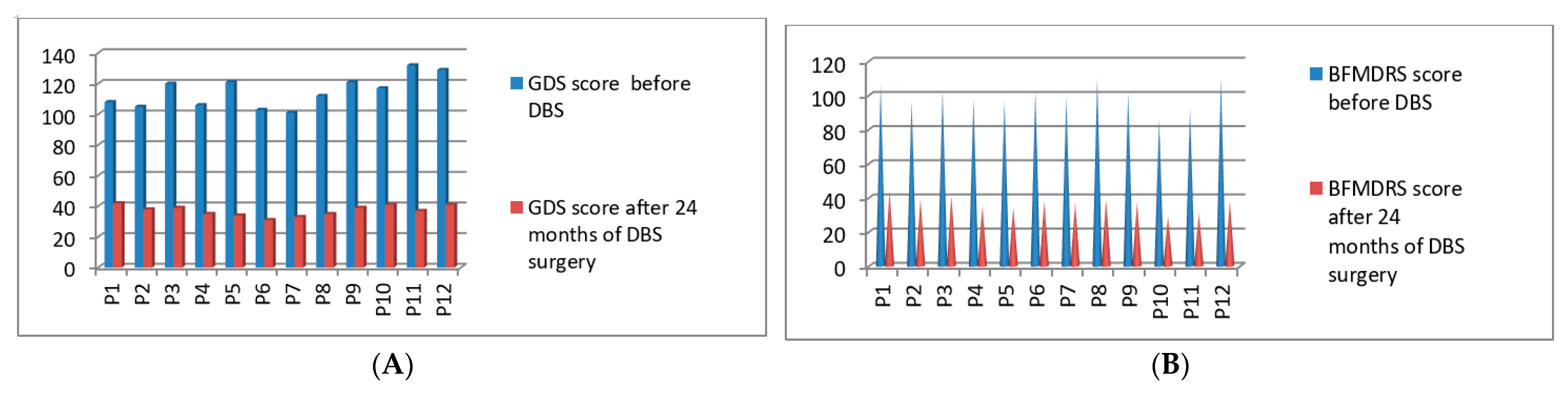
3.6. Post-Operative 2019 Batch Total 12 Patients’ Improvement (3 Months to 24 Months Post-DBS)
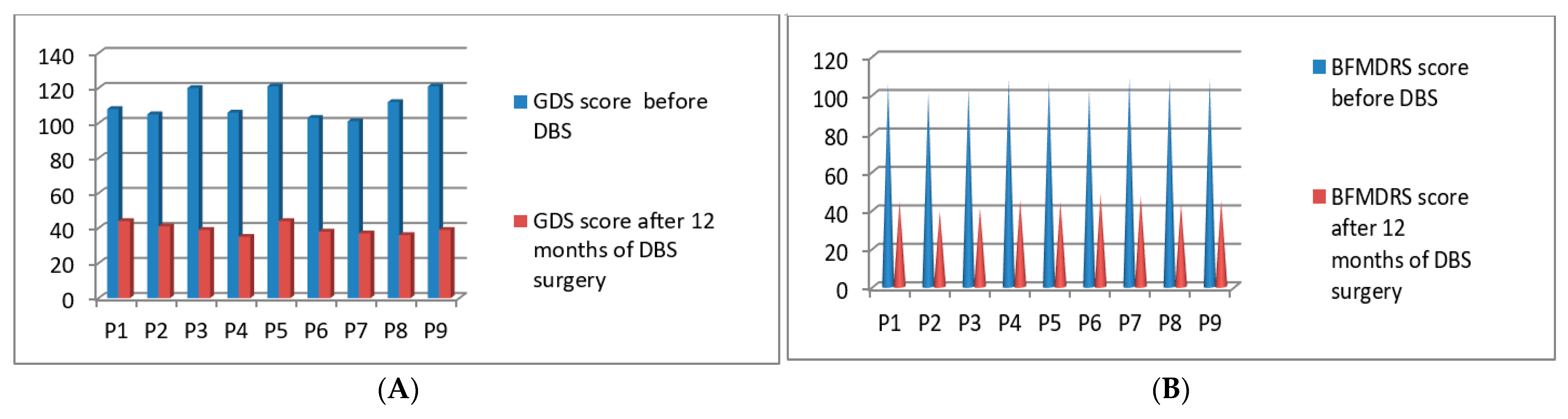
3.7. Post-Operative 2020 Batch of 9 Patients’ Improvement (3 Months to 16 Months Post-DBS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albanese, A.; Bhatia, K.; Bressman, S.B.; DeLong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, N.; Butler, A.; Jahanshahi, M. Quality of life in focal, segmental, and generalized dystonia. Mov. Disord. 2007, 22, 341–347. [Google Scholar] [CrossRef]
- Ramirez-Castaneda, J.; Jankovic, J. Long-term efficacy and safety of botulinumtoxin injections in dystonia. Toxins 2013, 5, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Termsarasab, P.; Thammongkolchai, T.; Frucht, S.J. Medical treatment of dystonia. J. Clin. Mov. Disord. 2016, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Volkmann, J.; Mueller, J.; Deuschl, G.; Kühn, A.A.; Krauss, J.K.; Poewe, W.; Timmermann, L.; Falk, D.; Kupsch, A.; Kivi, A.; et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: A randomised, sham-controlled trial. Lancet Neurol. 2014, 13, 875–884. [Google Scholar] [CrossRef]
- Mueller, J.; Skogseid, I.M.; Benecke, R.; Kupsch, A.; Trottenberg, T.; Poewe, W.; Schneider, G.H.; Eisner, W.; Wolters, A.; Müller, J.; et al. Pallidal deep brain stimulation improves quality of life in segmental and generalized dystonia: Results from a prospective, randomized sham-controlled trial. Mov. Disord. 2008, 23, 131–134. [Google Scholar] [CrossRef]
- Halbig, T.D.; Gruber, D.; Kopp, U.A.; Schneider, G.H.; Trottenberg, T.; Kupsch, A. Pallidal stimulation in dystonia: Effects on cognition, mood, and quality of life. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, J.; Wolters, A.; Kupsch, A.; Müller, J.; Kühn, A.A.; Schneider, G.-H.; Poewe, W.; Hering, S.; Eisner, W.; Müller, J.-U.; et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 2012, 11, 1029–1038. [Google Scholar] [CrossRef]
- Valldeoriola, F.; Regidor, I.; Mínguez-Castellanos, A.; Lezcano, E.; Ruiz, P.J.G.; Rojo, A.; Salvador, A.; Castro, A.; Grandas, F.; Kulisevsky, J.; et al. Efficacy and safety of pallidal stimulation in primary dystonia: Results of the Spanish multicentric study. J. Neurol. Neurosurg. Psychiatry 2009, 81, 65–69. [Google Scholar] [CrossRef]
- Albanese, A.; Barnes, M.P.; Bhatia, K.P.; Fernandez-Alvarez, E.; Filippini, G.; Gasser, T.; Newton, K.A.; Rektor, I.; Savoiardo, M.; Valls-Sole, J. A systematic review on the diagnosis and treatment of primary(idiopathic) dystonia and dystonia plus syndromes: Report of an EFNS/ MDS-ES task force. Eur. J. Neurol. 2006, 13, 433–444. [Google Scholar] [CrossRef]
- Albanese, A.; Asmus, F.; Bhatia, K.; Elia, A.E.; Elibol, B.; Filippini, G.; Gasser, T.; Krauss, J.K.; Nardocci, N.; Newton, A.; et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur. J. Neurol. 2010, 18, 5–18. [Google Scholar] [CrossRef]
- Comella, C.L.; Leurgans, S.; Wuu, J.; Stebbins, G.T.; Chmura, T. The Dystonia Study Group Rating scales for dystonia: A multicenter assessment. Mov. Disord. 2003, 18, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Moro, E.; LeReun, C.; Krauss, J.K.; Albanese, A.; Lin, J.-P.; Autiero, S.W.; Brionne, T.C.; Vidailhet, M. Efficacy of pallidal stimulation in isolated dystonia: A systematic review and meta-analysis. Eur. J. Neurol. 2017, 24, 552–560. [Google Scholar] [CrossRef]
- Walsh, R.A.; Sidiropoulos, C.; Lozano, A.; Hodaie, M.; Poon, Y.-Y.; Fallis, M.; Moro, E. Bilateral pallidal stimulation in cervical dystonia: Blinded evidence of benefit beyond 5 years. Brain 2013, 136, 761–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loher, T.J.; Capelle, H.-H.; Kaelin-Lang, A.; Weber, S.; Weigel, R.; Burgunder, J.M.; Krauss, J.K. Deep brain stimulation for dystonia: Outcome at long-term follow-up. J. Neurol. 2008, 255, 881–884. [Google Scholar] [CrossRef]
- Panov, F.; Gologorsky, Y.; Connors, G.; Tagliati, M.; Miravite, J.; Alterman, R.L. Deep brain stimulation in DYT1 dystonia: A 10-year experience. Neurosurgery 2013, 73, 93. [Google Scholar] [CrossRef] [Green Version]
- Vidailhet, M.; Vercueil, L.; Houeto, J.-L.; Krystkowiak, P.; Lagrange, C.; Yelnik, J.; Bardinet, E.; Benabid, A.-L.; Navarro, S.; Dormont, D.; et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: A prospective 3 year follow-up study. Lancet Neurol. 2007, 6, 223–229. [Google Scholar] [CrossRef]
- Bronte-Stewart, H.; Taira, T.; Valldeoriola, F.; Merello, M.; Marks, W.J.; Albanese, A.; Bressman, S.; Moro, A.E. Inclusion and exclusion criteria for DBS in dystonia. Mov. Disord. 2011, 26, S5–S16. [Google Scholar] [CrossRef]
- Isaias, I.U.; Alterman, R.L.; Tagliati, M. Outcome predictors of pallidal stimulation in patients with primary dystonia: The role of disease duration. Brain 2008, 131, 1895–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, J.; Starr, P.A.; Ostrem, J.L. Use of pallidal deep brain stimulation in post infarct hemidystonia. Stereotact. Funct. Neurosurg. 2013, 91, 243–247. [Google Scholar] [CrossRef]
- Jitkritsadakul, O.; Bhidayasiri, R.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Fasano, A. Systematic review of hardware-related complications of deep brain stimulation: Do new indications pose an increased risk. Brain Stimul. 2017, 10, 967–976. [Google Scholar] [CrossRef]
- Yianni, J.; Nandi, D.; Shad, A.; Bain, P.; Gregory, R.; Aziz, T. Increased risk of lead fracture and migration in dystonia compared with other movement disorders following deep brain stimulation. J. Clin. Neurosci. 2004, 11, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.Y.; Abosch, A.; Kim, S.H.; Lang, A.E.; Lozano, A.M. Long-term hardware related complications of deep brain stimulation. Neurosurgery 2002, 50, 6. [Google Scholar]
- Tagliati, M.; Krack, P.; Volkmann, J.; Aziz, T.; Krauss, J.K.; Kupsch, A.; Vidailhet, A.M. Long-Term management of DBS in dystonia: Response to stimulation, adverse events, battery changes, and special considerations. Mov. Disord. 2011, 26, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Pauls, K.; Bröckelmann, P.; Hammesfahr, S.; Becker, J.; Hellerbach, A.; Visser-Vandewalle, V.; Dembek, T.A.; Meister, I.; Timmermann, L. Dysarthria in pallidal Deep Brain Stimulation in dystonia depends on the posterior location of active electrode contacts: A pilot study. Park. Relat. Disord. 2018, 47, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bour, L.J.; Contarino, M.F.; Foncke, E.M.J.; De Bie, R.M.A.; Munckhof, P.V.D.; Speelman, J.D.; Schuurman, P.R. Long-term experience with intraoperative microrecording during DBS neurosurgery in STN and GPi. Acta Neurochir. 2010, 152, 2069–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, J.; Gonzalez, V.; Cif, L.; Cyprien, F.; Chan-Seng, E.; Coubes, P. Rechargeable or Nonrechargeable deep brain stimulation in dystonia: A cost analysis. Neuromodulation Technol. Neural Interface 2017, 20, 243–247. [Google Scholar] [CrossRef]
- Blahak, C.; Capelle, H.-H.; Baezner, H.; Kinfe, T.M.; Hennerici, M.G.; Krauss, J.K. Battery lifetime in pallidal deep brain stimulation for dystonia. Eur. J. Neurol. 2010, 18, 872–875. [Google Scholar] [CrossRef]
- Meoni, S.; Fraix, V.; Castrioto, A.; Benabid, A.L.; Seigneuret, E.; Vercueil, L.; Pollak, P.; Krack, P.; Chevrier, E.; Chabardes, S.; et al. Pallidal deep brain stimulation for dystonia: A long term study. J. Neurol. Neurosurg. Psychiatry 2017, 88, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Skogseid, I.M.; Ramm-Pettersen, J.; Volkmann, J.; Kerty, E.; Dietrichs, E.; Røste, G.K. Good long-term efficacy of pallidal stimulation in cervical dystonia: A prospective, observer-blinded study. Eur. J. Neurol. 2011, 19, 610–615. [Google Scholar] [CrossRef]
- Reese, R.; Fasano, A.; Knudsen, K.; Herzog, J.; Falk, D.; Mehdorn, H.M.; Deuschl, G.; Volkmann, J. Full Parkinsonian Triad Induced by Pallidal High-Frequency Stimulation in Cervical Dystonia. Mov. Disord. Clin. Pract. 2014, 2, 99–101. [Google Scholar] [CrossRef]
- Zauber, S.E.; Watson, N.; Comella, C.L.; Bakay, R.A.E.; Metman, L.V. Stimulation-induced parkinsonism after posteroventral deep brain stimulation of the globus pallidus internus for craniocervical dystonia. J. Neurosurg. 2009, 110, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Pahwa, R. Effects of bilateral subthalamic nucleus stimulation on sleep, daytime sleepiness, and early morning dystonia in patients with Parkinson disease. J. Neurosurg. 2006, 104, 502–505. [Google Scholar] [CrossRef]
- Kleiner-Fisman, G.; Liang, G.S.L.; Moberg, P.J.; Ruocco, A.C.; Hurtig, H.I.; Baltuch, G.H.; Jaggi, J.L.; Stern, M.B. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: Impact on severity, neuropsychological status, and quality of life. J. Neurosurg. 2007, 107, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrem, J.L.; Racine, C.A.; Glass, G.A.; Grace, J.K.; Volz, M.M.; Heath, S.L.; Starr, P.A. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology 2011, 76, 870–878. [Google Scholar] [CrossRef]
- Ostrem, J.L.; San Luciano, M.; Dodenhoff, K.A.; Ziman, N.; Markun, L.C.; Racine, C.A.; de Hemptinne, C.; Volz, M.M.; Heath, S.L.; Starr, P.A. Subthalamic nucleus deep brain stimulation in isolated dystonia: A 3-year follow-up study. Neurology 2017, 88, 25–35. [Google Scholar] [CrossRef]
- Volkmann, J.; Benecke, R. Deep brain stimulation for dystonia: Patient selection and evaluation. Mov. Disord. 2002, 17, S112–S115. [Google Scholar] [CrossRef]
- Brüggemann, N.; Kühn, A.; Schneider, S.A.; Kamm, C.; Wolters, A.; Krause, P.; Moro, E.; Steigerwald, F.; Wittstock, M.; Tronnier, V.; et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology 2015, 84, 895–903. [Google Scholar] [CrossRef]
- Panov, F.; Tagliati, M.; Ozelius, L.J.; Fuchs, T.; Gologorsky, Y.; Cheung, T.; Avshalumov, M.; Bressman, S.B.; Saunders-Pullman, R.; Weisz, D.; et al. Pallidal deep brain stimulation for DYT6 dystonia. J. Neurol. Neurosurg. Psychiatry 2011, 83, 182–187. [Google Scholar] [CrossRef]
- Tisch, S.; Zrinzo, L.; Limousin, P.; Bhatia, K.P.; Quinn, N.; Ashkan, K.; Hariz, M. Effect of electrode contact location on clinical efficacy of pallidal deep brain stimulation in primary generalised dystonia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1314–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reese, R.; Reich, M.M.; Falk, D.; Deuschl, G.; Mehdorn, H.M.; Volkmann, J. Intraoperative Thresholds for Capsular Stimulation Are Reliable for Chronic Pallidal Deep Brain Stimulation in Dystonia. Ster. Funct. Neurosurg. 2017, 95, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.D.; Starr, P.A.; Marks, W.J., Jr.; Ostrem, J.L. Induction of Bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact. Funct. Neurosurg. 2009, 87, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blahak, C.; Capelle, H.-H.; Baezner, H.; Kinfe, T.M.; Hennerici, M.G.; Krauss, J.K. Micrographia induced by pallidal DBS for segmental dystonia: A subtle sign of hypokinesia? J. Neural Transm. 2011, 118, 549–553. [Google Scholar] [CrossRef]
- Schrader, C.; Capelle, H.H.; Kinfe, T.M.; Blahak, C.; Baezner, H.; Dressler, D.; Krauss, J. Pallidal deep brain stimulation may induce freezing of gait in patients with focal and segmental dystonia. Mov. Disord. 2010, 25 (Suppl. 1), S466–S467. [Google Scholar]



| Year of the Surgery | Total Number of Patients | Placements of the Electrodes |
|---|---|---|
| January 2014 to December 2014 | 10 (7 males and 3 females) | Bilateral GPi |
| January 2015 to December 2015 | 9 (5 males and 4 females) | Bilateral GPi |
| January 2016 to December 2016 | 11 (6 males and 5 females) | Bilateral GPi |
| January 2017 to December 2017 | 7 (2 males and 5 females) | Bilateral GPi |
| January 2018-December 2018 | 9 (7 males and 2 females) | Bilateral GPi |
| January 2019 to December 2019 | 12 (10 males and 2 females) | Bilateral GPi |
| January 2020 to December 2020 | 9 (5 males and 4 females) | Bilateral GPi |
| Diagnosis | Total Number of Patients |
|---|---|
| Generalized Dystonia | 30 (20 patients DYT-1 positive and 10 patients DYT-3) |
| CERVICAL DYSTONIA | 27 (17 patients DYT-5 positive and 10 patients DYT-6) |
| Blepharospasm with PISA syndrome associated with Parkinson’s disease | 5 |
| Post-stroke Hemi Dystonia | 5 |
| Quality of Life with Dystonia | Total Number of Patients with Dystonia |
|---|---|
| Completely dependent and wheelchair bound | 20 |
| Partially able to do social things with dependency | 30 |
| Completely independent with dystonia | 17 |
| Total Number of Patients | Neuropsychological Assessment | Scale Performed |
|---|---|---|
| 11 | Severe depression with total social withdrawal | WEMWBS and KGSAS |
| 4 | Moderate depression with suicidal tendency | WEMWBS and KGSAS |
| 1 | Severe depression with suicidal tendency | WEMWBS and KGSAS |
| 51 | Minimal depression associated with dystonia | WEMWBS and KGSAS |
| Patients No. (Total Patients No. = 67) | Year DBS Undergone | Sex | Age | Dystonia Types | GDS Base Score before DBS | BFMDRS Base Score before DBS | GDS Base Score after DBS | BFMDRS Base Score after DBS | Post DBS Total Timeline (GDS and BFMDRS Scaling Done Post DBS) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 2014 | M | 44 | Generalized | 118/140 | 98/140 | 36/140 | 24/120 | 84 months |
| P2 | 2014 | M | 56 | Generalized | 115/140 | 95/120 | 29/140 | 21/120 | 84 months |
| P3 | 2014 | F | 48 | Generalized | 129/140 | 110/120 | 39/140 | 29/120 | 84 months |
| P4 | 2014 | M | 39 | Generalized | 106/140 | 106/120 | 24/140 | 25/120 | 84 months |
| P5 | 2014 | M | 53 | Generalized | 131/140 | 111/120 | 34/140 | 24/120 | 84 months |
| P6 | 2014 | F | 65 | Generalized | 103/140 | 103/120 | 38/140 | 28/120 | 84 months |
| P7 | 2014 | F | 55 | Cervical | 101/140 | 101/120 | 28/140 | 27/120 | 84 months |
| P8 | 2014 | M | 67 | Cervical | 99/140 | 102/120 | 21/140 | 31/120 | 84 months |
| P9 | 2014 | M | 78 | Cervical | 126/140 | 101/120 | 18/140 | 29/120 | 84 months |
| P10 | 2014 | M | 70 | Cervical | 110/140 | 95/120 | 23/140 | 19/120 | 84 months |
| P1 | 2015 | M | 68 | Generalized | 108/140 | 108/120 | 32/140 | 34/120 | 72 months |
| P2 | 2015 | F | 55 | Generalized | 105/140 | 95/120 | 33/140 | 31/120 | 72 months |
| P3 | 2015 | F | 48 | Generalized | 120/140 | 105/120 | 39/140 | 39/120 | 72 months |
| P4 | 2015 | M | 38 | Generalized | 106/140 | 106/120 | 25/140 | 35/120 | 72 months |
| P5 | 2015 | M | 41 | Cervical | 121/140 | 101/120 | 34/140 | 34/120 | 72 months |
| P6 | 2015 | M | 70 | Cervical | 103/140 | 103/120 | 31/140 | 28/120 | 72 months |
| P7 | 2015 | F | 59 | Cervical | 101/140 | 101/120 | 28/140 | 37/120 | 72 months |
| P8 | 2015 | F | 61 | Cervical | 109/140 | 102/120 | 21/140 | 31/120 | 72 months |
| P9 | 2015 | M | 40 | Generalized | 116/140 | 101/120 | 19/140 | 29/120 | 72 months |
| P1 | 2016 | M | 70 | Generalized | 108/140 | 108/120 | 32/140 | 34/120 | 60 months |
| P2 | 2016 | M | 67 | Post Stroke Hemi dystonia | 105/140 | 95/120 | 33/140 | 31/120 | 60 months |
| P3 | 2016 | M | 53 | Generalized | 120/140 | 105/120 | 39/140 | 39/120 | 60 months |
| P4 | 2016 | F | 46 | Generalized | 106/140 | 106/120 | 25/140 | 35/120 | 60 months |
| P5 | 2016 | M | 58 | Generalized | 121/140 | 101/120 | 34/140 | 34/120 | 60 months |
| P6 | 2016 | F | 39 | Blepharospasm With PISA syndrome | 103/140 | 103/120 | 31/140 | 28/120 | 60 months |
| P7 | 2016 | F | 48 | Cervical | 101/140 | 101/120 | 28/140 | 37/120 | 60 months |
| P8 | 2016 | M | 50 | Cervical | 109/140 | 102/120 | 21/140 | 31/120 | 60 months |
| P9 | 2016 | F | 51 | Generalized | 116/140 | 101/120 | 19/140 | 29/120 | 60 months |
| P10 | 2016 | F | 65 | Generalized | 112/140 | 98/120 | 23/140 | 32/120 | 60 months |
| P11 | 2016 | M | 42 | Generalized | 115/140 | 110/120 | 18/140 | 31/120 | 60 months |
| P1 | 2017 | M | 51 | Generalized | 108/140 | 106/120 | 32/140 | 34/120 | 48 months |
| P2 | 2017 | M | 48 | Generalized | 105/140 | 97/120 | 33/140 | 39/120 | 48 months |
| P3 | 2017 | F | 53 | Generalized | 120/140 | 103/120 | 39/140 | 39/120 | 48 months |
| P4 | 2017 | F | 43 | Cervical | 106/140 | 98/120 | 25/140 | 35/120 | 48 months |
| P5 | 2017 | F | 45 | Blepharospasm With PISA syndrome | 121/140 | 97/120 | 34/140 | 34/120 | 48 months |
| P6 | 2017 | F | 65 | Cervical | 103/140 | 103/120 | 31/140 | 38/120 | 48 months |
| P7 | 2017 | F | 66 | Cervical | 101/140 | 99/120 | 28/140 | 37/120 | 48 months |
| P1 | 2018 | F | 51 | Generalized | 108/140 | 106/120 | 42/140 | 34/120 | 36 months |
| P2 | 2018 | M | 56 | Generalized | 105/140 | 97/120 | 38/140 | 39/120 | 36 months |
| P3 | 2018 | M | 41 | Generalized | 120/140 | 103/120 | 39/140 | 39/120 | 36 months |
| P4 | 2018 | M | 42 | Post Stroke Hemi dystonia | 106/140 | 98/120 | 35/140 | 35/120 | 36 months |
| P5 | 2018 | F | 63 | Cervical | 121/140 | 97/120 | 34/140 | 34/120 | 36 months |
| P6 | 2018 | M | 72 | Cervical | 103/140 | 103/120 | 31/140 | 38/120 | 36 months |
| P7 | 2018 | M | 63 | Post Stroke Hemi dystonia | 101/140 | 99/120 | 33/140 | 37/120 | 36 months |
| P8 | 2018 | M | 59 | Cervical | 112/140 | 110/120 | 35/140 | 39/120 | 36 months |
| P9 | 2018 | M | 71 | Generalized | 121/140 | 102/120 | 39/140 | 37/120 | 36 months |
| P1 | 2019 | M | 60 | Generalized | 108/140 | 106/120 | 42/140 | 44/120 | 24 months |
| P2 | 2019 | M | 61 | Generalized | 105/140 | 97/120 | 38/140 | 39/120 | 24 months |
| P3 | 2019 | M | 52 | Cervical | 120/140 | 103/120 | 39/140 | 41/120 | 24 months |
| P4 | 2019 | M | 43 | Cervical | 106/140 | 98/120 | 35/140 | 35/120 | 24 months |
| P5 | 2019 | F | 41 | Cervical | 121/140 | 97/120 | 34/140 | 34/120 | 24 months |
| P6 | 2019 | M | 48 | Blepharospasm With PISA syndrome | 103/140 | 103/120 | 31/140 | 38/120 | 24 months |
| P7 | 2019 | M | 63 | Cervical | 101/140 | 99/120 | 33/140 | 37/120 | 24 months |
| P8 | 2019 | F | 56 | Cervical | 112/140 | 110/120 | 35/140 | 39/120 | 24 months |
| P9 | 2019 | M | 55 | Post Stroke Hemi dystonia | 121/140 | 102/120 | 39/140 | 37/120 | 24 months |
| P10 | 2019 | M | 45 | Generalized | 117/140 | 86/120 | 41/140 | 29/120 | 24 months |
| P11 | 2019 | M | 39 | Cervical | 132/140 | 92/120 | 37/140 | 31/120 | 24 months |
| P12 | 2019 | M | 58 | Generalized | 129/140 | 110/120 | 41/140 | 38/120 | 24 months |
| P1 | 2020 | M | 38 | Cervical | 108/140 | 106/120 | 44/140 | 44/120 | 12 months |
| P2 | 2020 | F | 45 | Cervical | 105/140 | 101/120 | 41/140 | 39/120 | 12 months |
| P3 | 2020 | M | 52 | Post Stroke Hemi dystonia | 120/140 | 103/120 | 39/140 | 41/120 | 12 months |
| P4 | 2020 | M | 54 | Cervical | 106/140 | 108/120 | 35/140 | 45/120 | 12 months |
| P5 | 2020 | M | 65 | Blepharospasm with PISA syndrome | 121/140 | 107/120 | 44/140 | 44/120 | 12 months |
| P6 | 2020 | F | 75 | Cervical | 103/140 | 103/120 | 38/140 | 48/120 | 12 months |
| P7 | 2020 | M | 71 | Cervical | 101/140 | 109/120 | 37/140 | 47/120 | 12 months |
| P8 | 2020 | F | 69 | Blepharospasm With PISA syndrome | 112/140 | 108/120 | 36/140 | 43/120 | 12 months |
| P9 | 2020 | F | 62 | Generalized | 121/140 | 108/120 | 39/140 | 45/120 | 12 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamel, W.A.; Majumdar, P.; Matis, G.; Fenoy, A.J.; Balakrishnan, S.; Zirh, A.T.; Cevik, A.; Tomar, A.K.; Ouerchefani, N. Surgical Management for Dystonia: Efficacy of Deep Brain Stimulation in the Long Term. Neurol. Int. 2021, 13, 371-386. https://doi.org/10.3390/neurolint13030037
Kamel WA, Majumdar P, Matis G, Fenoy AJ, Balakrishnan S, Zirh AT, Cevik A, Tomar AK, Ouerchefani N. Surgical Management for Dystonia: Efficacy of Deep Brain Stimulation in the Long Term. Neurology International. 2021; 13(3):371-386. https://doi.org/10.3390/neurolint13030037
Chicago/Turabian StyleKamel, Walaa A., Pritam Majumdar, Georgios Matis, Albert J. Fenoy, Shankar Balakrishnan, Ali T. Zirh, Aslihan Cevik, Amit Kumar Tomar, and Naoufel Ouerchefani. 2021. "Surgical Management for Dystonia: Efficacy of Deep Brain Stimulation in the Long Term" Neurology International 13, no. 3: 371-386. https://doi.org/10.3390/neurolint13030037
APA StyleKamel, W. A., Majumdar, P., Matis, G., Fenoy, A. J., Balakrishnan, S., Zirh, A. T., Cevik, A., Tomar, A. K., & Ouerchefani, N. (2021). Surgical Management for Dystonia: Efficacy of Deep Brain Stimulation in the Long Term. Neurology International, 13(3), 371-386. https://doi.org/10.3390/neurolint13030037






