Median-to-Ulnar Nerve Communication in Carpal Tunnel Syndrome: An Electrophysiological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aims of the Study
2.2. Participants and Data Collection
2.3. Electrophysiology Procedures
2.4. Statistical Analyses
3. Results
3.1. Recordings from ADM Muscle
3.2. Recordings from FDI Muscle
3.3. Recordings from APB Muscle
3.4. Severity of CTS and Presence of MUC Subtypes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ten Donkelaar, H.J.; Kachlík, D.; Tubbs, R.S. An Illustrated Terminologia Neuroanatomica; Springer International Publishing: Basel, Switzerland, 2018; Volume XVII, p. 491. [Google Scholar]
- Roy, J.; Henry, B.M.; Pękala, P.A.; Vikse, J.; Saganiak, K.; Walocha, J.A.; Tomaszewski, K.A. Median and ulnar nerve anastomoses in the upper limb: A meta-analysis. Muscle Nerve 2015, 54, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Mannerfelt, L. Studies on the Hand in Ulnar Nerve Paralysis: A Clinical-Experimental Investigation in Normal and Anomalous Innervation. Acta Orthop. Scand. 1966, 37, 3–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crutchfield, C.A.; Gutmann, L. Hereditary aspects of median-ulnar nerve communications. J. Neurol. Neurosurg. Psychiatry 1980, 43, 53–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutmann, L. Median--ulnar nerve communications and carpal tunnel syndrome. J. Neurol. Neurosurg. Psychiatry 1977, 40, 982–986. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; Oh, C.-S.; Chung, I.-H.; Sunwoo, I.-N. An anatomic study of the Martin-Gruber anastomosis: Electrodiagnostic implications. Muscle Nerve 2004, 31, 95–97. [Google Scholar] [CrossRef]
- Sarikcioglu, L.; Sindel, M.; Ozkaynak, S.; Aydin, H. Median and ulnar nerve communication in the forearm: An anatomical and electrophysiological study. Med. Sci. Monit. 2003, 9, 29–35. [Google Scholar]
- Cavalheiro, C.S.; Filho, M.R.; Pedro, G.; Caetano, M.F.; Vieira, L.A.; Caetano, E.B. Clinical repercussions of Martin-Gruber anastomosis: Anatomical study. Rev. Bras. Ortop. 2016, 51, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, J.G.; Bouma, P.A.D. Recognition of the Martin-Gruber anastomosis. Muscle Nerve 1997, 20, 887–889. [Google Scholar] [CrossRef]
- Hefny, M.; Sallam, A.; Abdellatif, M.; Okasha, S.; Orabi, M. Electrophysiological Evaluation and Clinical Implication of Martin-Gruber Anastomosis in Healthy Subjects. J. Hand Surg. 2020, 25, 87–94. [Google Scholar] [CrossRef]
- Iyer, V.; Fenichel, G.M. Normal median nerve proximal latency in carpal tunnel syndrome: A clue to coexisting Martin-Gruber anastomosis. J. Neurol. Neurosurg. Psychiatry 1976, 39, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, S.; Krarup, C. Unusual ulnar sensory innervation and Martin-Gruber anastomosis in a patient with a carpal tunnel syndrome. J. Neurol. 2000, 247, 141–142. [Google Scholar] [CrossRef]
- Amoiridis, G.; Vlachonikolis, I.G. Verification of the median-to-ulnar and ulnar-to-median nerve motor fiber anastomosis in the forearm: An electrophysiological study. Clin. Neurophysiol. 2003, 114, 94–98. [Google Scholar] [CrossRef]
- Marras, C.; Midroni, G. Proximal Martin-Gruber anastomosis mimicking ulnar neuropathy at the elbow. Muscle Nerve 1999, 22, 1132–1135. [Google Scholar] [CrossRef]
- Uncini, A.; Lange, D.J.; Lovelace, R.E. Anomalous intrinsic hand muscle innervation in median and ulnar nerve lesions: An electrophysiological study. Neurol. Sci. 1988, 9, 497–503. [Google Scholar] [CrossRef]
- Oh, S.J. Clinical Electromyography: Nerve Conduction Studies, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; p. 848. [Google Scholar]
- Cho, N.S.; Kim, D.H.; Kim, M.Y.; Park, B.K. Electrophysiological and ultrasonographic findings in ulnar neuropathy with martin-gruber anastomosis. Muscle Nerve 2013, 47, 604–607. [Google Scholar] [CrossRef]
- Whitaker, C.H.; Felice, K.J. Apparent conduction block in patients with ulnar neuropathy at the elbow and proximal Martin-Gruber anastomosis. Muscle Nerve 2004, 30, 808–811. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, D.H. Ulnar neuropathy around the mid-arm combined with martin-gruber anastomosis. Ann. Rehabil. Med. 2012, 36, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Burakgazi, A.Z.; Russo, M.; Bayat, E.; Richardson, P.K. Underrecognized anomaly: Proximal martin-gruber anastomosis at the elbow. J. Clin. Neurophysiol. 2014, 31, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.I.; Dimberg, E.L. Martin-gruber anastomosis and carpal tunnel 42: Morphologic clues to identification. Muscle Nerve 2010, 42, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Kayamori, R. Electrodiagnosis in Martin-Gruber anastomosis. Nihon Seikeigeka Gakkai Zasshi 1987, 61, 1367–1372. [Google Scholar] [PubMed]
- Kimura, I.; Ayyar, D.R.; Lippmann, S.M. Electrophysiological verification of the ulnar to median nerve communications in the hand and forearm. Tohoku J. Exp. Med. 1983, 141, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Kimura, J. Electrodiagnosis in Diseases of Nerve and Muscle. In Electrodiagnosis in Diseases of Nerve and Muscle; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Rempel, D.; Evanoff, B.A.; Amadio, P.C.; De Krom, M.; Franklin, G.; Franzblau, A.; Gray, R.; Gerr, F.; Hagberg, M.; Hales, T.; et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am. J. Public Health 1998, 88, 1447–1451. [Google Scholar] [CrossRef] [Green Version]
- Uchida, Y.; Sugioka, Y. Electrodiagnosis of Martin-Gruber connection and its clinical importance in peripheral nerve surgery. J. Hand Surg. 1992, 17, 54–59. [Google Scholar] [CrossRef]
- Wilbourn, A.J.; Lambert, E. The Forearm Median-to-Ulnar Nerve Communication; Eiectrodiagnostic Aspects. Scientific Program of the American Academy of Neurology Twenty-Eighth Annual Meeting. In Neurology; Ovid Technologies (Wolters Kluwer Health): New York City, NY, USA, 1976; p. 342. [Google Scholar]
- Erdem, H.R.; Ergun, S.; Erturk, C.; Ozel, S. Electrophysiological Evaluation of the Incidence of Martin-Gruber Anastomosis in Healthy Subjects. Yonsei Med. J. 2002, 43, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Kate, N.; Teli, C.; Gajbhiye, R.; Ambareesha, K.; Suresh, M. A study to analyze the prevalence of nervous anastomosis (Martin-Gruber) in medical students. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 185. [Google Scholar] [CrossRef] [Green Version]
- Khosrawi, S.; Kianimehr, L.; Andalib, S. The prevalence of Martin-Gruber anastomosis in Iranian subjects by electrodiagnostic criteria. Iran. J. Neurol. 2015, 14, 231–232. [Google Scholar]
- Kimura, J.; Murphy, M.J.; Varda, D.J. Electrophysiological Study of Anomalous Innervation of Intrinsic Hand Muscles. Arch. Neurol. 1976, 33, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Kachlik, D.; Musil, V.; Baca, V. Contribution to the anatomical nomenclature concerning upper limb anatomy. Surg. Radiol. Anat. 2016, 39, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Kimura, J. Collision technique: Physiologic block of nerve impulses in studies of motor nerve conduction velocity. Neurology 1976, 26, 680. [Google Scholar] [CrossRef] [PubMed]
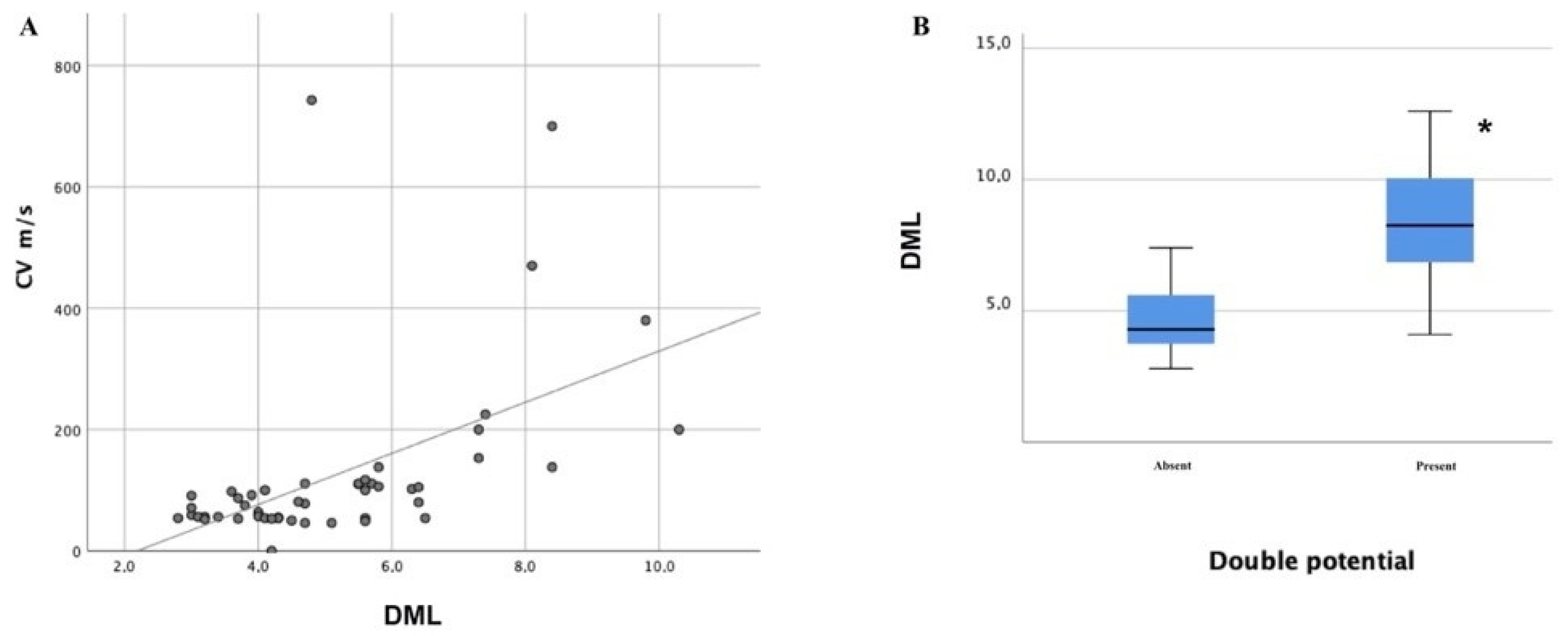
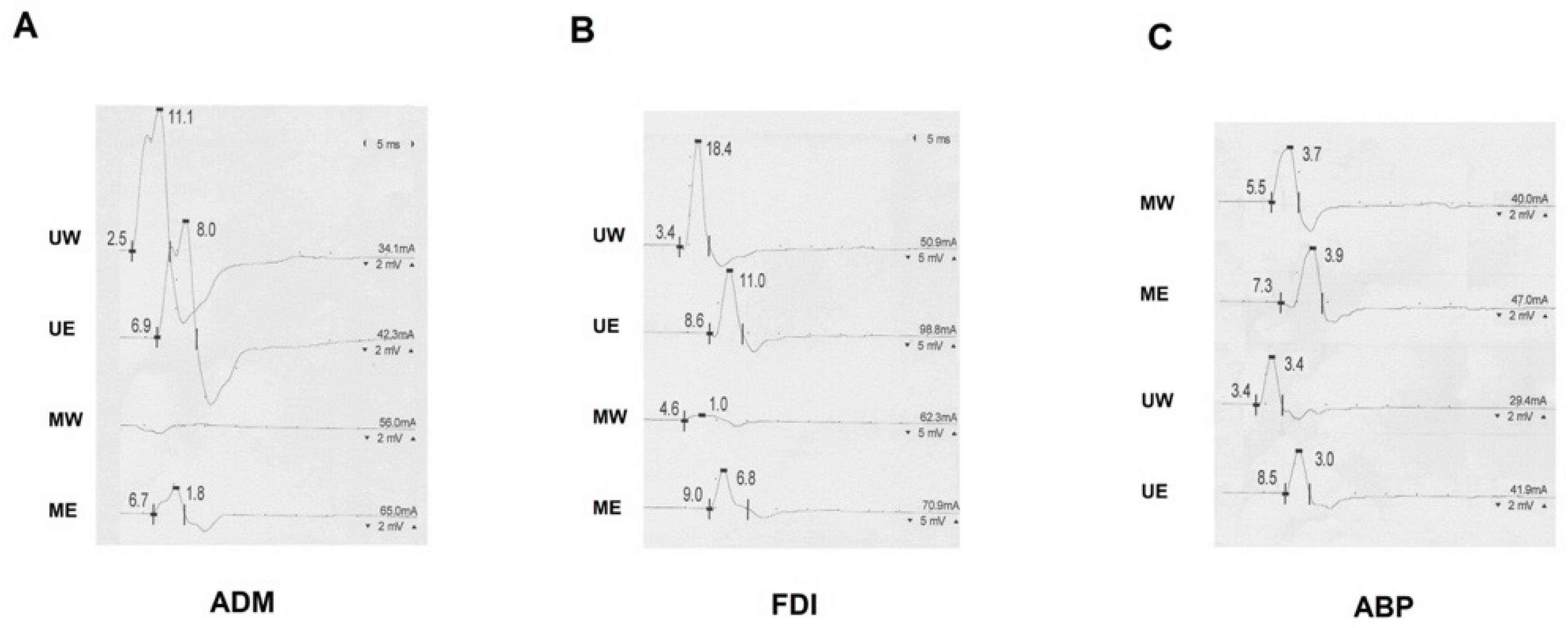

| Type of Communication | Frequency in Healthy Subjects | Distribution | Clinical Suspicion | NCS Findings | Possible Misdiagnosis |
|---|---|---|---|---|---|
| MUC type I | 2–44% [2,10,13,23,24,25,26,27] Coexisting MUC II in 14–100% of cases [10,23,25,26] | Proximal median to distal ulnar communication innervating the hypothenar muscles. | Absence of hypothenar involvement in the presence of ulnar nerve damage. | Greater CMAP amplitude over ADM recording when stimulating the ulnar nerve at the wrist compared to the elbow. | Ulnar neuropathy at the elbow/cubital tunnel syndrome |
| MUC type II | 8–58% [2,10,13,23,24,25,26,27] Coexisting MUC I in 9–31% of cases [10,23,25,26] | Proximal median to distal ulnar communication innervating the FDI muscle. | Absence of FDI involvement in the presence of ulnar nerve damage. | Greater CMAP amplitude over FDI recording when stimulating the ulnar nerve at the wrist compared to the elbow. | Ulnar neuropathy at the elbow/cubital tunnel syndrome |
| MUC type III | 0.01–30% [2,10,13,24,25,26,27] Coexisting MUC II in 20% of cases [10] | Proximal median to distal ulnar communication innervating the thenar muscles | Absence of thenar involvement in the presence of median nerve damage. | Greater CMAP amplitude over APB recording when stimulating the median nerve at the elbow compared to the wrist. | Carpal tunnel syndrome |
|
|
|
|
|
| MUC I | MUC II | MUC III | Total (Limbs) | |
|---|---|---|---|---|
| Limbs (n, %) | 18 (34%) | 39 (74%) | 32 (60%) | 53 |
| Sex (males, %) | 2 (11%) | 6 (15%) | 4 (13%) | 9 (17%) |
| Side (right, %) | 8 (44%) | 19 (49%) | 20 (63%) | 28 (53%) |
| Isolated communication (n, %) | 0 | 10 (26%) | 11 (34%) | 21 (40%) |
| Coexistent MUC I (n, %) | / | 18 (46%) | 9 (28%) | / |
| Coexistent MUC II (n, %) | 18 (100%) | / | 20 (63%) | / |
| Coexistent MUC III (n, %) | 9 (50%) | 20 (51%) | / | / |
| Recording Site | MUC I | MUC II | MUC III | Total Limbs |
|---|---|---|---|---|
| ADM ulnar nerve | ||||
| DML (ms) | 2.8 ± 0.6 | 2.8 ± 0.5 | 2.7 ± 0.5 | 2.7 ± 0.5 |
| CMAP-AW (mV) | 10.5 ± 2.3 | 10.5 ± 2.4 | 10.8 ± 2.0 | 10.6 ± 2.3 |
| CMAP-AE (mV) | 8.9 ± 2.4 | 9.1 ± 2.4 | 9.2 ± 2.2 | 9.1 ± 2.3 |
| CV (m/s) | 58.0 ± 7.6 | 57.8 ± 4.9 | 59.5 ± 4.7 | 58.7 ± 6.9 |
| Positive onset (n, %) | 2 (11%) | 2 (5%) | 1 (3%) | 4 (8%) |
| Double component (n, %) | 0 | 0 | 0 | 0 |
| Ulnar Gain in amplitude mV (%) | 1.7 ± 0.7 (21%) | 1.2 ± 0.9 (19%) | 1.4 ± 0.8 (22%) | 1.2 ± 0.9 (19%) |
| ADM median nerve | ||||
| DML (ms) | 5.6 ± 2.0 | 5.1 ± 1.9 | 5.2 ± 2.2 | 5.1 ± 1.9 |
| CMAP-AW (mV) | 0.2 ± 0.6 | 0.3 ± 0.6 | 0.4 ± 0.7 | 0.3 ± 0.6 |
| CMAP-AE (mV) | 1.1 ± 0.7 | 0.9 ± 0.7 | 0.7 ± 0.7 | 0.8 ± 0.7 |
| Positive onset (n, %) | 6 (33%) | 6 (15%) | 3 (9%) | 15 (28%) |
| Double component (n, %) | 0 | 0 | 0 | 0 |
| Median drop in amplitude mV (%) | 0.9 ± 0.4 (86%) | 0.5 ± 0.5 (73%) | 0.3 ± 0.4 (63%) | 0.4 ± 0.5 (73%) |
| FDI ulnar nerve | ||||
| DML (ms) | 3.4 ± 0.3 | 3.5 ± 0.4 | 3.6 ± 0.5 | 3.5 ± 0.4 |
| CMAP-AW (mV) | 11.9 ± 4.7 | 11.2 ± 4.4 | 10.4 ± 3.8 | 11.3 ± 4.4 |
| CMAP-AE (mV) | 7.1 ± 4.0 | 7.0 ± 3.7 | 6.8 ± 3.3 | 7.3 ± 3.9 |
| CV (m/s) | 55.8 ± 5.8 | 54.3 ± 7.1 | 52.6 ± 6.6 | 54.6 ± 6.9 |
| Positive onset (n, %) | 2 (11%) | 3 (8%) | 0 | 4 (8%) |
| Double component (n, %) | 0 | 0 | 0 | 0 |
| Ulnar Gain in amplitude mV (%) | 4.9 ± 2.4 (93%) | 4.1 ± 2.0 (80%) | 3.4 ± 1.5 (67%) | 3.9 ± 1.1 (73%) |
| FDI median nerve | ||||
| DML (ms) | 4.7 ± 0.8 | 4.9 ± 1.1 | 5.3 ± 1.5 | 4.8 ± 1.1 |
| CMAP-AW (mV) | 0.6 ± 4.0 | 0.7 ± 0.6 | 0.8 ± 1.2 | 0.8 ± 0.9 |
| CMAP-AE (mV) | 4.0 ± 2.6 | 3.6 ± 2.2 | 3.5 ± 2.0 | 3.5 ± 2.2 |
| Positive onset (n, %) | 0 | 0 | 0 | 0 |
| Double component (n, %) | 0 | 0 | 0 | 0 |
| Median drop in amplitude mV (%) | 3.3 ± 2.0 (81%) | 2.9 ± 1.7 (79%) | 2.6 ± 1.4 (73%) | 2.7 ± 1.6 (74%) |
| APB median nerve | ||||
| DML (ms) | 5.3 ± 2.6 | 5.2 ± 2.2 | 6.2 ± 2.4 | 5.3 ± 2.2 |
| CMAP-AW (mV) | 7.1 ± 3.1 | 7.0 ± 3.5 | 5.0 ± 3.4 | 6.3 ± 3.7 |
| CMAP-AE (mV) | 7.1 ± 3.0 | 7.4 ± 3.6 | 5.4 ± 3.4 | 6.6 ± 4.0 |
| CV (m/s) | 99.9 ± 58.7 | 103.1 ± 118.7 | 172.8 ± 113.8 | 123 ± 145 |
| Positive onset (n, %) | 12 (67%) | 22 (56%) | 28 (88%) | 35 (66%) |
| Double component (n, %) | 3 (17%) | 5 (13%) | 11 (34%) | 12 (23%) |
| Median drop in amplitude mV (%) | 0.1 ± 0.5 (1%) | 0.4 ± 0.7 (5%) | 0.5 ± 0.7 (6%) | 0.5 ± 0.7 (5%) |
| APB ulnar nerve | ||||
| DML (ms) | 3.1 ± 0.8 | 3.3 ± 0.8 | 3.2 ± 0.7 | 3.2 ± 0.7 |
| CMAP-AW (mV) | 4.2 ± 1.5 | 4.5 ± 2.0 | 4.3 ± 1.8 | 4.6 ± 2.2 |
| CMAP-AE (mV) | 3.6 ± 1.4 | 3.7 ± 1.5 | 3.6 ± 1.4 | 3.8 ± 1.6 |
| Positive onset (n, %) | 3 (17%) | 9 (23%) | 9 (28%) | 11 (21%) |
| Double component (n, %) | 0 | 0 | 0 | 0 |
| Ulnar Gain in amplitude mV (%) | 0.5 ± 0.6 (19%) | 0.7 ± 0.7 (21%) | 0.8 ± 0.6 (24%) | 0.7 ± 0.7 (21%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, V.; Gagliardo, A.; Barbone, F.; Vitale, M.; Ferri, L.; Lupica, A.; Iacono, S.; Di Muzio, A.; Brighina, F. Median-to-Ulnar Nerve Communication in Carpal Tunnel Syndrome: An Electrophysiological Study. Neurol. Int. 2021, 13, 304-314. https://doi.org/10.3390/neurolint13030031
Di Stefano V, Gagliardo A, Barbone F, Vitale M, Ferri L, Lupica A, Iacono S, Di Muzio A, Brighina F. Median-to-Ulnar Nerve Communication in Carpal Tunnel Syndrome: An Electrophysiological Study. Neurology International. 2021; 13(3):304-314. https://doi.org/10.3390/neurolint13030031
Chicago/Turabian StyleDi Stefano, Vincenzo, Andrea Gagliardo, Filomena Barbone, Michela Vitale, Laura Ferri, Antonino Lupica, Salvatore Iacono, Antonio Di Muzio, and Filippo Brighina. 2021. "Median-to-Ulnar Nerve Communication in Carpal Tunnel Syndrome: An Electrophysiological Study" Neurology International 13, no. 3: 304-314. https://doi.org/10.3390/neurolint13030031
APA StyleDi Stefano, V., Gagliardo, A., Barbone, F., Vitale, M., Ferri, L., Lupica, A., Iacono, S., Di Muzio, A., & Brighina, F. (2021). Median-to-Ulnar Nerve Communication in Carpal Tunnel Syndrome: An Electrophysiological Study. Neurology International, 13(3), 304-314. https://doi.org/10.3390/neurolint13030031








