Highlights
What are the main findings?
- Simplified Dutch Lipid Clinic Network Scores (sDLCNS) for familial hypercholesterolemia (FH) were calculated in a prospective cohort of 245 patients studied in a Cardiac Rehabilitation Program (CRP) after myocardial infarction (MI) occurance.
- A third of the cohort was categorized with likelihoods rated as “possible” (n = 72, 29.4%) and “probable” (n = 11, 4.5%) for FH by sDLCNS.
- The rate of genetic testing for FH is low (n = 4, 1.6%), even in this high-risk subgroup of patients.
What is the implication of the main finding?
- Strategies to improve screening for FH should be prospectively implemented during CRP after MI.
- The implications of these strategies in enhancing lipid control in secondary prevention and cascade screening in first-degree relatives should be further studied.
Abstract
Familial hypercholesterolemia (FH) is relatively prevalent in myocardial infarction (MI) sufferers, and its diagnosis could improve preventive treatment in family members. We aim to analyze the diagnosis of FH and the rate of genetic testing in a prospective cohort of 245 patients submitted to our Cardiac Rehabilitation Program (CRP) after MI. Baseline characteristics were registered, and basal low-density lipoprotein cholesterol (LDL-C) was calculated after correction for lipid-lowering therapies (LLT) before or during admission. Simplified Dutch Lipid Clinic Network Scores (sDLCNS) were retrospectively calculated based on personal and familial history of premature cardiovascular disease and basal LDL-C levels. Mean age was 62.19 ± 13.93 years, and most patients were male (81.6%). Mean LDL-C before admission and basal LDL-C corrected for LLT were 131.79 ± 45.34 mg/dL and 162.87 ± 44.17 mg/dL, respectively. Patients in the cohort were retrospectively categorized in the “unlikely” (<3 points; n = 162, 66.1%), “possible” (3–5 points; n = 72, 29.4%) and “probable” (6–8 points; n = 11, 4.5%) sDLCNS categories. Genetic testing for FH was requested in four (1.6%) patients, and no clinically significant genetic variants were detected. Patients who underwent genetic testing depicted significantly higher basal LDL-C (233 ± 49.09 vs. 161.71 ± 43.25 mg/dL, p = 0.001). However, the rate of individuals undergoing genetic testing was negligible even in the “possible” (n = 2, 2.8%) and “probable” (n = 1, 9.1%) sDLCNS categories. In conclusion, genetic testing for FH in our CRP after MI is largely underutilized, even in patients with a “possible” or “probable” diagnosis based on sDLCNS criteria, which represent about a third of the cohort. Strategies to improve screening for FH should be prospectively implemented.
1. Introduction
Familial hypercholesterolemia (FH) is a heterogeneous genetically inherited condition characterized by markedly elevated serum levels of low-density lipoprotein cholesterol (LDL-C) and an increased risk of cardiovascular disease (CVD) [1]. FH is an autosomal-dominant disease, with an estimated prevalence of 1 in 250–300 cases in its heterozygous type and 1 in 250,000–360,000 cases in its homozygous type [2]. However, most cases in the population remain underdiagnosed and untreated.
FH diagnosis should be suspected in patients with a familial or personal history of markedly elevated LDL-C levels and/or cardiovascular disease, especially in cases of premature CVD. Patients who experience an acute myocardial infarction (MI), particularly at a young age, represent a subgroup with increased prevalence of FH [3,4,5]. In these instances, FH should be suspected, and the diagnosis should be confirmed, arguably for at least two different reasons: (1) to identify individuals in which optimal lipid control in secondary prevention may be harder to achieve, potentially requiring advanced lipid-lowering therapies (LLT) therapies or specific therapeutic alternatives [5,6,7]; and (2) to enable cascade screening in first-degree relatives, which can have significant implications for primary prevention in these individuals [6,8,9].
The diagnosis of FH is typically established using the DLCN criteria [2,10,11,12], although a simplified version of these criteria can be useful in instances with limited availability to specific clinical information [4,13]. Given that genetic testing is associated with increased costs, universal screening after MI would be unfeasible, so risk stratification using FH criteria could be used to select patients for genetic testing.
After an MI, patients should be submitted to a Cardiac Rehabilitation Program (CRP), which provides the optimal setting for control of cardiovascular risk factors, therapeutic optimization, exercise prescription, and improvement of prognosis and quality of life [14]. Moreover, dedicated follow-up during Phase 2 CRP could be an ideal scenario for FH suspicion, genetic testing in suspected cases [15], and even cascade screening in first-degree relatives, if available.
The aim of our study is to analyze, in a CRP after MI, the suspicion of FH based on retrospectively calculated simplified Dutch Lipid Clinic Network Score (sDLCNS) criteria. Additionally, we also aim to examine real-world genetic testing to confirm the diagnosis of FH, with the goal of suggesting improvement strategies.
2. Materials and Methods
2.1. Population
The study population comprised patients with ST-segment elevation myocardial infarction (STEMI) or occlusion myocardial infarction (OMI) submitted to our CRP after discharge between January 2022 and July 2024. All STEMI/OMI patients in our institution, a high-complexity tertiary care hospital, are included in CRP unless contraindicated. Reasons for exclusion were severe functional limitation or life expectancy (n = 9), patient rejection for CRP (n = 7), contraindication for CRP (n = 3), and follow-up losses during CRP (n = 4).
We registered baseline clinical characteristics such as age, sex, cardiovascular risk factors, infarct location, Killip class during admission, Global Registry of Acute Coronary Events (GRACE) risk score, and echocardiographic left ventricular ejection fraction (LVEF) before discharge. We also registered the personal history of CVD (ischemic heart disease, ischemic stroke, and/or peripheral artery disease) and the familial history of CVD. Premature CVD was considered if onset was <55 years in men and <60 years in women.
The lipid profile was analyzed during admission, and we also registered the most recent lipid profile prior to admission, if available. The following variables were studied: fasting glucose (mg/dL), total cholesterol (mg/dL), triglycerides (mg/dL), HDL cholesterol (HDL-C, mg/dL), and LDL-C (mg/dL). Additionally, lipoprotein (a) levels were analyzed during admission or shortly after discharge.
The final study group comprised 245 patients. The study flowchart can be consulted in Figure 1.
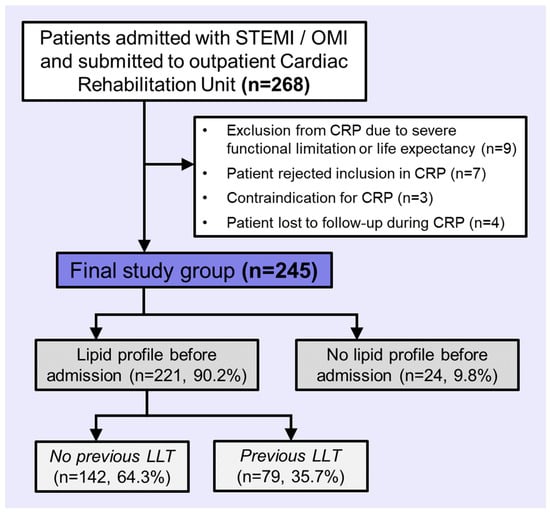
Figure 1.
Flowchart of patients included in the study. Abbreviations: CRP = Cardiac Rehabilitation Program. LLT = lipid-lowering therapy. OMI = occlusion myocardial infarction. STEMI = ST-segment elevation acute myocardial infarction.
2.2. Lipid Profile and Basal LDL-C
Basal LDL-C was analyzed in patients with at least one lipid profile before admission (n = 221, 90.2%) and in patients without any lipid profile before admission (n = 24, 9.8%; Figure 1).
In patients not receiving LLT before admission (n = 142, 64.3%), their most recent lipid testing before admission was used to determine their basal LDL-C.
In patients receiving LLT before admission (n = 79, 35.7%), their corrected basal LDL-C was calculated by an extrapolation using their measured LDL-C levels before admission and the estimated potency of the LLT which they were being treated with at that time [16]. We used the following formula:
Finally, in patients without any blood testing before admission, basal LDL-C was extrapolated by correcting their LDL-C during admission, accounting for the prescribed lipid-lowering therapy (i.e., oral atorvastatin 80 mg o.d. in all patients) and the days of treatment until blood testing. As previously reported, we estimated a 10% reduction in LDL-C if the blood test was sampled on the first day of admission, 30% during days 1 and 2, 40% in days 3 and 4, and a 45% reduction in subsequent days [16]. The same formula was applied.
2.3. Screening for Familial Hypercholesterolemia
During Phase 2 CRP, opportunistic screening for FH was performed based on basal LDL-C levels [16]. Genetic testing was requested, and familial screening was recommended in individuals with suspected FH.
In this study, we retrospectively calculated the sDLCNS, which are described in Figure 2, based on personal and familial history of premature CVD and basal LDL-C levels [4,13]. FH was categorized as “unlikely” if <3 points, “possible” if 3 to 5 points, “probable” if 6 to 8 points, and “confirmed” if >8 points.
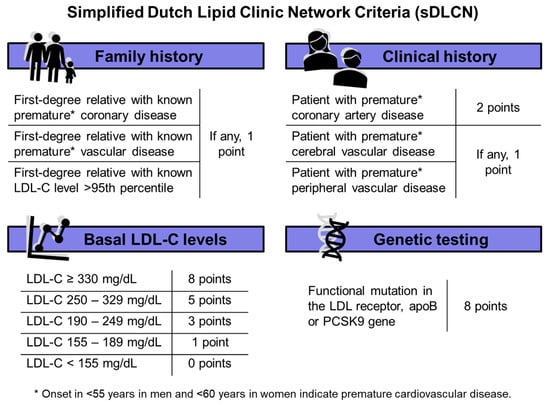
Figure 2.
Simplified Dutch Lipid Clinic Network Criteria (sDLCN). Abbreviations: LDL-C = low-density lipoprotein cholesterol. apoB = apolipoprotein B. PCSK9 = proprotein convertase subtilisin/kexin type 9.
2.4. Genetic Testing for Familial Hypercholesterolemia
We analyzed the percentage of patients in which genetic testing for FH was requested, specifically in the “possible”, “probable” and “confirmed” categories. Genetic testing was performed in a centralized laboratory (Health in Code S.L., A Coruña, Spain) by next-generation sequencing. Specific hybridization SureSelect XT HS Low Input (Agilent Technologies Spain, S.L., Madrid, Spain) probes for Illumina multiplex paired-end sequencing were used, and fragments were sequenced in the sequencing system NovaSeq 6000 (Illumina, Madrid, Spain). Sequencing data were analyzed using a proprietary bioinformatic pipeline. The basic profile included 6 genes (APOB, APOE, LDLR, LDLRAP1, PCSK9, and SLCO1B1), and the advanced profile included 12 genes (ABCG5, ABCG8, APOB, APOE, CRABP2, EPHX2, LDLR, LDLRAPI, LIPA, LPA, PCSK9, and PNPLAS).
2.5. Ethics
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Drug Research (CEIm) of Hospital Clinico Universitario de Valencia (protocol code: 2019/262 and date of approval: 26 May 2020). Informed consent was obtained from all subjects involved in the study. A specific informed consent was obtained for genetic testing, following local policies and procedures in our institution.
2.6. Statistical Analysis
One-sample Kolmogorov–Smirnov test was used to test the normal data distribution. For continuous parametric variables, data are expressed as mean ± standard deviation and analyzed by Student’s t test. Continuous non-parametric variables are shown as median plus interquartile range and compared with Mann–Whitney U test. Qualitative variables are presented as percentage and compared by chi-square test or Fisher’s exact test. Statistical significance was considered for 2-tailed p-values < 0.05. The SPSS statistical package version 26.0 was used.
3. Results
3.1. Cohort Description
The final cohort comprised 245 patients included in our CRP after a STEMI/OMI. Baseline characteristics of the cohort are depicted in Table 1.

Table 1.
Baseline characteristics and lipid and metabolic profile of patients included in the Cardiac Rehabilitation Program.
The mean age was 62.19 ± 13.93 years, and most patients were male (81.6%). Hypercholesterolemia was the most prevalent cardiovascular risk factor (89.4%), followed by hypertension (56.7%) and smoking habit (49.8%). The mean GRACE risk score was 119.09 ± 29.45 points, indicative of an intermediate risk. The mean LVEF was 51.77 ± 10.58%, and 38% depicted a LVEF < 50%.
Previous history of chronic coronary syndrome, peripheral artery disease, or cerebral vascular disease were relatively uncommon in the cohort (8.6, 7.8 and 5.1%, respectively). Mean LDL-C before admission was 131.79 ± 45.34 mg/dL, and a significant reduction in LDL-C levels during admission was noted (mean: 101.68 ± 36.6 mg/dL), as previously reported [16]. However, basal LDL-C levels were higher after correction for LLT (mean: 162.87 ± 44.17 mg/dL).
3.2. Screening for FH
We used simplified Dutch Lipid Clinic Network Score (sDLCNS) for FH, as depicted in Table 2. Premature coronary artery disease was relatively prevalent in the cohort (n = 72, 29.4%), as well as markedly elevated LDL-C—52 patients (21.2%) depicted basal LDL-C between 190 and 249 mg/dL, and 9 patients (3.7%) had basal LDL-C between 250 and 329 mg/d. Family history of premature coronary artery disease in first-degree relatives was uncommon (n = 17, 6.9%).

Table 2.
Simplified Dutch Lipid Clinic Network Score for familial hypercholesterolemia in the cohort.
Patients in the cohort were retrospectively categorized in the “unlikely” (<3 points; n = 162, 66.1%), “possible” (3–5 points; n = 72, 29.4%), and “probable” (6–8 points; n = 11, 4.5%) sDLCNS categories (Figure 3). No patients were categorized as “definite” FH.

Figure 3.
Categories in simplified Dutch Lipid Clinic Network Score for familial hypercholesterolemia.
3.3. Genetic Testing for FH
Genetic testing for FH was requested in four patients (1.6% of the cohort). Two patients underwent basic testing, and two patients underwent advanced testing. No clinically significant genetic variants regarding FH were detected in any of the studies.
Patients who underwent genetic testing (Table 1) depicted higher levels of total cholesterol (272 ± 60.16 vs. 199.54 ± 54.38 mg/DL, p = 0.009), HDL-C (61.75 ± 12.95 vs. 46.38 ± 11.73 mg/dL, p = 0.01), and LDL-C (184.25 ± 44.91 vs. 130.84 ± 44.88 mg/dL, p = 0.02) before admission, and also during admission (total cholesterol: 225.25 ± 64.32 vs. 161.3 ± 42.17 mg/dL, p = 0.003; HDL-C: 53 ± 10.68 vs. 38.39 ± 10.1, p = 0.005; and LDL-C: 145 ± 52.31 vs. 100.93 ± 35.97, p = 0.02). After correction for LLT before admission, these patients also had significantly higher basal LDL-C (233 ± 49.09 vs. 161.71 ± 43.25 mg/dL, p = 0.001).
Amongst sDLCNS categories, the proportion of patients undergoing genetic testing was low in all categories. In patients with “unlikely” FH, genetic testing was seldom requested (n = 1, 0.6%), also being uncommon in the “possible” (n = 2, 2.8%) and “probable” (n = 1, 9.1%) FH categories.
4. Discussion
In our study, we show that about one-third of MI sufferers included in our CRP could be categorized as “possible” or “probable” FH based on sDLCNS criteria. However, genetic testing for FH was infrequent, even in patients with higher scores on sDLCNS.
It is generally accepted that FH affects about 1 in 300 people in the general population [17], but its prevalence can be up to 18 times higher in individuals with atherosclerotic cardiovascular disease, especially in coronary artery disease patients [5,18]. Indeed, a premature MI combined with elevated basal LDL-C levels and/or family history of premature CVD confers at least a “possible” or “probable” likelihood of FH as per the Dutch Lipid Clinic Network (DLCN) score criteria. LDL-C concentrations is the highest scoring variable and confers independent risk of CVD [10].
Clinical diagnosis of FH using diagnostic algorithms can help identify patients with high cardiovascular risk and increased difficulty in achieving target lipid control [5,6,7]. The diagnosis of FH is typically established using the DLCN criteria [2,10,11,12], although other scores such as the Simon Broome criteria [19] or Make Early Diagnosis to Prevent Early Deaths (MEDPED) criteria [20] are also used for this purpose. However, these criteria share a common limitation, i.e., they require detailed clinical history information, which complicates their application in research and in routine clinical practice. Consequently, several research studies, such as ours, use a simplified version of DLCN criteria [4,13].
Even though FH is a genetic disorder and causal genetic mutations for FH virtually confirm the disease on these algorithms, genetic testing is rarely used in clinical practice. According to available evidence and expert consensus recommendations, genetic testing for FH offers several advantages. Firstly, it provides a definitive diagnosis. Secondly, it serves as an indicator of high cardiovascular risk, prompting more aggressive preventive treatment in affected individuals and increased therapeutic adherence by patients. Finally, it facilitates cascade screening of at-risk family members, which can be highly effective in identifying asymptomatic individuals with FH who may need lifestyle changes and pharmacological treatment [6,8,9,21,22]. For instance, when FH is detected and treated at a young age, the risk of atherosclerotic cardiovascular disease can be drastically reduced, potentially to general population levels [23,24]. Additionally, several studies have shown that genetic testing for FH is a cost-effective strategy [25,26].
In our study, about one-third of MI sufferers included in our CRP could be categorized as “possible” or “probable” FH based on sDLCNS criteria. In these cases, genetic testing could be useful to confirm the FH diagnosis. Indeed, it has been observed that carriers of a FH mutation have a significantly higher risk of coronary artery disease compared to non-carriers within similar LDL-C levels [27]. Also, FH is more common than generally believed, especially in patients with a history of ischemic heart disease. In a prospective cohort of 223 patients with acute coronary syndrome and LDL-C ≥135.3 mg/dL (3.5 mmol/L), causal genetic mutations for FH were documented in 26% of the population [7], although a lower prevalence (8.7%) has also been described in a similar cohort [28]. Similarly to our results, in a study in young MI sufferers (<50 years) 37.5% met criteria for “possible” FH, 6.4% for “probable” FH, and 2.6% for “confirmed” FH [5].
However, in our CRP, genetic testing for familial hypercholesterolemia was performed in only four patients (1.6%), with no clinically significant genetic variants detected. These results highlight the underutilization of genetic testing in clinical practice to diagnose FH, even in high-risk individuals, which has been previously reported in the literature [5,29,30]. For example, one study investigated the underutilization of genetic testing for FH in a real-world setting based on a single laboratory measurement of LDL-C ≥ 99.5th percentile (adjusted for sex and age), which was associated with undertreatment of these patients [31].
Specifically in our CRP, we believe that genetic testing for FH is seldom requested due to the lack of established protocols and the challenges associated with the application of existing diagnostic algorithms in daily clinical practice. Nevertheless, strategies should be implemented to enhance screening for FH, e.g., by systematically incorporating the prospective calculation of DLCN criteria into routine clinical practice and by granting genetic testing on an individualized basis in high-risk individuals. Given that universal screening with genetic testing in all MI sufferers is infeasible, patients with “probable” or “possible” FH on DLCN criteria, which represent approximately a third of the cohort, could be prioritized.
Our study has several limitations that should be commented on. Our cohort derives for a relatively small, single-center registry in a high-complexity tertiary hospital, and the results may not be applicable to other clinical settings. However, close follow-up and continuous monitoring of the response to lipid-lowering therapy to achieve optimal lipid control during CRP would have created an ideal setting for FH suspicion and genetic testing. We retrospectively calculated the sDLCNS criteria and did not collect information regarding tendinous xanthomata and arcus cornealis, which might yield different results compared to the original DLCN score. Also, in some patients, basal LDL-C levels were unavailable, so a correction considering LLT was performed as previously described [5,16]; however, this extrapolation could have implied some inherent calculation error. Finally, we had no information regarding patients’ diet and fat consumption before MI, which may have influenced their basal LDL-C.
5. Conclusions
We conclude that the rate of genetic testing for FH in our CRP after MI is low, even in about the third of patients with a “possible” or “probable” diagnosis based on sDLCNS criteria. Strategies to improve screening for FH should be implemented, such as prospective analysis of DLCN score and genetic testing in patients with high likelihood of FH.
Author Contributions
Conceptualization: C.B.-B., V.M.-G., H.M.-G., M.L.M.M. and V.B.; Data curation: C.B.-B., V.M.-G., H.M.-G., M.L.M.M., J.I.C.A., N.P., L.L.B., M.C.E.A., M.V.R., A.A.B., A.P.R., C.R.-N., E.d.D., J.G., M.F.J.-N., F.J.C., J.S. and V.B.; Formal analysis: C.B.-B., V.M.-G., H.M.-G. and V.B.; Funding acquisition: V.M.-G., F.J.C., J.S. and V.B.; Investigation: C.B.-B., V.M.-G., H.M.-G., M.L.M.M., J.I.C.A., N.P., L.L.B., M.C.E.A., M.V.R., A.A.B., A.P.R. and V.B.; Methodology: C.B.-B., V.M.-G., H.M.-G., M.L.M.M., J.I.C.A., N.P., L.L.B., M.C.E.A., M.V.R., A.A.B., A.P.R., C.R.-N., E.d.D., J.G., M.F.J.-N., F.J.C., J.S. and V.B.; Project administration: C.B.-B., V.M.-G., C.R.-N., E.d.D., J.G. and V.B.; Resources: C.B.-B., V.M.-G., H.M.-G., A.P.R., M.F.J.-N., F.J.C., J.S. and V.B.; Software: C.B.-B., V.M.-G. and V.B.; Supervision: V.M.-G., A.P.R., F.J.C., J.S. and V.B.; Validation: C.B.-B., V.M.-G. and V.B.; Visualization: C.B.-B., V.M.-G. and V.B.; Writing—original draft: C.B.-B. and V.M.-G.; Writing—review and editing: C.B.-B., V.M.-G., H.M.-G., M.L.M.M., J.I.C.A., N.P., L.L.B., M.C.E.A., M.V.R., A.A.B., A.P.R., C.R.-N., E.d.D., J.G., M.F.J.-N., F.J.C., J.S. and V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from “Instituto de Salud Carlos III”, “Fondos Europeos de Desarrollo Regional FEDER” and “Fondo Social Europeo Plus (FSE+)” (grant numbers PI20/00637, PI23/01150, and CIBERCV16/11/00486, a postgraduate contract FI18/00320 to C.R.-N., CM23/00246 to H.M.-G., and CM21/00175 and JR23/00032 to V.M.-G.) and Conselleria de Educación—Generalitat Valenciana (PROMETEO/2021/008). C.R.-N. and J.G. acknowledge funding from the Conselleria de Educación–Generalitat Valenciana and co-funding from Fondo Social Europeo Plus (grant numbers CIAPOS/2023/247, and CIAPOS2023/248).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Drug Research (CEIm) of Hospital Clinico Universitario de Valencia (protocol code: 2019/262 and date of approval: 26 May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to ethical restrictions.
Acknowledgments
We would like to acknowledge the multidisciplinary team of healthcare professionals that contributed to the creation and development of the Cardiac Rehabilitation Unit in our hospital.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sniderman, A.D.; Glavinovic, T.; Thanassoulis, G. Key Questions About Familial Hypercholesterolemia. J. Am. Coll. Cardiol. 2022, 79, 1023–1031. [Google Scholar] [CrossRef]
- Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Unveiling Familial Hypercholesterolemia—Review, Cardiovascular Complications, Lipid-Lowering Treatment and Its Efficacy. Int. J. Mol. Sci. 2024, 25, 1637. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Hauguel-Moreau, M.; Aïdan, V.; Hergault, H.; Beauchet, A.; Pépin, M.; Prati, G.; Pillière, R.; Ouadahi, M.; Josseran, L.; Rodon, C.; et al. Prevalence of Familial Hypercholesterolaemia in Patients Presenting with Premature Acute Coronary Syndrome. Arch. Cardiovasc. Dis. 2022, 115, 87–95. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, A.; Collins, B.L.; Qamar, A.; Monda, K.L.; Biery, D.; Lopez, J.A.G.; De Ferranti, S.D.; Plutzky, J.; Cannon, C.P.; et al. Familial Hypercholesterolemia Among Young Adults with Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 2439–2450. [Google Scholar] [CrossRef]
- Sturm, A.C.; Knowles, J.W.; Gidding, S.S.; Ahmad, Z.S.; Ahmed, C.D.; Ballantyne, C.M.; Baum, S.J.; Bourbon, M.; Carrié, A.; Cuchel, M.; et al. Clinical Genetic Testing for Familial Hypercholesterolemia. J. Am. Coll. Cardiol. 2018, 72, 662–680. [Google Scholar] [CrossRef]
- Wang, C.; Yu, P.; Hu, L.; Liang, M.; Mao, Y.; Zeng, Q.; Wang, X.; Huang, K.; Yan, J.; Xie, L.; et al. Prevalence and Prognosis of Molecularly Defined Familial Hypercholesterolemia in Patients with Acute Coronary Syndrome. Front. Cardiovasc. Med. 2022, 9, 921803. [Google Scholar] [CrossRef] [PubMed]
- Umans-Eckenhausen, M.A.; Defesche, J.C.; Sijbrands, E.J.; Scheerder, R.L.; Kastelein, J.J. Review of First 5 Years of Screening for Familial Hypercholesterolaemia in the Netherlands. Lancet 2001, 357, 165–168. [Google Scholar] [CrossRef]
- Hadfield, S.G.; Horara, S.; Starr, B.J.; Yazdgerdi, S.; Marks, D.; Bhatnagar, D.; Cramb, R.; Egan, S.; Everdell, R.; Ferns, G.; et al. Family Tracing to Identify Patients with Familial Hypercholesterolaemia: The Second Audit of the Department of Health Familial Hypercholesterolaemia Cascade Testing Project. Ann. Clin. Biochem. Int. J. Lab. Med. 2009, 46, 24–32. [Google Scholar] [CrossRef]
- Séguro, F.; Bongard, V.; Bérard, E.; Taraszkiewicz, D.; Ruidavets, J.-B.; Ferrières, J. Dutch Lipid Clinic Network Low-Density Lipoprotein Cholesterol Criteria Are Associated with Long-Term Mortality in the General Population. Arch. Cardiovasc. Dis. 2015, 108, 511–518. [Google Scholar] [CrossRef]
- Ruel, I.; Brisson, D.; Aljenedil, S.; Awan, Z.; Baass, A.; Bélanger, A.; Bergeron, J.; Bewick, D.; Brophy, J.M.; Brunham, L.R.; et al. Simplified Canadian Definition for Familial Hypercholesterolemia. Can. J. Cardiol. 2018, 34, 1210–1214. [Google Scholar] [CrossRef]
- McGowan, M.P.; Hosseini Dehkordi, S.H.; Moriarty, P.M.; Duell, P.B. Diagnosis and Treatment of Heterozygous Familial Hypercholesterolemia. J. Am. Heart Assoc. 2019, 8, e013225. [Google Scholar] [CrossRef]
- Bellows, B.K.; Khera, A.V.; Zhang, Y.; Ruiz-Negrón, N.; Stoddard, H.M.; Wong, J.B.; Kazi, D.S.; De Ferranti, S.D.; Moran, A.E. Estimated Yield of Screening for Heterozygous Familial Hypercholesterolemia With and Without Genetic Testing in US Adults. J. Am. Heart Assoc. 2022, 11, e025192. [Google Scholar] [CrossRef]
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The Role of Cardiac Rehabilitation in Improving Cardiovascular Outcomes. Nat. Rev. Cardiol. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Lee, W.-J.; Chuang, H.-N.; Hsiao, T.-H.; Lee, W.-L.; Wu, J.-P.; Sheu, W.H.-H.; Liang, K.-W. Prevalence and Prognosis of Genetically Proven Familial Hypercholesterolemia in Subjects with Coronary Artery Disease and Reduced Ejection Fraction. Sci. Rep. 2023, 13, 16942. [Google Scholar] [CrossRef]
- Marcos-Garcés, V.; Merenciano-González, H.; Martínez Mas, M.L.; Palau, P.; Climent Alberola, J.I.; Perez, N.; López-Bueno, L.; Esteban Argente, M.C.; Valls Reig, M.; Muñoz Alcover, R.; et al. Short-Course High-Intensity Statin Treatment during Admission for Myocardial Infarction and LDL-Cholesterol Reduction—Impact on Tailored Lipid-Lowering Therapy at Discharge. J. Clin. Med. 2024, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef]
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.T.; Sharabiani, M.T.A.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.K.; Vallejo-Vaz, A.J. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef]
- Humphries, S.E.; Cooper, J.A.; Seed, M.; Capps, N.; Durrington, P.N.; Jones, B.; McDowell, I.F.W.; Soran, H.; Neil, H.A.W. Coronary Heart Disease Mortality in Treated Familial Hypercholesterolaemia: Update of the UK Simon Broome FH Register. Atherosclerosis 2018, 274, 41–46. [Google Scholar] [CrossRef]
- Williams, R.R.; Hunt, S.C.; Schumacher, M.C.; Hegele, R.A.; Leppert, M.F.; Ludwig, E.H.; Hopkins, P.N. Diagnosing Heterozygous Familial Hypercholesterolemia Using New Practical Criteria Validated by Molecular Genetics. Am. J. Cardiol. 1993, 72, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Wang, M.; Patel, A.P.; Ajufo, E.; Maamari, D.J.; Aragam, K.G.; Brockman, D.G.; Vosburg, T.; Ellinor, P.T.; Ng, K.; et al. Association of the Interaction Between Familial Hypercholesterolemia Variants and Adherence to a Healthy Lifestyle With Risk of Coronary Artery Disease. JAMA Netw. Open 2022, 5, e222687. [Google Scholar] [CrossRef]
- Mszar, R.; Mszar, E. Familial Hypercholesterolemia and Our Family’s Heart History: From Atherosclerosis and Angina to Awareness and Advocacy. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e010864. [Google Scholar] [CrossRef] [PubMed]
- Mirzai, S.; Chevli, P.A.; Rikhi, R.; Shapiro, M.D. Familial Hypercholesterolemia: From Clinical Suspicion to Novel Treatments. Rev. Cardiovasc. Med. 2023, 24, 311. [Google Scholar] [CrossRef]
- Campbell-Salome, G.; Walters, N.L.; Ladd, I.G.; Sheldon, A.; Ahmed, C.D.; Brangan, A.; McMinn, M.N.; Rahm, A.K.; Schwartz, M.L.B.; Tricou, E.; et al. Motivating Cascade Testing for Familial Hypercholesterolemia: Applying the Extended Parallel Process Model for Clinician Communication. Transl. Behav. Med. 2022, 12, 800–809. [Google Scholar] [CrossRef]
- Marquina, C.; Morton, J.I.; Lloyd, M.; Abushanab, D.; Baek, Y.; Abebe, T.; Livori, A.; Dahal, P.; Watts, G.F.; Ademi, Z. Cost-Effectiveness of Screening Strategies for Familial Hypercholesterolaemia: An Updated Systematic Review. PharmacoEconomics 2024, 42, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Ademi, Z.; Watts, G.F.; Pang, J.; Sijbrands, E.J.G.; Van Bockxmeer, F.M.; O’Leary, P.; Geelhoed, E.; Liew, D. Cascade Screening Based on Genetic Testing is Cost-Effective: Evidence for the Implementation of Models of Care for Familial Hypercholesterolemia. J. Clin. Lipidol. 2014, 8, 390–400. [Google Scholar] [CrossRef]
- Khera, A.V.; Won, H.-H.; Peloso, G.M.; Lawson, K.S.; Bartz, T.M.; Deng, X.; Van Leeuwen, E.M.; Natarajan, P.; Emdin, C.A.; Bick, A.G.; et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J. Am. Coll. Cardiol. 2016, 67, 2578–2589. [Google Scholar] [CrossRef]
- Amor-Salamanca, A.; Castillo, S.; Gonzalez-Vioque, E.; Dominguez, F.; Quintana, L.; Lluís-Ganella, C.; Escudier, J.M.; Ortega, J.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Genetically Confirmed Familial Hypercholesterolemia in Patients with Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2017, 70, 1732–1740. [Google Scholar] [CrossRef]
- Ahmad, Z.S.; Andersen, R.L.; Andersen, L.H.; O’Brien, E.C.; Kindt, I.; Shrader, P.; Vasandani, C.; Newman, C.B.; deGoma, E.M.; Baum, S.J.; et al. US Physician Practices for Diagnosing Familial Hypercholesterolemia: Data from the CASCADE-FH Registry. J. Clin. Lipidol. 2016, 10, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial Hypercholesterolaemia is Underdiagnosed and Undertreated in the General Population: Guidance for Clinicians to Prevent Coronary Heart Disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- Ibrahim, S.; Nurmohamed, N.S.; Nierman, M.C.; De Goeij, J.N.; Zuurbier, L.; Van Rooij, J.; Schonck, W.A.M.; De Vries, J.; Hovingh, G.K.; Reeskamp, L.F.; et al. Enhanced Identification of Familial Hypercholesterolemia Using Central Laboratory Algorithms. Atherosclerosis 2024, 393, 117548. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).