Medically Refractory Neuroborreliosis Case Presented with Coexistance Involvements of Cranial 7 and 8 Nerves
Abstract
1. Introduction
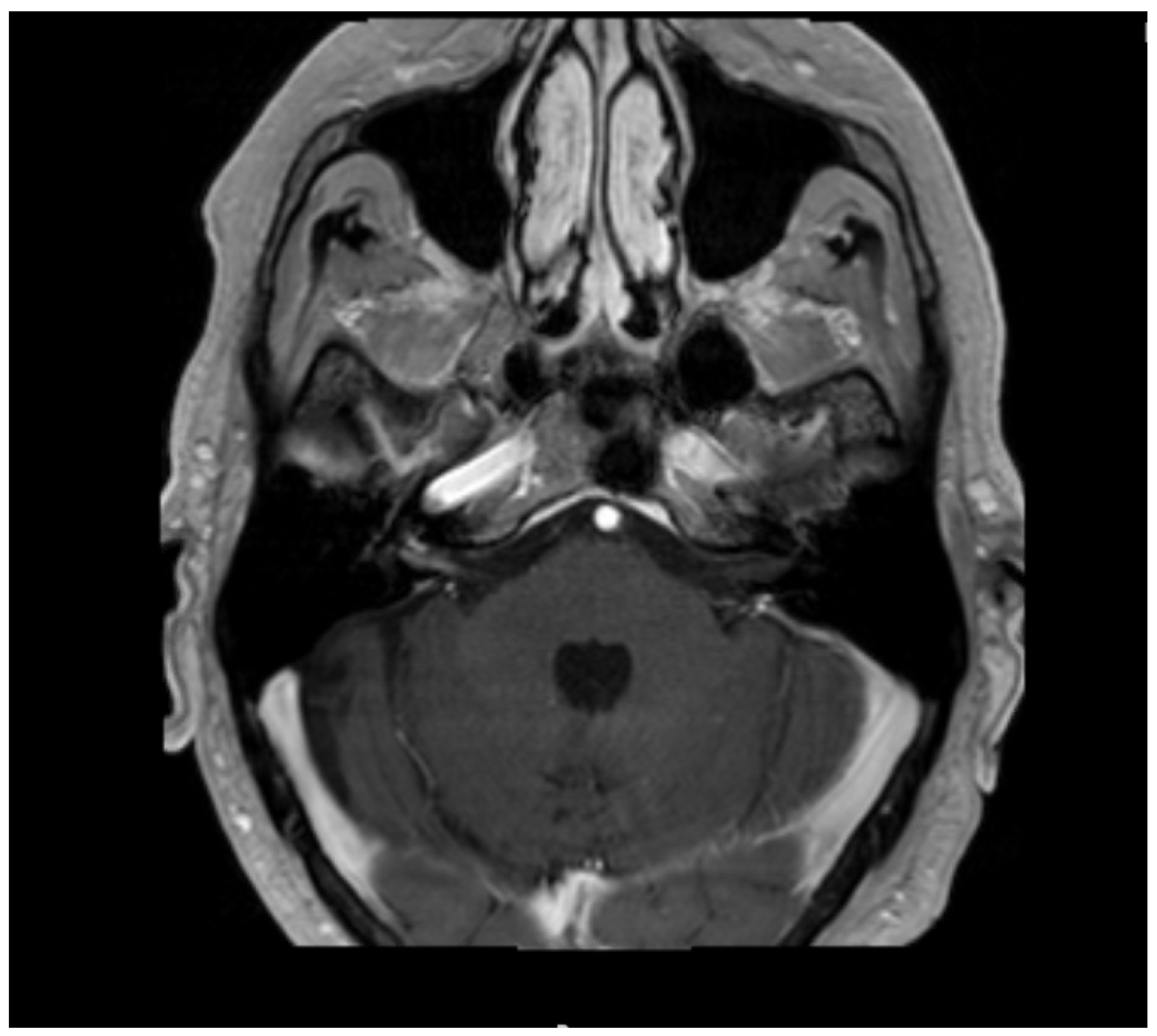
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 1995, 44, 590–591. [Google Scholar]
- Centers for Disease Control and Prevention. Lyme disease--United States, 2003–2005. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 573–576. [Google Scholar]
- Cook, M.J. Lyme borreliosis: A review of data on transmission time after tick attachment. Int. J. Gen. Med. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Halperin, J.J. Lyme neuroborreliosis. Curr. Opin. Infect. Dis. 2019, 32, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.J. Neuroborreliosis. Neurol. Clin. 2018, 36, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Burakgazi, A.Z. Lyme disease-induced polyradiculopathy mimicking amyotrophic lateral sclerosis. Int. J. Neurosci. 2014, 124, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Burakgazi, A.Z.; Henderson, C.S. Unusual Presentation of Unilateral Isolated Probable Lyme Optic Neuritis. Case Rep. Neurol. Med. 2016, 2016, 7471842. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.J. Neuroborreliosis and Neurosyphilis. Continuum (Minneap Minn) 2018, 24, 1439–1458. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.J. Neuroborreliosis. J. Neurol. 2017, 264, 1292–1297. [Google Scholar] [CrossRef]
- Nadelman, R.B.; Wormser, G.P. Lyme borreliosis. Lancet 1998, 352, 557–565. [Google Scholar] [CrossRef]
- Halperin, J.J. Oral treatment of parenchymal central nervous system neuroborreliosis—Are we there yet? Eur. J. Neurol. 2014, 21, 1147–1148. [Google Scholar] [CrossRef]
- Auwaerter, P.G.; Bakken, J.S.; Dattwyler, R.J.; Dumler, J.S.; Halperin, J.J.; McSweegan, E.; Nadelman, R.B.; O’Connell, S.; Shapiro, E.D.; Sood, S.K.; et al. Antiscience and ethical concerns associated with advocacy of Lyme disease. Lancet Infect. Dis. 2011, 11, 713–719. [Google Scholar] [CrossRef]
- Halperin, J.J. Diagnosis and management of Lyme neuroborreliosis. Expert Rev. Anti-Infect. Ther. 2018, 16, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.J. Nervous system lyme disease. Curr. Infect. Dis. Rep. 2015, 17, 445. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.J. Nervous system Lyme disease. Infect. Dis. Clin. N. Am. 2015, 29, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Luft, B.J.; Halperin, J.J.; Volkman, D.J.; Dattwyler, R.J. Ceftriaxone—An effective treatment of late Lyme borreliosis. J. Chemother. 1989, 1 (Suppl. 4), 917–919. [Google Scholar]
- Wormser, G.P.; Halperin, J.J. Oral doxycycline for neuroborreliosis. Lancet Neurol. 2008, 7, 665–666. [Google Scholar] [CrossRef]
- Halperin, J.J. Nervous system lyme disease: Diagnosis and treatment. Curr. Treat. Options Neurol. 2013, 15, 454–464. [Google Scholar] [CrossRef]
- Klempner, M.S.; Halperin, J.J.; Baker, P.J.; Shapiro, E.D.; O’Connell, S.; Fingerle, V.; Wormser, G.P. Lyme borreliosis: The challenge of accuracy. Neth. J. Med. 2012, 70, 3–5. [Google Scholar]
- Halperin, J.J. Diagnosis and treatment of the neuromuscular manifestations of lyme disease. Curr. Treat. Options Neurol. 2007, 9, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G. Fall and rise of Lyme disease and other Ixodes tick-borne infections in North America and Europe. Br. Med. Bull. 1998, 54, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug combinations against Borrelia burgdorferi persisters in vitro: Eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS ONE 2015, 10, e0117207. [Google Scholar] [CrossRef]
- Dattwyler, R.J.; Volkman, D.J.; Luft, B.J.; Halperin, J.J.; Thomas, J.; Golightly, M.G. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N. Engl. J. Med. 1988, 319, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Halperin, J.J. Toward a better understanding of European lyme neuroborreliosis. Clin. Infect. Dis. 2013, 57, 510–512. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hareem, A.; Dabiri, I.; Zaheer, N.; Burakgazi, A.Z. Medically Refractory Neuroborreliosis Case Presented with Coexistance Involvements of Cranial 7 and 8 Nerves. Neurol. Int. 2021, 13, 125-129. https://doi.org/10.3390/neurolint13010012
Hareem A, Dabiri I, Zaheer N, Burakgazi AZ. Medically Refractory Neuroborreliosis Case Presented with Coexistance Involvements of Cranial 7 and 8 Nerves. Neurology International. 2021; 13(1):125-129. https://doi.org/10.3390/neurolint13010012
Chicago/Turabian StyleHareem, Anam, Iman Dabiri, Nida Zaheer, and Ahmet Z. Burakgazi. 2021. "Medically Refractory Neuroborreliosis Case Presented with Coexistance Involvements of Cranial 7 and 8 Nerves" Neurology International 13, no. 1: 125-129. https://doi.org/10.3390/neurolint13010012
APA StyleHareem, A., Dabiri, I., Zaheer, N., & Burakgazi, A. Z. (2021). Medically Refractory Neuroborreliosis Case Presented with Coexistance Involvements of Cranial 7 and 8 Nerves. Neurology International, 13(1), 125-129. https://doi.org/10.3390/neurolint13010012



