MicroRNA and DNA Methylation Adaptation Mechanism to Endurance Training in Cardiovascular Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection Process and Data Extraction
2.4. Quality Assessment
3. Results
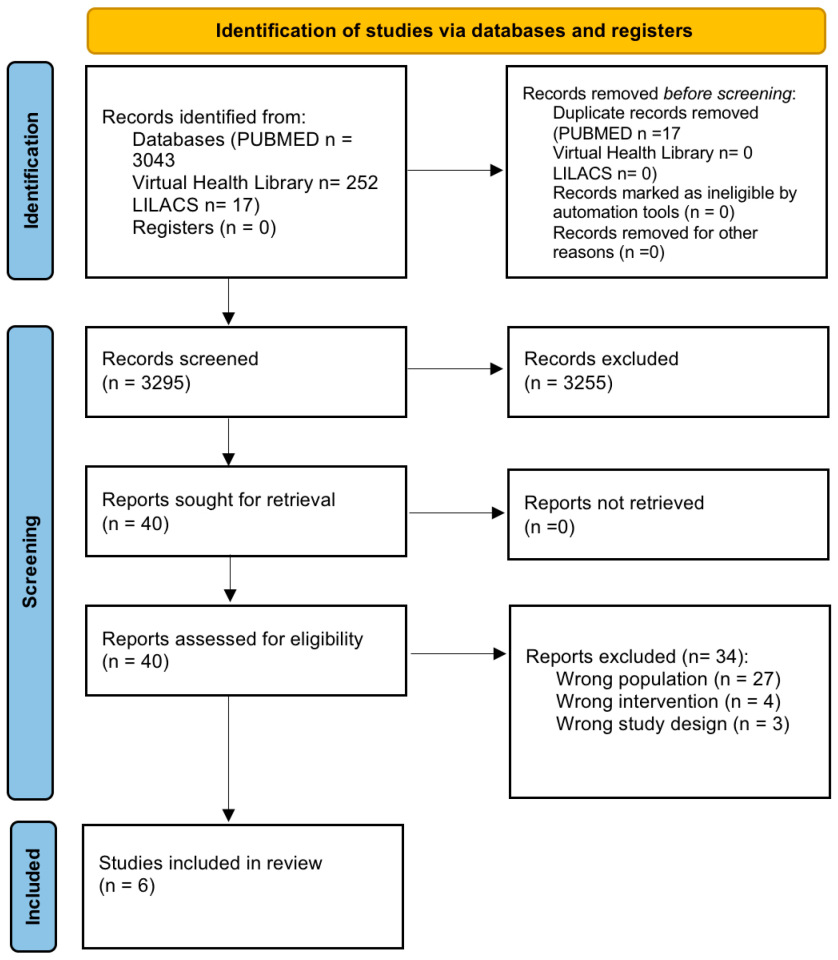
3.1. Selection Process
3.2. Study Characteristics
| First Author, (Year) | Study Design | Population Disease | Sample Size (Groups) | Sex Distribution M/F | Age (Years) Mean ± SD |
|---|---|---|---|---|---|
| Yamada et al. [24] (2020) | Non RCT pre- and post-intervention | HF | IG: n = 3 (HF) | 3 M | 60.0 ± 12.2 |
| CG: n = 3 (healthy controls) | 3 M | 58.7 ± 0.6 | |||
| Ferrari et al. [20] (2019) | Non RCT pre- and post-intervention | Hypertension | IG: n = 44 (hypertensive) | 33 M/11 F | 49.5 (no SD) |
| RG: n = 24 (normotensive) | 17 M/7 F | Not specified | |||
| Mayr et al. [22] (2021) | Non RCT pre- and post- intervention | CAD | IG: n = 10 (male) | 10 M | 53.2 ± 4.1 |
| IG: n = 10 (female) | 10 F | 62.7 ± 7.6 | |||
| Taurino et al. [23] (2010) | Mixed: cross-sectional and pre- and post-intervention | CAD | n = 12 (CAD CABG) | 12 M | 66 ± 11 |
| CG: n =12 (healthy) | 12 M | 59 ± 7 | |||
| IG: n = 10 (CAD CABG performing CRP) | 10 M | 69 ± 9 | |||
| Hsu et al. [21] (2023) | Non RCT pre- and post-intervention | HF | IG: n = 12 (HF) | 11 M/1 F | 56.5 ± 3.9 |
| Masoumi-Ardakani et al. [25] (2022) | Double blind RCT | Hypertension | IG: n = 13 (placebo) | 13 M | 49 ± 0.7 |
| IG: n = 13 (MitoQ) | 13 M | 49 ± 0.7 | |||
| IG: n = 13 (ET) | 13 M | 48 ± 0.9 | |||
| IG: n = 13 (MitoQ + ET) | 13 M | 47 ± 1.1 |
3.3. Intervention Characteristics
| First Author, (Year) | Type of Intervention | Duration and Frequency of Intervention |
|---|---|---|
| Yamada et al. [24] (2020) | Cardiac rehabilitation programme (bicycle ergometer) | 20 min, 2 x/day, 5 x/week 2 weeks Pre-determined anaerobic threshold intensity |
| Ferrari et al. [20] (2019) | Aerobic exercise-training programme (stationary bike or jogging) | 40 min (minimum 30 min of HR at anaerobic threshold) 4 x/week 12 continuous weeks |
| Mayr et al. [22] (2021) | Maximal ergospirometry | Increasing intensity by 10–20 watts every minute to reach maximal fatigue after 12.44 ± 3.23 min 1 session |
| Taurino et al. [23] (2010) | CABG surgery Cardiac rehabilitation programme (4–6 weeks post-CABG procedure) | / 60 min exercise (15 min warm-up, 30 min cardiovascular training, 15 min warming down) 2 x/week for 10 weeks |
| Hsu et al. [21] (2023) | HIIT programme using a bicycle ergometer | 3-min intervals of 80% VO2peak and 3-min intervals of 40% VO2peak for 30 min 36 sessions, 2–3 x/week |
| Masoumi-Ardakani et al. [25] (2022) | Placebo capsules MitoQ capsules Endurance training on cycle ergometer Simultaneous MitoQ (capsules) + ET (cycle ergometer) | 6 weeks 20 mg/day for 6 weeks 40–60% VO2peak, 3 x/week for 6 weeks MitoQ: 20 mg/day for 6 weeks ET: 40–60% VO2peak, 3 x/week for 6 weeks First session for 15 min, in subsequent sessions, an average of 2 min was added to the training time until the duration reached ~45 min |
3.4. Outcome Results
3.4.1. Molecular Outcomes
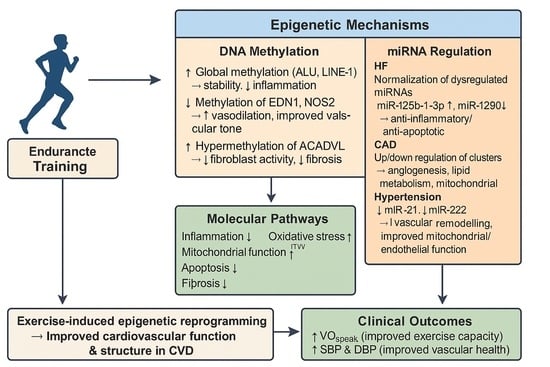
DNA Methylation
miRNA Expression
3.4.2. Clinical Parameters
Body Composition
Biochemical Markers
Cardiovascular Function
Cardiovascular Structure
| Author | Molecular Outcomes | Results | Clinical Parameters | Results |
|---|---|---|---|---|
| Yamada et al. (2020) [24] | miRNA expression | Body structure | ||
| hsa-miR-125b-1-3p | ↓↓ (pre vs. post CR in HF) | Body weight | ↓ (CR) | |
| hsa-miR-200c-3p | ↓↓ (pre vs. post CR in HF) | BMI | ↓ (CR) | |
| hsa-miR-3181 | ↓↓ (pre vs. post CR in HF) | Cardiovascular function | ||
| hsa-miR-1290 | ↑↑ (pre vs. post CR in HF) | SBP | ↓↓ (CR) | |
| hsa-miR-196b-3p | ↑↑ (pre vs. post CR in HF), ↑ (pre vs. post CR) | DBP | ↓ (CR) | |
| hsa-miR-24-3p | ↓ (pre vs. post CR) | HR | ↓ (CR) | |
| hsa-miR-3661 | ↓ (pre vs. post CR) | |||
| hsa-miR-30c-1-3p | ↑ (pre vs. post CR) | |||
| hsa-miR-3945 | ↑ (pre vs. post CR) | |||
| hsa-miR-7151-3p | ↑ (pre vs. post CR) | |||
| Ferrari et al. (2019) [20] | DNA methylation | Cardiovascular function | ||
| ALU | ↑↑ (ETP) | VO2peak | ↑ (ETP) | |
| LINE-1 | ↑↑ (ETP) | SBP | ↓(ETP) | |
| EDN1 | ↓↓ (ETP) | DBP | ↓ (ETP) | |
| NOS2 | ↓↓ (ETP) | |||
| NOS3 | ↑ (ETP) | |||
| ICAM1 | ↑ (ETP) | |||
| TLR2 | ↑ (ETP) | |||
| TNF | ↑ (ETP) | |||
| Mayr et al. (2021) [22] | miRNA expression | |||
| miR-338-3p | ↑↑ (ME) | |||
| miR-223-3p | ↑↑ (ME) | |||
| miR-197-3p | ↑↑ (ME) | |||
| miR-199a-3p | ↑↑ (ME) | |||
| miR-99b-5p | ↑↑ (ME) | |||
| let-7f-5p | ↑↑ (ME) | |||
| miR-146a-5p | ↑↑ (ME) | |||
| miR-342-3p | ↑↑ (ME) | |||
| miR-23b-3p | ↑↑ (ME) | |||
| miR-150-5p | ↑↑ (ME) | |||
| miR-23a-3p | ↑↑ (ME) | |||
| miR-24-3p | ↑↑ (ME) | |||
| miR-30b-5p | ↑↑ (ME) | |||
| miR-26a-5p | ↑↑ (ME) | |||
| miR-192-5p | ↓↓ (ME) | |||
| miR-22-3p | ↓↓ (ME) | |||
| let-7i-5p | ↓↓ (ME) | |||
| miR-186-6p | ↓↓ (ME) | |||
| miR-423-5p | ↓↓ (ME) | |||
| miR-25-3p | ↓↓ (ME) | |||
| miR-92a-3p | ↓↓ (ME) | |||
| miR-185-5p | ↓↓ (ME) | |||
| miR-17-5p | ↓↓ (ME) | |||
| miR-16-5P | ↓↓ (ME) | |||
| miR-425-5P | ↓↓ (ME) | |||
| miR-320a | ↓↓ (ME) | |||
| miR-130a-3p | ↓↓ (ME) | |||
| miR-140-3p | ↓↓ (ME) | |||
| miR-363-3p | ↓↓ (ME) | |||
| let-7b-5p | ↓↓ (ME) | |||
| miR-16-2-3p | ↓↓ (ME) | |||
| miR-451a | ↓↓ (ME) | |||
| miR-101-3p | ↓↓ (ME) | |||
| Taurino et al. (2010) [23] | miRNA expression | |||
| hsa-miR-140-3p | ↑ (CAD vs. controls | |||
| hsa-miR182 | ↑ (CAD vs. controls) | |||
| hsa-miR-92a | ↑ (EP) | |||
| hsa-miR-92b | ↑ (EP) | |||
| Gene expression profiling | ||||
| NDUFB3 | ↑↑ (CAD vs. controls) | |||
| COX7C | ↑↑ (CAD vs. controls), ↓ (EP) | |||
| UQCRQ | ↑↑ (CAD vs. controls), ↓ (EP) | |||
| ATP5I | ↑↑ (CAD vs. controls), ↓ (EP) | |||
| NDUFA1 | ↓ (EP) | |||
| CASP3, | ↓ (EP) | |||
| ATP5L | ↓ (EP) | |||
| Hsu et al. (2023) [21] | DNA methylation | Cardiovascular function | ||
| DNMT1 | ↑↑ (pre vs. post HIIT) | VO2peak | ↑↑ | |
| ACADVL | ↑↑↑ | LVESV | ↓↓ | |
| After knockdown of ACADVL: | LVEDV | ↓↓ | ||
| ↓↓VLCAD | COex | ↑↑ | ||
| ↓↓Β-actin | LVEF | ↑↑ | ||
| ↑ Cyto C | BNP | ↓ | ||
| ↑ CASP3 | Cardiovascular structure | |||
| Heart size | ↓↓ | |||
| ECV | ↓↓ (middle, apex, total LV segments), = (LV base) | |||
| Masoumi-Ardakani et al. (2022) [25] | miRNA expression | Body structure | ||
| miR-21 | ↓↓ (MitoQ, ET, MitoQ + ET) | Body weight | ↓↓ (ET, MitoQ + ET) | |
| miR-222 | ↓↓ (MitoQ, MitoQ + ET) | BMI | ↓↓ (ET, MitoQ + ET) | |
| Body fat percentage | ↓↓ (ET, MitoQ + ET) | |||
| Biochemical makers | ||||
| TAC | ↑↑ (MitoQ, ET, MitoQ + ET) | |||
| MDA | ↓↓ (MitoQ, ET, MitoQ + ET) | |||
| IL-6 | ↓↓ (MitoQ, ET, MitoQ + ET) | |||
| Cardiovascular function | ||||
| EF | = | |||
| LV filling | = | |||
| SBP | ↓↓ (ET, MitoQ + ET) | |||
| DBP | ↓ (MitoQ + ET) | |||
| HR | = | |||
| VO2 | = | |||
| Cardiovascular structure | ||||
| LV mass | ↓↓ (MitoQ + ET) | |||
| LVESD | ↓↓ (MitoQ + ET) | |||
| LV mass index | = | |||
| LVEDD | = | |||
| RWT | = |
3.5. Article Appraisal
| Study | Yamada et al. [24] | Ferrari et al. [20] | Mayr et al. [22] | Taurino et al. [23] | Hsu et al. [21] | ||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 |  |  |  |  |  | ||||
| Q2 |  | 4 | 9 |  | 11 |  | |||
| Q3 | 1 | 5 |  |  | 12 |  | |||
| Q4 | 2 | 6 |  | 13 | 14 |  | |||
| Q5 |  | 3 | 7 |  | 10 |  |  |  | |
| Q6 |  |  |  |  |  | ||||
| Q7 |  |  |  |  |  | ||||
| Q8 |  |  | 8 |  | 15 | 16 |  | ||
| Q9 |  |  |  |  |  | ||||
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 |
| Masoumi-Ardakani et al. [25] |  |  |  |  |  |  |  |
| Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | ||
 |  |  |  |  |  |
4. Discussion
4.1. DNA Methylation
4.2. MiRNA Expression
4.3. Clinical Parameters
4.4. Quality and Limitations
4.5. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK535419/ (accessed on 16 June 2025).
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Morici, G.; Gruttad’Auria, C.I.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Bonsignore, M.R. Endurance training: Is it bad for you? Breathe Sheff. Engl. 2016, 12, 140–147. [Google Scholar] [CrossRef]
- Isath, A.; Koziol, K.J.; Martinez, M.W.; Garber, C.E.; Martinez, M.N.; Emery, M.S.; Baggish, A.L.; Naidu, S.S.; Lavie, C.J.; Arena, R.; et al. Exercise and cardiovascular health: A state-of-the-art review. Prog. Cardiovasc. Dis. 2023, 79, 44–52. [Google Scholar] [CrossRef]
- Huang, G.; Gibson, C.A.; Tran, Z.V.; Osness, W.H. Controlled Endurance Exercise Training and VO2max Changes in Older Adults: A Meta-Analysis. Prev. Cardiol. 2005, 8, 217–225. [Google Scholar] [CrossRef]
- Wilmore, J.H.; Knuttgen, H.G. Aerobic Exercise and Endurance: Improving Fitness for Health Benefits. Phys. Sportsmed. 2003, 31, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Kwak, H.-B.; Kim, A.H.; Park, S.H.; Heo, J.W.; Kim, H.K.; Ko, J.R.; Lee, S.J.; Bang, H.S.; Sim, J.W.; et al. Cardiac adaptation to exercise training in health and disease. Pflugers Arch. 2020, 472, 155–168. [Google Scholar] [CrossRef]
- Soci, U.P.R.; Melo, S.F.S.; Gomes, J.L.P.; Silveira, A.C.; Nóbrega, C.; de Oliveira, E.M. Exercise Training and Epigenetic Regulation: Multilevel Modification and Regulation of Gene Expression. Adv. Exp. Med. Biol. 2017, 1000, 281–322. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Pan, L.; Zhou, L.; Mozaffaritabar, S.; Gu, Y.; Pinho, R.A.; Zheng, X.; Ba, X.; Boldogh, I. Epigenetic and “redoxogenetic” adaptation to physical exercise. Free Radic. Biol. Med. 2024, 210, 65–74. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, X.; Gao, F. The epigenetic landscape of exercise in cardiac health and disease. J. Sport Health Sci. 2021, 10, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Izquierdo, D.; Torres-Martos, Á.; Baig, A.T.; Aguilera, C.M.; Ruiz-Ojeda, F.J. Impact of Physical Activity and Exercise on the Epigenome in Skeletal Muscle and Effects on Systemic Metabolism. Biomedicines 2022, 10, 126. [Google Scholar] [CrossRef]
- Fernandes, T.; Baraúna, V.G.; Negrão, C.E.; Phillips, M.I.; Oliveira, E.M. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H543–H552. [Google Scholar] [CrossRef]
- Fulghum, K.; Hill, B.G. Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling. Front. Cardiovasc. Med. 2018, 5, 127. [Google Scholar] [CrossRef]
- Gibb, A.A.; Hill, B.G. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Z.; Liang, H. Role of physical activity in cardiovascular disease prevention: Impact of epigenetic modifications. Front. Cardiovasc. Med. 2025, 12, 1511222. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The revised JBI critical appraisal tool for the assessment of risk of bias for quasi-experimental studies. JBI Evid. Synth. 2024, 22, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Vicenzi, M.; Tarantini, L.; Barretta, F.; Sironi, S.; Baccarelli, A.A.; Guazzi, M.; Bollati, V. Effects of Physical Exercise on Endothelial Function and DNA Methylation. Int. J. Environ. Res. Public Health 2019, 16, 2530. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Wang, J.-S.; Shyu, Y.-C.; Fu, T.-C.; Juan, Y.-H.; Yuan, S.-S.; Wang, C.-H.; Yeh, C.-H.; Liao, P.-C.; Wu, H.-Y.; et al. Hypermethylation of ACADVL is involved in the high-intensity interval training-associated reduction of cardiac fibrosis in heart failure patients. J. Transl. Med. 2023, 21, 187. [Google Scholar] [CrossRef]
- Mayr, B.; Müller, E.E.; Schäfer, C.; Droese, S.; Schönfelder, M.; Niebauer, J. Exercise-induced changes in miRNA expression in coronary artery disease. Clin. Chem. Lab. Med. 2021, 59, 1719–1727. [Google Scholar] [CrossRef]
- Taurino, C.; Miller, W.H.; McBride, M.W.; McClure, J.D.; Khanin, R.; Moreno, M.U.; Dymott, J.A.; Delles, C.; Dominiczak, A.F. Gene expression profiling in whole blood of patients with coronary artery disease. Clin. Sci. Lond. Engl. 1979 2010, 119, 335–343. [Google Scholar] [CrossRef]
- Yamada, R.; Okumura, S.; Kono, Y.; Miyazaki, A.; Niwa, Y.; Ito, T.; Ueda, S.; Ishiguro, T.; Yoshinaga, M.; Fujiwara, W.; et al. Effect of cardiac rehabilitation on circulating microRNA expressknown for its implication inion in heart failure: A preliminary study. Fujita Med. J. 2021, 7, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Masoumi-Ardakani, Y.; Najafipour, H.; Nasri, H.R.; Aminizadeh, S.; Jafari, S.; Safi, Z. Moderate Endurance Training and MitoQ Improve Cardiovascular Function, Oxidative Stress, and Inflammation in Hypertensive Individuals: The Role of miR-21 and miR-222: A Randomized, Double-Blind, Clinical Trial. Cell J. 2022, 24, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Newell-Price, J.; Clark, A.J.L.; King, P. DNA Methylation and Silencing of Gene Expression. Trends Endocrinol. Metab. 2000, 11, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Knottnerus, S.J.G.; Bleeker, J.C.; Ferdinandusse, S.; Houtkooper, R.H.; Langeveld, M.; Nederveen, A.J.; Strijkers, G.J.; Visser, G.; Wanders, R.J.A.; Wijburg, F.A.; et al. Subclinical effects of long-chain fatty acid β-oxidation deficiency on the adult heart: A case-control magnetic resonance study. J. Inherit. Metab. Dis. 2020, 43, 969–980. [Google Scholar] [CrossRef]
- Ghazal, R.; Wang, M.; Liu, D.; Tschumperlin, D.J.; Pereira, N.L. Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure. Circ. Res. 2025, 136, 773–802. [Google Scholar] [CrossRef]
- Shang, F.; Wang, S.-C.; Hsu, C.-Y.; Miao, Y.; Martin, M.; Yin, Y.; Wu, C.-C.; Wang, Y.-T.; Wu, G.; Chien, S.; et al. MicroRNA-92a Mediates Endothelial Dysfunction in CKD. J. Am. Soc. Nephrol. JASN 2017, 28, 3251–3261. [Google Scholar] [CrossRef]
- Das, D.; Kumar, A.; Sharma, M. A systematic review of work-related musculoskeletal disorders among handicraft workers. Int. J. Occup. Saf. Ergon. JOSE 2020, 26, 55–70. [Google Scholar] [CrossRef]
- Bernstein, D.L.; Jiang, X.; Rom, S. let-7 microRNAs: Their Role in Cerebral and Cardiovascular Diseases, Inflammation, Cancer, and Their Regulation. Biomedicines 2021, 9, 606. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, J.; Wang, L.; Pei, G.; Cheng, H.; Zhang, Q.; Wang, S.; He, C.; Fu, C.; Wei, Q. MiR-125 Family in Cardiovascular and Cerebrovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 799049. [Google Scholar] [CrossRef] [PubMed]
- Guz, M.; Jeleniewicz, W.; Cybulski, M. An Insight into miR-1290: An Oncogenic miRNA with Diagnostic Potential. Int. J. Mol. Sci. 2022, 23, 1234. [Google Scholar] [CrossRef]
- Xu, H.; Cui, Y.; Liu, X.; Zheng, X.; Liu, J.; Hu, X.; Gao, F.; Hu, X.; Li, M.; Wei, X.; et al. miR-1290 promotes IL-8-mediated vascular endothelial cell adhesion by targeting GSK-3β. Mol. Biol. Rep. 2022, 49, 1871–1882. [Google Scholar] [CrossRef]
- miRBase Entry: hsa-mir-200c. Available online: https://mirbase.org/hairpin/MI0000650?acc=MI0000650 (accessed on 20 June 2025).
- Klimczak-Tomaniak, D.; Haponiuk-Skwarlińska, J.; Kuch, M.; Pączek, L. Crosstalk between microRNA and Oxidative Stress in Heart Failure: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 15013. [Google Scholar] [CrossRef] [PubMed]
- Pernaute, B.; Spruce, T.; Smith, K.M.; Sánchez-Nieto, J.M.; Manzanares, M.; Cobb, B.; Rodríguez, T.A. MicroRNAs control the apoptotic threshold in primed pluripotent stem cells through regulation of BIM. Genes Dev. 2014, 28, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, D.; Clauss, S.; Weckbach, L.T.; Brunner, S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells 2019, 8, 1128. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delhez, J.; Ougier, J.; de Araujo, F.X.; de Abreu, R.M.; Corbellini, C. MicroRNA and DNA Methylation Adaptation Mechanism to Endurance Training in Cardiovascular Disease: A Systematic Review. Cardiogenetics 2025, 15, 28. https://doi.org/10.3390/cardiogenetics15040028
Delhez J, Ougier J, de Araujo FX, de Abreu RM, Corbellini C. MicroRNA and DNA Methylation Adaptation Mechanism to Endurance Training in Cardiovascular Disease: A Systematic Review. Cardiogenetics. 2025; 15(4):28. https://doi.org/10.3390/cardiogenetics15040028
Chicago/Turabian StyleDelhez, Jil, Jeanne Ougier, Francisco Xavier de Araujo, Raphael Martins de Abreu, and Camilo Corbellini. 2025. "MicroRNA and DNA Methylation Adaptation Mechanism to Endurance Training in Cardiovascular Disease: A Systematic Review" Cardiogenetics 15, no. 4: 28. https://doi.org/10.3390/cardiogenetics15040028
APA StyleDelhez, J., Ougier, J., de Araujo, F. X., de Abreu, R. M., & Corbellini, C. (2025). MicroRNA and DNA Methylation Adaptation Mechanism to Endurance Training in Cardiovascular Disease: A Systematic Review. Cardiogenetics, 15(4), 28. https://doi.org/10.3390/cardiogenetics15040028