Abstract
Medical economics is essential in cardiac genetics for the clinical application and development of research results. However, related economic evaluations are unclear, and limited systematic reviews are available on the cost-effectiveness of drug selection based on the CYP2C19 LOF allele. This review analyzed research in the MEDLINE database from January 2012 to June 2023 using more evidence than a well-designed cohort study, owing to the lack of relevant research in the database. For example, cost-effectiveness analyses are often reported as simulation assays, and were included in this analysis. No conditions related to patient background or antiplatelet drug therapy were selected. This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (2020). Twenty-one cardiac genetic studies were selected, of which nineteen involved antiplatelet therapy after PCI. A universal group consisting of clopidogrel and other drugs was used as the baseline and compared with the drug selection groups based on the CYP2C19 LOF allele. The incremental cost–effectiveness ratio was generally below 50,000 (US$/Qaly), and drug selection based on the CYP2C19 LOF allele was the most cost-effective, followed by universal clopidogrel. Although cardiac genetic and economic data are rudimentary, this review indicates that antiplatelet therapy (drug selection based on the CYP2C19 LOF allele) after PCI is generally cost-effective.
1. Introduction
Cardiovascular diseases develop because of a combination of genetic and environmental factors [1]. Although “heart failure” is a common phenotype, the timing of onset and degree of severity vary by case. Therefore, the identification of pathogenic variants using genome analysis could allow for stratification of the disease state and individualized therapeutic interventions based on genetic information. Genetic factors associated with congenital long QT syndrome and essential hypertension have long been discussed. The genetic factors associated with dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, and lifestyle-related cardiovascular diseases (ischemic heart disease, atrial fibrillation, and hypercholesterolemia) have recently been studied. For example, DCM is caused by genes encoding proteins important for maintaining the function of cardiac muscle cells, such as titin, lamin A/C, and desmoplakin [2]. Further genetic studies are being conducted in the field of ischemic heart disease, focusing on common variants in single nucleotide polymorphisms (SNP) regions [3].
The clinical application and development of cardiac genetics research have introduced new financial burdens, such as R&D investments, infrastructure development, and additional testing and diagnosis costs. In addition, the socioeconomic effects of these factors must be considered. In general, cost-effectiveness analysis, which is part of health technology assessment (HTA), has become popular worldwide, as a method for conducting an economic evaluation of medical interventions [4]. Therefore, this study surveyed research on the cost-effectiveness of cardiovascular disease treatment based on genetic information and examined methods for evaluating the economic potential of cardiac genetics. The expected effect of this study was to encourage the implementation of these results in practice to reduce financial burden. Large racial differences in genetics and the considerable number of rare diseases have limited the accumulation of clinical evidence in medical economics, including treatment strategies based on cardiac genetics. Therefore, the focus remains on major diseases with established genetic testing methods that have a strong track record of research involving treatment strategies based on genetic information in the present study.
Percutaneous coronary intervention (PCI or stent placement) is a widely used revascularization technique for ischemic heart diseases such as angina pectoris, in which antiplatelet therapy suppresses platelet aggregation after surgery. Although adenosine diphosphate (ADP) P2Y12 receptor inhibitors and aspirin are the most commonly used therapies, the pharmacogenetic mechanisms of action of ADP (P2Y12) receptor inhibitors are subject to debate regarding the pharmacogenetic mechanisms of action, including CYP2C19. Protocols have been considered to determine which intervention (such as drug therapy) is appropriate based on whether a patient carries the CYP2C19 loss-of-function (LOF) allele [5]. From a socioeconomic perspective, the cost-effectiveness of genetic testing and diagnosis has been verified in parallel with systematic reviews of simulation studies [6]. Many of these reports indicated good cost-effectiveness.
However, some studies have denied the usefulness of drug selection based on the CYP2C19 LOF allele (genotype-guided therapy); therefore, these contrasting results require further updates using the most current information on this topic [7]. There are also reports showing that, in patients undergoing primary PCI, the strategy of selecting oral P2Y12 inhibitors based on the CYP2C19 genotype was non-inferior (lower incidence of bleeding) to standard therapy with ticagrelor or prasugrel, with respect to thrombotic events at 12 months [8]. In cases where these additional clinical efficacies are not different or minimal, the balance between clinical benefit and increased intervention costs is a point of contention in health economics. In other words, it is desirable to examine whether a cost-effectiveness analysis can adequately address such issues.
In light of the above, this study involved a systematic review of the cost-effectiveness of drug selection based on the CYP2C19 LOF allele (genotype-guided therapy) and used the results as material to discuss the ideal medical economic evaluation method in this area, with the aim of contributing to the development of future health economic evaluation methods related to cardiac genetics.
2. Method
2.1. Basic Concept of This Review
This study aimed to consolidate evidence and advance the discussion on economic factors of healthcare related to cardiac genetics, with a focus on P2Y12 inhibitor selection based on the CYP2C19 loss-of-function allele. Furthermore, based on the information obtained, the procedural aspects of cost-effectiveness evaluations were considered, focusing on the characteristics of cardiac genetics while factoring in the trends in oncology cardiology practice. The literature reviewed in this review was limited to studies with evidence from clinical trials and similar protocols. Therefore, in examining the comparisons of outcomes between medical technologies, reports of complementary simulation studies (model calculations) were excluded if no comparison of test results from clinical trials or other studies were included. This exclusion was due to the limited ability to rigorously discuss the level of evidence and the difficulty of meta-analysis ([9,10]; Supplementary Material S1, Supplementary Material S2). Therefore, the area of genetic screening for familial hypercholesterolemia FH (identification of subjects by genetic screening), which is discussed later in this review, was positioned as a reference, and only simulation studies that specifically discussed clinical impact were addressed.
2.2. Related PCI and Antiplatelet Therapy
PCI destroys atherosclerotic lesions within the coronary arteries, resulting in platelet adherence to the rupture site of the blood vessel wall and thrombus formation. Organ ischemia symptoms occur when a thrombus is sufficiently enlarged to occlude the coronary artery or embolize it peripherally. Antiplatelet therapy is administered to treat the symptoms. Since local thrombosis in the coronary arteries after PCI is common, combination antiplatelet therapy (DAPT) consisting of aspirin and ADP (P2Y12) receptor inhibitors is preferable to aspirin alone [11]. Antiplatelet combination therapy, particularly after stent placement, is the standard PCI treatment used worldwide. ADP (P2Y12) receptor inhibitors include thienopyridine (e.g., ticlopidine, clopidogrel, and prasugrel) and non-thienopyridine (e.g., ticagrelor) drugs. Thienopyridine drugs are prodrugs that exhibit their effects after metabolism, whereas non-thienopyridine drugs act directly on P2Y12 receptors. Therefore, non-thienopyridine drugs exert more rapid effects than thienopyridine drugs.
2.3. Genotype-Guided Therapy to Be Evaluated
The CYP2C19 protein, which belongs to the cytochrome P450 group of the mixed-function oxidase system, is involved in the metabolism of many xenobiotics and is encoded by CYP2C19. Genetic polymorphisms exist in the expression of CYP2C19, with approximately 3–5% of Caucasians and 15–20% of Asians lacking CYP2C19 function, resulting in weak metabolism [12]. Thienopyridine clopidogrel is a prodrug that, when metabolized by cytochrome P450, becomes an active metabolite that exhibits its effects. Therefore, CYP2C19 gene polymorphisms may inhibit clopidogrel from achieving its pharmacological effects. Genotype-guided therapy (GGT) has been discussed as an alternative to antiplatelet therapy for PCI. Guided treatment involves the selection of an intervention based on the presence or absence of the CYP2C19 LOF allele (degree of poor metabolism). The analytical comparison populations in many studies included universal DAPT for clopidogrel, universal DAPT for prasugrel, or ticagrelor and GGT-based DAPT. Furthermore, prasugrel and ticagrelor are the first-line drugs used in Europe [13].
2.4. Health Economic Evaluation (Cost-Effectiveness Analysis)
Methods for measuring and analyzing the utility values of patients based on their health-related quality of life (QOL) have been developed and are continuously improving. A cost–utility analysis (CUA) applies this concept to a cost-effectiveness analysis (CEA). When a utility function is applied to a cost-effectiveness calculation, medical value is determined as a “resource consumption (mainly direct medical costs)/health recovery (patient outcomes such as utility)” [14], with one outcome being the global index “quality-adjusted life years” (QALYs). This concept integrates the utility value for a patient and the number of years of life and discusses both quantitative (life prognosis) and qualitative outcomes (QOL) [14]. The incremental cost–effectiveness ratio (ICER) can be a “yardstick” for judging whether cost-effectiveness is superior or inferior. The formula for the ICER is the difference between the cost (b) of similar technology β and the cost (a) of new medical technology α (that is, (a)–(b)) divided by the difference between the effectiveness (B) of similar technology β and the effectiveness (A) of new medical technology α (that is, (A)-(B)) (Figure 1) [15,16,17]. These concepts are included in cardiovascular field guidelines, such as those of the American Heart Association (AHA) in the United States [15].
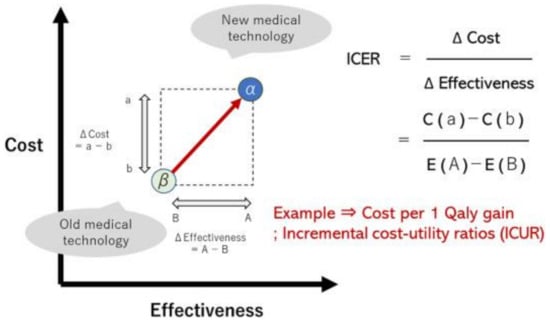
Figure 1.
Concept of cost–effectiveness evaluation (incremental cost–effectiveness ratio); ICER is denoted as ICUR (incremental cost–utility ratio) when utility (Qaly) is applied as an effectiveness measure.
2.5. Simulation Study
Uncertainty in the data of clinical trials and clinical studies is due to variability in the efficacy and cost of treatment for each patient as well as variability in the original background factors of the patients. This uncertainty can be reduced by ensuring that an appropriate number of cases are included in the study and by designing a trial or study. However, a health economic evaluation as a simulation experiment, which is not a real clinical study, is greatly affected by the uncertainty of the parameters, making it important to verify the robustness of the results. In particular, to evaluate the uncertainty of multiple parameters simultaneously, a Probabilistic Sensitivity Analysis, in which the evaluation is based on the simultaneous distribution of those parameters, is desirable. Note that when considering the simultaneous distribution of multiple parameters, the probability density function of the simultaneous distribution becomes complex and difficult to evaluate analytically; therefore, Monte Carlo simulations and computerized arithmetic estimation of the Markov Chain Monte Carlo method are often used in the analysis [18]. By plotting and analyzing a large amount of hypothetical data obtained from these simulations on an incremental cost–effectiveness plane, it was possible to calculate the confidence intervals for the ICER. However, when extrapolating the outcomes (indirect comparison of interventions, statistically nonsignificant) of different trials and discussing the superiority or inferiority of medical technologies, the ability to discuss the validity of the estimated values rigorously is limited. This is a useful approach to predict the effectiveness of new medical technologies that require time to clarify the results of clinical trials.
2.6. Review Design
The paper search question for this study, which took a trial approach, was “Is the cost effectiveness of P2Y12 inhibitor selection based on the CYP2C19 loss-of-function allele superior?” to contribute to the development of future economic evaluation methods for cardiac genetics. The next question was “Are there other disease areas for which there are more than a certain number of cost-effectiveness reports related to cardiac genetics?” This study sets a standard for objectively discussing the level of evidence as an inclusion criterion for papers. In particular, I aimed to collect papers that had a certain degree of representativeness in terms of the target population, intervention techniques, and sample size, while taking into account the heterogeneity between trials. However, regional differences, such as system characteristics, patient differences, such as disease characteristics, and inter-intervention differences, such as treatment characteristics, including unrecognizable uncertainties, were considered impossible to discuss rigorously. To select databases that affected the comprehensiveness of the search, one main database was used for trial selection. However, there was a report [19] that MEDLINE alone can retrieve only 55% of the documents needed for review, and EMBASE alone can retrieve only 49%. Therefore, in this study, I set the following conditions as a countermeasure. This study incorporated the results of one or more systematic reviews that included at least one database other than the one selected.
2.7. Literature Review Methods and Conditions
This study used the MEDLINE (Medical Literature Analysis and Retrieval System Online) database, accessed through PubMed (National Center for Biotechnology Information). The keywords searched were related to the target disease [cardiogenetics or ischemic heart disease], related technology [PCI or antiplatelet therapy], evaluation technology [genotype-guided therapy OR CYP2C19], and evaluation methods [cost-effectiveness, health economy, and economic evaluation]. The target period was from January 2012 to June 2023 (Figure 2). Based on the limited number of results, the criteria for selecting the literature were set at a higher evidence level than that of a well-designed cohort study. When selecting the literature, duplicates of the constituent literature in the systematic review articles were avoided as much as possible while considering the characteristics of the reported content. In addition, considering the characteristics of cost-effectiveness analysis, reports on model calculations (simulation studies that cannot discuss the level of evidence) were included. Studies published in languages other than English were excluded. The search process involved surveying the database using keywords and narrowing down the results based on the strategy requirements and abstracts. No conditions related to patient background or drug therapy were used when selecting the antiplatelet therapy studies. Although this study was not a rigorous systematic review, it was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (2020) [20,21].
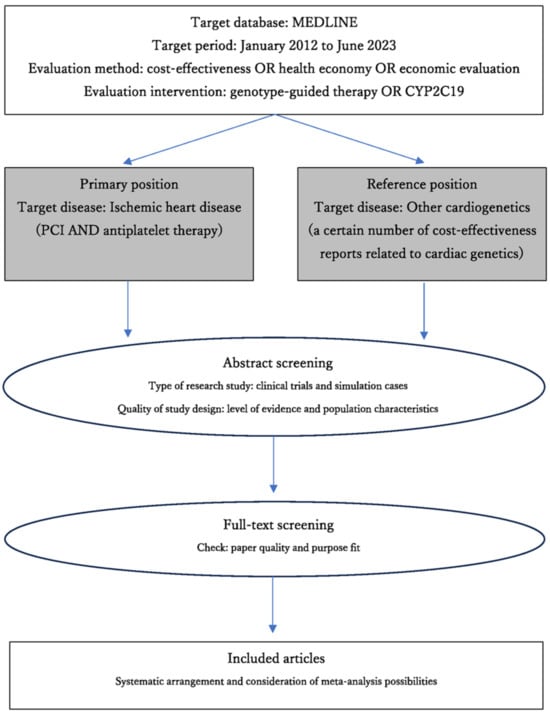
Figure 2.
Overview of review flow.
2.8. Meta-Analysis
Problems with meta-analyses have been discussed, including the integration of studies with different participants and intervention backgrounds, the risk of including low-quality studies, and the tendency not to publish negative results [22]. Approaches for estimating the integration effect in meta-analyses can be broadly classified into two types. There were two types of models: the fixed-effects model (when there was no heterogeneity between studies) and the random-effects model (when there was heterogeneity between studies). The former assumes that the true effect size is the same in each study and that differences in effect estimates between studies are due to error variation. The latter assumes that the true effect size is distributed around a certain value across the studies. If there is heterogeneity in the reported content between studies and the cause is thought to be the population, intervention, or outcome, it is desirable to conduct a subgroup analysis for each item that may be the cause of the heterogeneity [23]. Funnel plots are often used to assess publication bias. In other words, it has been suggested that when there is a high possibility of publication bias or selection bias, the results of meta-analysis are inconsistent with the results of large-scale clinical trials [24]. Generally, the greater the number of included papers, the more accurate the discussion. In this study, while excluding simulation studies because meta-analysis is difficult, I assumed that there would be heterogeneity between studies and explored the possibility of a meta-analysis.
3. Results
3.1. Literature Search Results
During our literature review, I identified 34 medical reports related to cardiac genetics (including those on ischemic heart disease) (Figure 3). Of these, 13 were excluded because of their research content and design. Two studies focused on the medical economics of treatment strategies based on cardiac genetics (FH), whereas the others concentrated on the cost-effectiveness of drug selection (GGT) based on the CYP2C19 LOF allele. I finalized nineteen reports related to antiplatelet therapy after PCI and two reports (treated as references) on genetic screening for FH [6,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
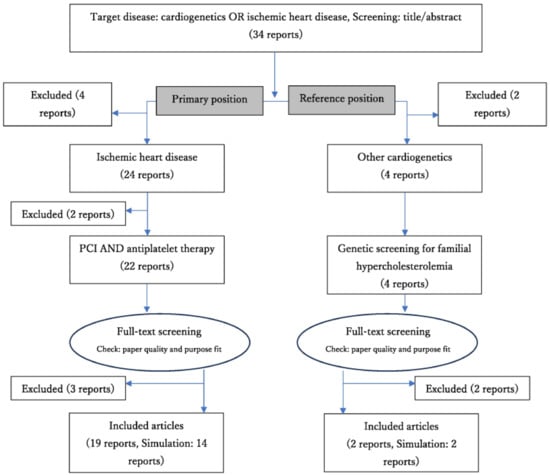
Figure 3.
Review flow diagram (results).
Post-PCI antiplatelet therapy (including GGT) articles consisted of two review papers, one RCT (application to simulation study), two cohort studies, and 14 simulation (model calculation) reports (Table 1). The cost-effectiveness evaluation used cost–utility analysis with QALY in 18 reports. Among ADP (P2Y12) receptor inhibitors, clopidogrel, prasugrel, and ticagrelor were identified in 18, 14, and 13 reports, respectively. Two selected papers on genetic screening for FH were a CUA based on simulations (model calculations).

Table 1.
Literature review results (number of papers); drug selection based on CYP2C19 LOF allele.
3.2. Antiplatelet Therapy (GGT) after PCI
Many studies have evaluated medical economics using the general clopidogrel DAPT group as the baseline and a drug selection (GGT) group based on the CYP2C19 LOF allele. The analysis period is often longer than 12 months. Regarding the treatment strategies and patient characteristics, first-time PCI for myocardial infarctions was common (Table 2).

Table 2.
List of literature types surveyed; excludes simulation studies.
In most cases, the treatment outcomes (QALY) of the GGT intervention group improved or the treatment costs decreased compared to those of the general DAPT group. In all included articles, drug selection based on the CYP2C19 LOF allele (GGT) treatment strategy was more cost-effective than that based on universal clopidogrel, universal prasugrel, or ticagrelor (Figure 4). The ICUR of the GGT group was generally less than 50,000 (US$/Qaly). GGT interventions showed the greatest cost-effectiveness, followed by clopidogrel (including generics), ticagrelor, and prasugrel. Owing to the long survey period, changes in drug generation may have influenced our results. Some analyses (in review papers) have found that ticagrelor is highly cost-effective.
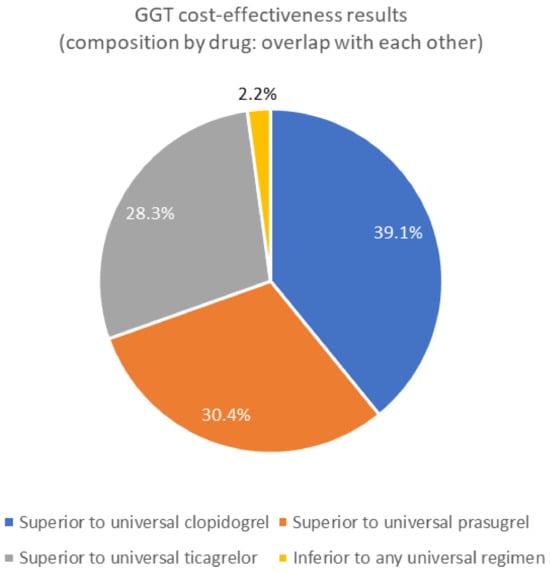
Figure 4.
Cost-effectiveness results of genotype-guided therapy (drug selection based on CYP2C19 LOF allele); GGT: Genotype-guided therapy.
In a meta-analysis, it is important to consider not only the quality of the paper, but also selection bias. However, a funnel plot was not possible due to the small number of included studies [43]. Therefore, publication bias could not be determined. Furthermore, the information required for the meta-analysis (e.g., calculation of weighted averages) and the number of studies were highly restricted, making it difficult to conduct a meta-analysis. Considering that the MEDLINE database, used in this study, has a relatively high coverage of representative studies, it is desirable to further examine in detail whether a sufficient amount of information for meta-analysis can be obtained, even if the number of databases applied is expanded.
3.3. Genetic Screening for Familial Hypercholesterolemia
No clinical trial studies have rigorously discussed genetic screening for familial hypercholesterolemia (FH). Simulation studies (model calculations) have been conducted in the United States and Australia. Studies with relatively high contributions to clinical strategies were selected. Specifically, articles that discussed the significance of detecting heterozygous FH, including the status of statin pharmacotherapy and other clinical practices (LDL-C levels and stroke incidence), were selected. Articles on screening strategies for children were excluded from the analysis. The two resulting articles were used as references [44,45]. One of these studies was conducted in Australia and used a Markov model for decision analysis to evaluate the utility of population genomic screening in adults aged 18–40 years. The results of that study indicated that population genomic screening for FH was cost-effective if the cost per test was less than AU$250 and the ICUR per Qaly obtained was less than AU$28,000. The second article involved model calculations from the US that reported an ICUR at age 20 of US$181,000 per Qaly, with a 38% probability of cost-effectiveness at the willingness-to-pay threshold of US$100,000 per Qaly. Based on this, the authors concluded that population screening for FH is not cost-effective under the current willingness-to-pay threshold. However, the cost-effectiveness of genetic screening for FH is ongoing, and further discussions focusing on younger populations are required.
4. Discussion
This study aimed to examine the healthcare economics of cardiac genetics, with a focus on determining the cost-effectiveness of antiplatelet therapy (GGT; drug selection based on the CYP2C19 LOF allele) post-PCI and compiling related evidence. The results showed that drug selection based on the CYP2C19 LOF allele had superior healthcare economic benefits compared to other methods for any universal regimen. Some studies have discussed the usefulness of CYP2C19 genotypes in platelet reactivity-guided antiplatelet therapy (PG-PRT) [39,41]. Additionally, although used only as a reference, a limited literature review was conducted to evaluate the cost-effectiveness of genetic screening for FH. As medical economics in this area have not been fully evaluated, an accumulation of evidence supported by clinical trials is desired. In contrast, cost-effectiveness analysis, which is widely used worldwide and has been applied in policymaking, is commonly used to evaluate the economic efficiency of healthcare without major restrictions.
The CLASSICS trial (2000) compared “clopidogrel plus aspirin” with “ticlopidine plus aspirin” in patients treated with stents. The primary endpoint rates were 4.6% in the clopidogrel group and 9.1% in the ticlopidine group. Adverse effects were less in the clopidogrel group [46]. This trend did not change significantly in the subsequent studies. However, the use of clopidogrel has been associated with drug resistance. This resistance is not due to individual differences in the action of the P2Y12 receptor but to variations in the production of the active metabolite. Prasugrel, a third-generation thienopyridine derivative, has a single-step metabolic pathway and less effect on the CYP2C19 genotype. In the GRAPE registry (2016), the prasugrel group had fewer MACE and more overall bleeding events (interpreted as minor bleeding) at 1 year than the clopidogrel group among patients undergoing PCI for acute coronary syndromes [47]. On the other hand, prasugrel is generally associated with an increased cost burden (drug price) in terms of medical economics. Therefore, in post-PCI antiplatelet therapy, it is desirable to improve the cost-effectiveness of the entire population by using GGT. The results of the present study show that post-PCI antiplatelet therapy combined with GGT improved healthcare economics.
A meta-analysis could not be performed in this study because of the limited number of quality reports. To evaluate the clinical aspects underlying cost-effectiveness, a meta-analysis was recently reported in patients with acute coronary syndrome (ACS). A GGT-based P2Y12 inhibitor prescription strategy compared to a non-GGT-based strategy was shown to have a potential benefit in reducing MACE and improving cardiovascular (CV) mortality, myocardial infarction (MI), and stent thrombosis [48]. It has also been reported that GGT-based P2Y12 inhibitor selection for patients with ACS achieved the most favorable balance between safety and efficacy compared to the routine choice of potent P2Y12 inhibitor selection (prasugrel or ticagrelor) [49]. These reports support the significance of the wider dissemination of GGT-based P2Y12 inhibitor selection approaches in patients with ACS.
Several studies have demonstrated non-significant benefits of drug selection based on the CYP2C19 LOF allele [7,8]. In contrast, the present study shows that genotype-guided therapy is cost-effective. The difference between these results could be influenced by the characteristics of cost-effectiveness, which includes both clinical and economic benefits, as well as the time lag in updating the most recent reports. In other words, cost-effectiveness improves when costs are reduced despite a lack of change in clinical outcomes. Therefore, as a cost-minimization analysis, health economic performance increases, leading to the conclusion presented in this study. In addition, simulation studies involve calculations that extrapolate the results of multiple studies (e.g., mean values of subjects or controls); therefore, a clinical trial may show no statistically significant difference between groups, whereas an extrapolated analysis may identify an apparent variation. Although there are limitations in explaining the level of evidence, these results must be verified by conducting additional procedures such as a sensitivity analysis.
Simulation studies account for a high percentage of the identified cost-effective-related studies, which is a characteristic of this type of research in various fields. Simulation research, which includes model calculations, is considered an effective approach for stakeholders to make rational decisions under certain assumptions. Therefore, this method is suitable for preparing a health economic evaluation from a policy standpoint for new medical methodologies such as cardiac genetics, for which clinical applications have not been fully developed. Although this study incorporated reports from simulation studies, if the target methodology is deployed in clinical practice and considered for inclusion in medical practice guidelines, the level of evidence should be discussed based on causal inferences. Further validation of this hypothesis is required through clinical trials.
The limitations of this study include the lack of a rigorous systematic review method, singular use of the MEDLINE database, limited comprehensiveness, and the use of simulation studies, which hinder discussions on the level of evidence. In cases such as cardiac genetics, where research results have not been fully applied in clinical practice and the number of reports is limited, a preliminary exploratory review is important. The results of this study revealed several studies using drug selection based on the CYP2C19 LOF allele; however, an extended systematic review and further meta-analyses are necessary for the development of this field. Further research on the stratification (targeting) of genetic screening for FH according to age and other factors related to public health programs and services is required.
Although not included in this study because of the limited sample size, several other studies have focused on the cost-effectiveness of cardiac genetics. For example, statin therapy based on the polygenic risk score (PRS), which is the sum of the contributions of SNPs to disease development, has been shown to be cost-effective [50]. Thus, health economic evaluations related to cardiac genetics should be further developed as they can greatly aid clinical practice and contribute to socioeconomic conditions. To support the advancement of medical technology, a social system that encourages and accepts technological progress is necessary [51]. A system design that harmonizes people, technology, and society to a greater degree must be developed into a socioeconomic mechanism (e.g., medical and industrial policies). Medical science, economics, and policy studies must collaborate to promote behavioral changes in the people involved and advance medical innovation and national welfare (Figure 5). Health economic evaluations associated with the further development of cardiac genetics can serve as catalysts for such discussions.
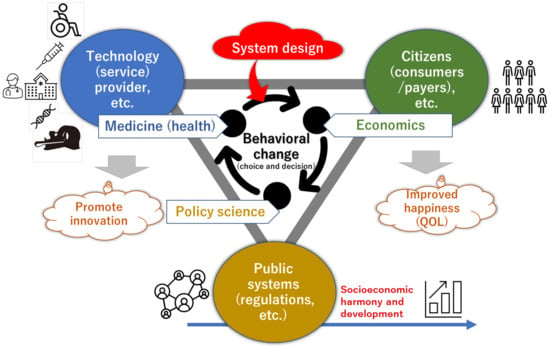
Figure 5.
Social medical system design: Concept of applying system design to societies where behavior change is desired (health care, etc.); Management of system design represents the academic system and practice of creatively designing and reliably managing all kinds of large-scale, complex, and uncertain social medicine, from the design and evaluation of medical technology and practice systems to conceptual recommendations for social systems.
5. Conclusions
Research on the health economic potential of cardiac genetics is still in its infancy compared with the state of research, development, and clinical applications in the field. However, research results have accumulated in certain areas (e.g., the identification of subjects by genetic screening and gene-guided treatment selection). Post-PCI antiplatelet therapy (GGT; drug selection based on the CYP2C19 LOF allele), which has a substantial number of research reports, is extremely cost-effective. However, genetic screening for FH has not been fully evaluated, and further evidence supported by clinical trials is necessary. The cost–effectiveness analysis method is considered to have no technical limitations in the application to the area concerned. However, there is a lack of quality clinical studies to further promote accurate decision-making and meta-analyses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cardiogenetics14020005/s1, The Oxford Center for Evidence-Based Medicine’s 2011 Levels of Evidence and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (Supplementary Materials S1 and S2).
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this study.
Acknowledgments
The authors thank Naoko Tsukamoto and Yoshie Syomura for their administrative support.
Conflicts of Interest
The authors declare that they have no personal circumstances or interests that could improperly influence the representation or interpretation of the reported study results.
Abbreviation
| ACS | acute coronary syndrome |
| CYP2C19 LOF allele | Cytochrome P4502C19 loss-of-function allele |
| DAPT | dual antiplatelet therapy |
| FH | familial hypercholesterolemia |
| GGT | genotype-guided therapy |
| HTA | health technology assessment |
| ICER | incremental cost–effectiveness ratio |
| ICUR | incremental cost–utility ratio |
| MACE | major adverse cardiovascular events |
| PCI | percutaneous coronary intervention |
| PG-PRT | genotype plus platelet reactivity-guided antiplatelet therapy |
| SNP | single nucleotide polymorphism |
| QOL | quality of life |
| QALY | quality-adjusted life year |
| RCT | randomized control trial |
References
- Walter, C.W. Balancing life-style and genomics research for disease prevention. Science 2002, 296, 695–698. [Google Scholar]
- Rosenbaum, A.N.; Agre, K.E.; Pereira, N.L. Genetics of dilated cardiomyopathy: Practical implications for heart failure management. Nat. Rev. Cardiol. 2020, 17, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Ito, K.; Terao, C.; Akiyama, M.; Horikoshi, M.; Momozawa, Y.; Matsunaga, H.; Ieki, H.; Ozaki, K.; Onouchi, Y.; et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat Genet. 2020, 52, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Mela, A.; Lis, D.; Rdzanek, E.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Drop, B.; Blicharski, T.; Niewada, M. AOTMiT reimbursement recommendations compared to other HTA agencies. Eur. J. Health Econ, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Beitelshees, A.L.; Thomas, C.D.; Empey, P.E.; Stouffer, G.A.; Angiolillo, D.J.; Franchi, F.; Tuteja, S.; Limdi, N.A.; Lee, J.C.; Duarte, J.D.; et al. CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention in diverse clinical settings. J. Am. Heart Assoc. 2022, 11, e024159. [Google Scholar] [CrossRef] [PubMed]
- AlMukdad, S.; Elewa, H.; Al-Badriyeh, D. Economic evaluations of CYP2C19 genotype-guided antiplatelet therapy compared to the universal use of antiplatelets in patients with acute coronary syndrome: A systematic review. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.H.; Jeong, M.H.; Chavez, I.; et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: The TAILOR-PCI randomized clinical trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Claassens, D.M.F.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van’t Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kimura, K.; Kimura, T.; Ishihara, M.; Otsuka, F.; Kozuma, K.; Shinke, T.; Nakagawa, Y.; Natsuaki, M.; Yasuda, S. JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients with Coronary Artery Disease. Circ. J. 2020, 84, 831–865. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z.; Zhao, X.; Shin, J.G.; Flockhart, D.A. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 2002, 41, 913–958. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Damman, P.; Zwart, B.; Appelman, Y.; Voskuil, M.; de Vos, A.; van Royen, N.; Jukema, J.W.; Waalewijn, R.; Hermanides, R.S.; et al. ESC Guidelines on Acute Coronary Syndrome without ST-Segment Elevation. Neth. Heart J. 2021, 29, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.M.; Eraker, S.A. Parameter Estimates for a QALY Utility Model. Med. Decis. Mak. 1985, 5, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Heidenreich, P.A.; Barnett, P.G.; Creager, M.A.; Fonarow, G.C.; Gibbons, R.J.; Halperin, J.L.; Hlatky, M.A.; Jacobs, A.K.; Mark, D.B.; et al. ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation 2014, 129, 2329–2345. [Google Scholar] [CrossRef] [PubMed]
- About the Immediate Operation of the Cost-Effectiveness Evaluation System-Central Social Insurance Medical Council: Total No. 9. Available online: https://www.mhlw.go.jp/content/12404000/000736552.pdf (accessed on 8 January 2022).
- Takura, T.; Komuro, I.; Ono, M. Trends in the Cost-Effectiveness Level of Percutaneous Coronary Intervention: Macro Socioeconomic Analysis and Health Technology Assessment. J. Cardiol. 2023, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Baio, G. Bayesian Methods in Health Economics; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Hopewell, S.; Clarke, M.; Lefebvre, C.; Scherer, R. Handsearching Versus Electronic Searching to Identify Reports of Randomized Trials. Cochrane Database Syst. Rev. 2007, 2007, MR000001. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Criticisms of Meta-analysis. In Introduction to Meta Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 377–387. [Google Scholar]
- Murad, M.H.; Montori, V.M.; Ioannidis, J.P.; Jaeschke, R.; Devereaux, P.J.; Prasad, K.; Neumann, I.; Carrasco-Labra, A.; Agoritsas, T.; Hatala, R.; et al. How to Read a Systematic Review and Meta-Analysis and Apply the Results to Patient Care: Users’ Guides to the Medical Literature. JAMA 2014, 312, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Dong, O.M.; Friede, K.A.; Chanfreau-Coffinier, C.; Voora, D. Cost-Effectiveness of CYP2C19-Guided P2Y12 Inhibitors in Veterans Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndromes. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Claassens, D.M.F.; van Dorst, P.W.M.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van’T Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; et al. Cost effectiveness of a CYP2C19 eenotype-guided strategy in patients with acute myocardial infarction: Results from the POPular genetics trial. Am. J. Cardiovasc. Drugs 2022, 22, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Panattoni, L.; Brown, P.M.; Te Ao, B.; Webster, M.; Gladding, P. The Cost Effectiveness of Genetic Testing for CYP2C19 Variants to Guide Thienopyridine Treatment in Patients with Acute Coronary Syndromes: A New Zealand Evaluation. Pharmacoeconomics 2012, 30, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Limdi, N.A.; Cavallari, L.H.; Lee, C.R.; Hillegass, W.B.; Holmes, A.M.; Skaar, T.C.; Pisu, M.; Dillon, C.; Beitelshees, A.L.; Empey, P.E.; et al. Cost-Effectiveness of CYP2C19-Guided Antiplatelet Therapy in Patients with Acute Coronary Syndrome and Percutaneous Coronary Intervention Informed by Real-World Data. Pharmacogenomics J. 2020, 20, 724–735. [Google Scholar] [CrossRef] [PubMed]
- AlMukdad, S.; Elewa, H.; Arafa, S.; Al-Badriyeh, D. Short- and Long-Term Cost-Effectiveness Analysis of CYP2C19 Genotype-Guided Therapy, Universal Clopidogrel, Versus Universal Ticagrelor in Post-percutaneous Coronary Intervention Patients in Qatar. Int. J. Cardiol. 2021, 331, 27–34. [Google Scholar] [CrossRef]
- Fragoulakis, V.; Bartsakoulia, M.; Díaz-Villamarín, X.; Chalikiopoulou, K.; Kehagia, K.; Ramos, J.G.S.; Martínez-González, L.J.; Gkotsi, M.; Katrali, E.; Skoufas, E.; et al. Cost-Effectiveness Analysis of Pharmacogenomics-Guided Clopidogrel Treatment in Spanish Patients Undergoing Percutaneous Coronary Intervention. Pharmacogenomics J. 2019, 19, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.P.; Liew, D.; Lee, V.W.Y. Cost-Effectiveness of Cytochrome P450 2C19 *2 Genotype-Guided Selection of Clopidogrel or Ticagrelor in Chinese Patients with Acute Coronary Syndrome. Pharmacogenomics J. 2018, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; You, J.H. CYP2C19 LOF and GOF-Guided Antiplatelet Therapy in Patients with Acute Coronary Syndrome: A Cost-Effectiveness Analysis. Cardiovasc. Drugs Ther. 2017, 31, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Deiman, B.A.; Tonino, P.A.; Kouhestani, K.; Schrover, C.E.; Scharnhorst, V.; Dekker, L.R.; Pijls, N.H. Reduced Number of Cardiovascular Events and Increased Cost-Effectiveness by Genotype-Guided Antiplatelet Therapy in Patients Undergoing Percutaneous Coronary Interventions in the Netherlands. Neth. Heart J. 2016, 24, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; You, J.H. Review of Pharmacoeconomic Evaluation of Genotype-Guided Antiplatelet Therapy. Expert Opin. Pharmacother. 2015, 16, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Lin, F.J.; Ojo, O.; Rao, S.; Yu, S.; Zhan, L.; Touchette, D.R. Cost-Utility Analysis of Genotype-Guided Antiplatelet Therapy in Patients with Moderate-to-High Risk Acute Coronary Syndrome and Planned Percutaneous Coronary Intervention. Pharm. Pract. 2014, 12, 438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lala, A.; Berger, J.S.; Sharma, G.; Hochman, J.S.; Scott Braithwaite, R.; Ladapo, J.A. Genetic Testing in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention: A Cost-Effectiveness Analysis. J. Thromb. Haemost. 2013, 11, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Reese, E.S.; Daniel Mullins, C.; Beitelshees, A.L.; Onukwugha, E. Cost-Effectiveness of Cytochrome P450 2C19 Genotype Screening for Selection of Antiplatelet Therapy with Clopidogrel or Prasugrel. Pharmacotherapy 2012, 32, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kazi, D.S.; Garber, A.M.; Shah, R.U.; Dudley, R.A.; Mell, M.W.; Rhee, C.; Moshkevich, S.; Boothroyd, D.B.; Owens, D.K.; Hlatky, M.A. Cost-effectiveness of genotype-guided and dual antiplatelet therapies in acute coronary syndrome. Ann. Intern. Med. 2014, 160, 221–232. [Google Scholar] [CrossRef]
- Kim, K.; Touchette, D.R.; Cavallari, L.H.; Ardati, A.K.; DiDomenico, R.J. Cost-Effectiveness of Strategies to Personalize the Selection of p2y12 Inhibitors in Patients with Acute Coronary Syndrome. Cardiovasc. Drugs Ther. 2019, 33, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; You, J.H. CYP2C19 Genotype plus Platelet Reactivity-Guided Antiplatelet Therapy in Acute Coronary Syndrome Patients: A Decision Analysis. Pharmacogenet. Genom. 2015, 25, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; You, J.H. Cost-Effectiveness Analysis of Personalized Antiplatelet Therapy in Patients with Acute Coronary Syndrome. Pharmacogenomics 2016, 17, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.G.; Gruntowicz, D.; Chua, T.; Morlock, R.J. Financial Analysis of CYP2C19 Genotyping in Patients Receiving Dual Antiplatelet Therapy Following Acute Coronary Syndrome and Percutaneous Coronary Intervention. J Manag Care Spec Pharm 2015, 21, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P.; Marston, L. How to Read a Funnel Plot in a Meta-analysis. BMJ 2015, 351, h4718. [Google Scholar] [CrossRef] [PubMed]
- Marquina, C.; Lacaze, P.; Tiller, J.; Riaz, M.; Sturm, A.C.; Nelson, M.R.; Ference, B.A.; Pang, J.; Watts, G.F.; Nicholls, S.J.; et al. Population Genomic Screening of Young Adults for Familial Hypercholesterolaemia: A Cost-Effectiveness Analysis. Eur. Heart J. 2022, 43, 3243–3254. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.J.; Jones, L.K.; Guzauskas, G.F.; Hao, J.; Williams, M.S.; Peterson, J.F.; Veenstra, D.L. Cost-Effectiveness of Population-Wide Genomic Screening for Familial Hypercholesterolemia in the United States. J. Clin. Lipidol. 2022, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.E.; Rupprecht, H.J.; Urban, P.; Gershlick, A.H.; CLASSICS Investigators. Double-Blind Study of the Safety of Clopidogrel with and without a Loading Dose in Combination with Aspirin Compared with Ticlopidine in Combination with Aspirin After Coronary Stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000, 102, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, D.; Xanthopoulou, I.; Deftereos, S.; Hamilos, M.; Sitafidis, G.; Kanakakis, I.; Pentara, I.; Vavouranakis, M.; Davlouros, P.; Hahalis, G.; et al. Contemporary Antiplatelet Treatment in Acute Coronary Syndrome Patients Undergoing Percutaneous Coronary Intervention: 1-Year Outcomes from the Greek Antiplatelet (GRAPE) Registry. J. Thromb. Haemost. 2016, 14, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Gupta, R.; Chakraborty, S.; Mahajan, P.; Bandyopadhyay, D.; Yandrapalli, S.; Zaid, S.; Sreenivasan, J.; Chaturvedi, A.; Mehta, S.S.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection After Percutaneous Coronary Intervention: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Cardiovasc. Revasc. Med. 2022, 41, 115–121. [Google Scholar] [CrossRef]
- Galli, M.; Benenati, S.; Franchi, F.; Rollini, F.; Capodanno, D.; Biondi-Zoccai, G.; Vescovo, G.M.; Cavallari, L.H.; Bikdeli, B.; ten Berg, J.; et al. Comparative Effects of Guided vs. Potent P2Y12 Inhibitor Therapy in Acute Coronary Syndrome: A Network Meta-analysis of 61 898 Patients from 15 Randomized Trials. Eur. Heart J. 2022, 43, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Kiflen, M.; Le, A.; Mao, S.; Lali, R.; Narula, S.; Xie, F.; Paré, G. Cost-Effectiveness of Polygenic Risk Scores to Guide Statin Therapy for Cardiovascular Disease Prevention. Circ Genom Precis Med 2022, 15, e003423. [Google Scholar] [CrossRef] [PubMed]
- Takura, T.; Miura, H. Socioeconomic Determinants of Universal Health Coverage in the Asian Region. Int. J. Environ. Res. Public Health 2022, 19, 2376. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).