Abstract
Background. Pompe disease is a rare, severe, autosomal recessive genetic disorder caused by GAA gene mutations, which cause α-1,4-glucosidase enzyme deficiency. There are two forms of Pompe disease based on the age of onset, the infantile and the adult form (LOPD). Cardiac involvement, previously recognized only in infantile cases, is now also reported in adults. Cardiomyopathy remains an exceptional finding while heart rhythm disorders appear to be more frequent. Methods. We retrospectively evaluated cardiac involvement in 12 patients with late-onset Pompe disease (LOPD) followed for an overall period of 143 years (mean 12.7 ± 7.7) using ECG, Holter ECG, and echocardiography. Results. The mean age of patients (M8:F4) at the first visit was 40.7 ± 16.1 (range 14–63) and 53.7 ± 16.9 (range 21–76) at last visit. Conduction delay was present in three patients; one patient developed ascending aorta ectasia but had a history of hypertension, and one patient showed right heart enlargement on echocardiography, probably due to pulmonary hypertension. No patient died during the FU, nor developed cardiomyopathy. Ectopic supraventricular beats and repeated episodes of ablation-resistant atrial fibrillation were observed in only one patient (8.3%) who required PMK implantation. Conclusions. Benefitting from the long follow-up, this study allows us to state that primary myocardial involvement is rare in patients with LOPD, while rhythm disorders are more frequent and require monitoring to avoid the risk of possible life-threatening complications.
1. Introduction
Pompe disease (PD), also known as acid maltase deficiency or glycogenosis type II, is a rare, severe, autosomal recessive, and progressive genetic disorder belonging to the group of glycogenoses affecting both muscles and heart [1,2,3,4]. PD results from the absence or partial deficiency of the lysosomal acid α-glucosidase (GAA) activity due to mutations in the GAA gene, localized on chromosome 17. GAA (NM_000152.3) is approximately 18.3 kb long and contains 20 exons. Its cDNA has 2859 nucleotides of coding sequence, which encode the immature 952 amino acid enzyme. Currently, more than 560 mutations spread throughout GAA gene have been reported [5,6,7,8,9].
GAA is synthesized as a membrane-bound, catalytically inactive precursor, which is sequestered in the endoplasmic reticulum. It undergoes sugar chain modification in the Golgi complex, followed by transport into the (minor) secretory pathway, or into lysosomes where it is trimmed in a stepwise process at both the amino- and carboxyl-termini domains [10,11,12]. Phosphorylation of mannose residues ensures efficient transport of the enzyme to the lysosomes via the mannose 6-phosphate receptor. GAA catalyzes the hydrolysis of α1→4 glucosidic linkages in glycogen at acid pH. Specificity for the natural substrate (glycogen) is acquired during its maturation [11].
The deficiency of GAA results in progressive storage and accumulation of glycogen, initially within the lysosome but subsequently within the cytoplasm and into muscle inter-fibre free glycogen pools. The initial insult is due to the accumulation of the intra-lysosomal glycogen which causes glycogen burden in lysosomes and triggers cell malfunctions, especially in cardiac, smooth and skeletal muscle cells, and motor neurons [1,2,3,4]. However, recent studies showed that multiple other cellular abnormalities occur and that the pathophysiology of Pompe disease is far more complex than appreciated previously. In particular, the central role of autophagy is becoming more important [13,14].
1.1. Clinical Features
Two forms of Pompe disease have been described based on age of onset of the disease, the infantile or early onset (EOPD) form and the adult or late-onset form (LOPD). Classical Pompe disease at infantile onset (IOPD) is characterized by a more severe clinical course, age of onset of symptoms ≤ 12 months, rapidly progressive hypertrophic cardiomyopathy, left ventricular outflow obstruction, and respiratory muscle weakness, leading to respiratory failure. Untreated patients usually die within the first year of life [11,12].
LOPD, due to partial reduction in GAA enzyme activity, develops in adults, but it may also occur during childhood or adolescence, and may present with a multisystem involvement [15,16,17,18,19,20,21]. A limb-girdle phenotype with axial weakness can be the most common clinical presentation, although respiratory insufficiency or asymptomatic hyperCKemia may be the first indication [22,23].
Creatin kinase (CK) is a catalytic enzyme that combines creatine and ATP to form phosphocreatine and ADP. This reaction is crucial for cellular energy generation and metabolism. HyperCKemia is a persistent rise in serum creatine kinase l evels of at least 1.5 times the upper reference value, as evidenced by a minimum of two measurements at 30-day intervals. The term “asymptomatic hyperCKemia” is used in clinical practice to indicate patients presenting high levels of creatine kinase without any symptoms or signs of neuromuscular impairment [24,25,26].
In LOPD, respiratory muscle dysfunction may precede limb girdle weakness and ventilatory support is indicated prior to wheelchair dependence in about one-third of patients [27,28,29]. Recent evidence shows that diaphragmatic dysfunction cannot only be attributed to myopathic changes but also to accumulation of glycogen in cervical anterior horn cells and alterations of both phrenic nerve fibers and neuromuscular junctions, respectively [30,31].
Cardiac involvement in Pompe disease has long been considered a peculiarity of infantile forms only [15,16]. However, cardiac hypertrophy [32,33], isolated or in association with cerebral injury [34], heart rhythm disturbances—mainly SVT, episodes of atrial fibrillation and Wolff–Parkinson–White Syndrome [35,36,37], and aortic abnormalities [38] are now also recognized and reported in patients with LOPD. Accumulation of glycogen in smooth muscle is thought to be responsible for cerebral artery ectasia, aortic aneurysms, and increased aortic stiffness reported in some individuals [33,35]. Cardiomyopathy remains an exceptional finding, while heart rhythm disorders appear to be more frequent.
Severe cardiac involvement is rare in patients with Pompe disease sharing the common c.-32-13T>G genotype [39].
1.2. Diagnosis and Treatment
An early diagnosis [40,41,42,43], based on the quantitation of the enzyme tested by DBS assay [44] and confirmed by genetic investigation [5,6,7,8,9,45], is desirable given the possibility of a replacement treatment.
Since 2006, GAA deficiency has been treated with enzyme replacement therapy (ERT), which involves regular infusion of recombinant human alglucosidase alfa that prevents the accumulation of glycogen in tissues and muscles.
Long-term treatment with alfa-glucosidase has been shown to markedly slow disease progression and increase life expectancy in children with PD, extending survival as well as ventilation-free survival [46,47,48,49,50]. The most notable effect of ERT is observed on cardiac function regardless of the disease severity [51,52]. In contrast, the skeletal muscle response is variable and less important despite the high dosage in recombinant protein compared to treatments of other lisosomal storage disorders [46,47,48,49,50]. Very early treatment for infantile-onset Pompe disease contributes to better outcomes [53,54].
In adults with Pompe disease, the use of ERT demonstrated in all reports an improvement in the walking distance and a trend toward stabilization of respiratory function [55,56,57]. Semplicini et al. [58] have recently provided further evidence that ERT improves walking abilities and likely stabilizes respiratory function in adults with LOPD with a ceiling effect for the 6MWT in the first 3 years of treatment. These limitations are at least partially due to insufficient uptake into disease-relevant tissues [59]. This therapy is also ineffective in treating neurological aspects of the disorder because of enzymes’ inability to cross the blood–brain barrier. Moreover, severe anaphylactic and immunologic reactions are sometimes observed upon ERT treatment [60].
The limitations of ERT have stimulated the scientific community to investigate alternative therapeutic strategies against PD, independent or complementary to ERT. Among the therapies currently explored, gene therapy, substrate reduction therapy, and pharmacological chaperone (PC) therapy should be mentioned [61,62,63,64].
The main aim of this study was to investigate the occurrence of cardiac involvement in a group of LOPD patients followed for an overall period of 143 years (average FU 12.7 ± 7.7), and to compare the results with those reported so far
2. Materials and Methods
2.1. Patients
Patients reported in the study were selected from the Cardiomyology and Medical Genetics of the Luigi Vanvitelli Campania University Hospital internal registry. The inclusion criteria were diagnosis of late-onset Pompe disease, molecular investigation confirming the diagnosis, and follow-up of at least 3 years. The period of observation was between 1997 and 2023.
2.2. Methods
Scheduled periodic checks were carried out on all patients, and included myological and cardiological examination, as well as spirometric tests to evaluate forced vital capacity (FVC). Retrospectively evaluated cardiac follow-up data, including annual ECG, 24 h Holter monitoring for ECG abnormalities, and echocardiography, were extracted from medical records. The electrocardiographic parameters studied were heart rate (HR), ectopic supraventricular beats (ESVB), ectopic ventricular beats (EVB), supraventricular tachycardia (SVT), runs, and episodes of atrial fibrillation (AF), while the echocardiographic parameters studied were telediastolic volume (TDV) in mL, and left ventricle ejection fraction (LVEF) in percentage.
Informed consent for data collection and publication for research purposes was obtained by the patients, or tutors when minors in the occasion of the blood collection for the genetic test as a consolidated hospital practice.
2.3. Review of the Literature, Search and Selection
A systematic analysis of the literature was conducted to document cardiac manifestations in patients with late-onset Pompe disease. Cardiac involvement was defined as left ventricle hypertrophy, heart rhythm disorders, atrial fibrillation, atrio-ventricular block, and pacemaker placement needs.
The literature search was performed on 20 September 2023 and returned 41 results in PubMed, 0 in Scopus, and 0 in WoS. The PubMed database was queried for studies published through September 2023, by using the National Library of Medicine Medical Subject Heading terms “cardiac involvement”, “cardiac hypertrophy”, “left ventricle hypertrophy”, “rhythm disorders”, “atrio-ventricular block”, “atrial fibrillation” in late-onset Pompe disease. Articles that did not meet the selection criteria or did not answer the research question, were excluded. For a comprehensive analysis, relevant case reports were also included.
3. Results
Demographics and baseline clinical characteristics of the 12 patients included in the study are shown in Table 1.

Table 1.
Patient’s demographics and baseline clinical characteristics.
All patients are from Campania Region in southern Italy. Six patients are isolated cases, while the other six belong to two unrelated families, each with three affected individuals. All patients had a genetically confirmed diagnosis of late-onset Pompe disease. All were compound heterozygotes, and 11/12 (91.7%) had the common c.-32-13T>G variation on one of the two alleles. On the second allele, c. 784G>A variant was present in 2/12 (16.7%) unrelated patients; c.1124 G>T variant was present in 5/12 (41.7%) patients, three of whom were siblings; c.2237 G>A variation was present in the three affected members of the same family; c.956-6T>C and c.989G>A variations were present in isolated patients. Variants c. 784G>A and c.2237 G>A are usually reported in classic infantile Pompe disease.
The mean age at onset of symptoms was 35 ± 13.6 (range 16–54 years) while the average age at the first visit was 40.7 ± 16.1 (range 14–63 years). In the majority of patients (8/12; 66.7%) the presenting symptoms consisted of muscle weakness of the upper or lower limbs, and in one patient (8.3%) myalgia. Three patients (25%) sought medical attention due to increased CK values, ranging from two to five the upper reference limit, in the absence of signs and symptoms of neuromuscular impairment (asymptomatic hyperCKemia).
The average age at the last control was 53.6 ± 16.8 (range 21–76 years). The mean period of follow-up was 12.7 ± 7.7 years (range 3–26 years).
Overall, two out of 12 (16.7%) and 3/12 (25%) patients had respiratory insufficiency (FVC below 50%) at the first and last visit, respectively. Five of them were on noninvasive mechanical ventilation. Six patients (50%) agreed to undergo treatment with enzymatic replacement therapy (ERT) during the FU. The average period of treatment was 55.8 ± 25.6 months (range 6–84). Four patients (33.3%) were still on treatment at the last check-up while two had voluntarily suspended it, respectively after 45 and 57 months.
Table 2 shows the cardiologic parameters collected for all patients at baseline, and at the end of the follow-up.

Table 2.
Cardiologic parameters collected for all patients at baseline, and at the end of the follow-up.
The average heart rate at the first visit was 72.8 ± 11.8 b/m’ (range 50–88) and at the last visit 75.9 ± 8.9 b/m’ (range 55–90); the differences were not statistically different.
No patient, except one, presented arrhythmias. The patient with arrhythmias (NA3) had numerous supraventricular ectopic beats and a run of premature beats at the first Holter ECG check-up (Figure 1). Despite a prompt treatment with beta-blockers and ACE-inhibitors, she presented during the follow-up several episodes of atrial fibrillation, resistant to transcatheter ablation. At the age of 47, she had a pacemaker implanted.
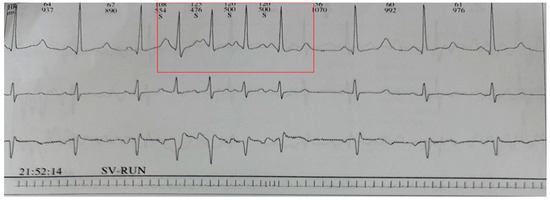
Figure 1.
SV-RUN episode recorded on Holter monitoring. The numbers at the top indicate heart rate and R-R interval, respectively. The letter “S” stands for systole.
No significant changes were observed in heart volumes, the mean value of TDV varying from 49.6 ± 4.6 mL (range 42.4–57.0) at baseline to 50.2 ± 2.5 (range 45.6–54.0) at the last visit.
There was a slight decrease in the percentage values of LVEF, which went from a mean value of 64.9 ± 5.1 (range 54.5–70.0) to 61.3 ± 5.5 (range 55.0–71.6); however, once again, the differences were not statistically significant. There was no cardiac death during the FU. No patient developed cardiomyopathy.
4. Discussion
Cardiac involvement in Pompe disease has long been considered a peculiarity of infantile forms only [15,16]. However, cardiac hypertrophy, heart rhythm disorders, and aortic abnormalities are now also recognized and reported in patients with LOPD [32,33,34,35,36,37,38].
In our cohort of patients, followed for a total of 143 years (mean 12.7 ± 7.7), episodes of arrhythmias and atrial fibrillation, resistant to transcatheter ablation, occurred in only one female (8.3%). She required pacemaker implantation at the age of 47. None developed cardiomyopathy, confirming that cardiomyopathy remains an exceptional finding in patients with LOPD. Interestingly, all but one patient shared the common c.-32-13T>G genotype on one of the two alleles, confirming that this allele is associated with absence of cardiomyopathy [39,65,66].
Review of Literature
Through literature analysis, we identified eight articles by title and abstract screening. After title and abstract screening, four studies and two clinical cases were eligible for full article review.
Van der Beek et al. [39] investigated the presence and extent of cardiac involvement in 22 children and 46 adults with Pompe disease presenting the common IVS1-32-13T>G variation to determine the usefulness of cardiac screening in these patients with relatively ‘milder’ phenotypes. Cardiac dimensions and function were assessed through echocardiography, electrocardiography and Holter monitoring. Among patients with LOPD, one—severely affected—had a mild hypertrophic cardiomyopathy that did not change during ERT. Furthermore, four patients (8.7%) showed minor cardiac abnormalities, which were attributed to advanced age, hypertension or pre-existing cardiac pathologies. The authors concluded that cardiac involvement is rare in patients with Pompe disease sharing the common c.-32-13T>G genotype; nevertheless, they suggested electrocardiographic evaluation as an appropriate initial screening tool, while reserving a thorough cardiac screening only in case of ECG abnormalities or history of cardiac disease.
Boentert M et al. [29] performed a comprehensive cardiovascular magnetic resonance (CMR) in a group of 17 patients with genetically proven late-onset Pompe disease (50 ± 18 years; 11M:6F) and in 18 age- and gender-matched healthy controls (44 ± 10 years; 12M:6F), in order to detect focal and diffuse fibrosis. All patients had normal left ventricular (LV) and right ventricular (RV) volumes and normal LV and RV ejection fraction. Three patients (18%) had non-ischemic LGE in the basal infero-lateral wall, and 21% demonstrated elevated global extracellular volume fraction (ECV) values suggestive of interstitial myocardial fibrosis. Non-specific abnormalities such as left atrial (LA) dilation were present in two patients, while LV hypertrophy was observed only in one. Two of the three LGE-positive patients were also hypertensive. After a median follow-up of 25 (11–29) months, only one cardiovascular event occurred: one of the late gadolinium enhancement (LGE)-positive patients with a high cardiovascular risk profile suffered an acute coronary syndrome. The authors concluded that, in contrast to EOPD, mild and rather non-specific cardiac abnormalities can be detected by CMR only in a small proportion of patients with LOPD, and that the observed structural abnormalities seem the result of an interplay between the storage disease and other comorbidities without affecting short-term to mid-term prognosis.
Herbert et al. [32] in 2018 performed a retrospective review of cardiological parameters (ECG and Echo) in a large cohort, which included 144 patients with LOPD and 40 patients with EOPD from the Duke University Pompe disease registry. Among the adult patients, five (3.5%) presented with arrhythmias: two had atrioventricular blocks of differing degrees, two had supraventricular tachycardia, and one had a right bundle branch block. Echocardiography showed a mild left ventricular hypertrophy (LVH) in 14 patients (9.7%), who had additional cardiovascular risk factors such as hypertension, restrictive lung disease, chronic respiratory failure, type 2 diabetes mellitus, or hyperlipidemia. Left atrial enlargement was seen in four patients (2.8%). Unlike van der Beek et al., the authors concluded that patients with LOPD sharing the common c.-32-13T>G variant require frequent cardiac follow-up for the risk of arrhythmias.
In a cohort of 131 French patients with LOPD, Sacconi et al. [34] retrospectively identified 4 patients (3%) with severe progressive atrio-ventricular blocks requiring pacemaker implantation, at an average age of 44 years (range 35–57). These patients had no other risk factors for cardiovascular diseases or cardiomyopathy. In one patient, the atrioventricular block was found while still asymptomatic. Although A-V blocks are relatively rare in LOPD and can occur even in the absence of cardiac symptoms or ECG abnormalities, the authors suggest that cardiac follow-up in patients with LOPD should include periodic Holter-ECG monitoring, due to the possible life-threatening complications associated with these conduction defects.
Occasional severe post-partum cardiomyopathy or a syncopal episode revealing an underlying dilated cardiomyopathy as isolated presenting features of LOPD have been reported by Mori et al. [35] in a female aged 35 after a pregnancy complicated by primary hyperparathyroidism, and by Walczak-Galezewska et al. [36] in a 54-year-old Caucasian sportsman. The endomyocardial biopsy revealed excess glycogen by PAS staining in one case, while the echo showed multiple storage materials located in the left ventricle with decreased EF in the other one.
5. Conclusions
We are aware that the study has some limitations, which include the small number of patients enrolled and use of retrospective data. However, despite these limitations, we believe that our study, benefitting from the long follow-up, may contribute to reassure clinicians routinely involved in the management of adult patients with LOPD that primary myocardial involvement remains very rare in these patients, while there is a relatively higher frequency of rhythm disturbances that should be monitored to avoid the risk of possible life-threatening complications.
Author Contributions
Conceptualization, L.P. (Luisa Politano); Data curation, A.P. and L.P. (Luisa Politano); Formal analysis, A.P., L.P. (Luisa Politano), M.S., S.M. and A.A.P.; Investigation, A.P., L.P. (Luigia Passamano), M.S., S.M., E.P., A.A.P. and G.N.; Methodology, G.N. and L.P. (Luisa Politano); Supervision, L.P. (Luisa Politano); Validation, G.N.; Writing—original draft, L.P. (Luisa Politano); Writing—review and editing, L.P. (Luisa Politano). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and. The data were de-identified and stored in an electronic file using numeric codes for each individual to facilitate retrospective data analysis, for which as per clause 50 of the new EU General Data Protection Regulation (Directive 95/46/EC) no additional consent is required.
Informed Consent Statement
Informed consent for data collection and publication for research purposes was obtained by all subjects involved in the study or their tutors if minors, in the occasion of the blood collection for the genetic test as a consolidated hospital practice.
Data Availability Statement
Data are available upon reasonable request from the corresponding author and may not become publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hannah, W.B.; Derks, T.G.J.; Drumm, M.L.; Grünert, S.C.; Kishnani, P.S.; Vissing, J. Glycogen storage diseases. Nat. Rev. Dis. Prim. 2023, 9, 46. [Google Scholar] [CrossRef]
- Hirschhorn, R.; Reuser, A. Glycogen storage disease type II: Acid alpha-glucosidase (acid maltase) deficiency. In The Metabolic and Molecular Bases of Inherited Disease; Scriver, C., Beaudet, A., Sly, W., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; Volume 3, pp. 3389–3420. [Google Scholar]
- Reuser, A.J.J.; Kroos, M.A.; Hermans, M.M.P.; Bijvoet, A.G.A.; Verbeet, M.P.; Van Diggelen, O.P.; Kleijer, W.J.; Van Der Ploeg, A.T. Glycogenosis type II (acid maltase deficiency). Muscle Nerve 1995, 18, S61–S69. [Google Scholar] [CrossRef]
- Di Rocco, M.D.; Buzzi, D.; Tarò, M. Glycogen storage disease type II: Clinical overview. Acta Myol. 2007, XXVI, 42–44. [Google Scholar]
- Kroos, M.; Pomponio, R.J.; van Vliet, L.; Palmer, R.E.; Phipps, M.; Van der Helm, R.; Halley, D.; Reuser, A.; The GAA Database Consortium. Update of the Pompe disease mutation database with 107 sequence variants and a format for severity rating. Hum. Mutat. 2008, 29, E13–E26. [Google Scholar] [CrossRef] [PubMed]
- Reuser, A.J.J.; Ploeg, A.T.; Chien, Y.; Llerena, J.; Abbott, M.; Clemens, P.R.; Kimonis, V.E.; Leslie, N.; Maruti, S.S.; Sanson, B.; et al. GAA variants and phenotypes among 1,079 patients with Pompe disease: Data from the Pompe Registry. Hum. Mutat. 2019, 40, 2146–2164. [Google Scholar] [CrossRef] [PubMed]
- Niño, M.Y.; In’t Groen, S.L.; Bergsma, A.J.; van der Beek, N.A.; Kroos, M.; Hoogeveen-Westerveld, M.; Van Der Ploeg, A.T.; Pijnappel, W.P. Extension of the Pompe mutation database by linking disease-associated variants to clinical severity. Hum. Mutat. 2019, 40, 1954–1967. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, Y.; Fuji, N.; Yamazaki, N.; Hirakiyama, A.; Kamioka, T.; Seo, J.-H.; Mashima, R.; Kosuga, M.; Okuyama, T. A molecular analysis of the GAA gene and clinical spectrum in 38 patients with Pompe disease in Japan. Mol. Genet. Metab. Rep. 2017, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- De Filippi, P.; Errichiello, E.; Toscano, A.; Mongini, T.; Moggio, M.; Ravaglia, S.; Filosto, M.; Servidei, S.; Musumeci, O.; Giannini, F.; et al. Distribution of Exonic Variants in Glycogen Synthesis and Catabolism Genes in Late Onset Pompe Disease (LOPD). Curr. Issues Mol. Biol. 2023, 45, 2847–2860. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, S. Trafficking of lysosomal enzymes in normal and disease states. J. Clin. Investig. 1986, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wisselaar, H.A.; Kroos, M.A.; Hermans, M.M.; van Beeumen, J.; Reuser, A.J. Structural and functional changes of lysosomal acid al-pha-glucosidase during intracellular transport and maturation. J. Biol. Chem. 1993, 268, 2223–2231. [Google Scholar] [CrossRef]
- Dasouki, M.; Jawdat, O.; Almadhoun, O.; Pasnoor, M.; McVey, A.L.; Abuzinadah, A.; Herbelin, L.; Barohn, R.J.; Dimachkie, M.M. Pompe Disease: Literature review and case series. Neurol. Clin. 2014, 32, 751–776. [Google Scholar] [CrossRef] [PubMed]
- Malicdan, M.C.V.; Nishino, I. Autophagy in Lysosomal Myopathies. Brain Pathol. 2011, 22, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Raben, N.; Wong, A.; Ralston, E.; Myerowitz, R. Autophagy and mitochondria in Pompe disease: Nothing is so new as what has long been forgotten. Am. J. Med. Genet. Part C Semin. Med. Genet. 2012, 160C, 13–21. [Google Scholar] [CrossRef] [PubMed]
- van den Hout, H.M.; Hop, W.; van Diggelen, O.P.; Smeitink, J.A.M.; Smit, G.P.A.; Poll-The, B.-T.T.; Bakker, H.D.; Loonen, M.C.B.; De Klerk, J.B.C.; Reuser, A.J.J.; et al. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics 2003, 112, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Hwu, W.-L.; Mandel, H.; Nicolino, M.; Yong, F.; Corzo, D.; Infantile-Onset Pompe Disease Natural History Study Group. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006, 148, 671–676.e2. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.P.F.; Hagemans, M.L.C.; Doorn, P.A.; Loonen, M.C.B.; Hop, W.J.C.; Reuser, A.J.J.; Ploeg, A.T. The natural course of non–classic Pompe’s disease; a review of 225 published cases. J. Neurol. 2005, 252, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Toscano, A.; Rodolico, C.; Musumeci, O. Multisystem late onset Pompe disease (LOPD): An update on clinical aspects. Ann. Transl. Med. 2019, 7, 284. [Google Scholar] [CrossRef]
- Hagemans, M.L.C.; Winkel, L.P.F.; Van Doorn, P.A.; Hop, W.J.C.; Loonen, M.C.B.; Reuser, A.J.J.; Van der Ploeg, A.T. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain 2005, 128, 671–677. [Google Scholar] [CrossRef]
- Filosto, M.; Todeschini, A.; Cotelli, M.S.; Vielmi, V.; Rinaldi, F.; Rota, S.; Scarpelli, M.; Padovani, A. Non-muscle involvement in late-onset Glycogenosis II. Acta Myol. 2013, XXXII, 91–94. [Google Scholar]
- Labella, B.; Piccinelli, S.C.; Risi, B.; Caria, F.; Damioli, S.; Bertella, E.; Poli, L.; Padovani, A.; Filosto, M. A Comprehensive Update on Late-Onset Pompe Disease. Biomolecules 2023, 13, 1279. [Google Scholar] [CrossRef]
- Preisler, N.; Lukacs, Z.; Vinge, L.; Madsen, K.L.; Husu, E.; Hansen, R.S.; Duno, M.; Andersen, H.; Laub, M.; Vissing, J. Late-onset Pompe disease is prevalent in unclassified limb-girdle muscular dystrophies. Mol. Genet. Metab. 2013, 110, 287–289. [Google Scholar] [CrossRef]
- Lukacs, Z.; Cobos, P.N.; Wenninger, S.; Willis, T.A.; Guglieri, M.; Roberts, M.; Quinlivan, R.; Hilton-Jones, D.; Evangelista, T.; Zierz, S.; et al. Prevalence of Pompe disease in 3,076 patients with hyperCKemia and limb-girdle muscular weakness. Neurology 2016, 87, 295–298. [Google Scholar] [CrossRef]
- Silvestri, N.J.; Wolfe, G.I. Asymptomatic/pauci-symptomatic creatine kinase elevations (hyperckemia). Muscle Nerve 2013, 47, 805–815. [Google Scholar] [CrossRef]
- Morandi, L.; Angelini, C.; Prelle, A.; Pini, A.; Grassi, B.; Bernardi, G.; Politano, L.; Bruno, C.; De Grandis, D.; Cudia, P.; et al. High plasma creatine kinase: Review of the literature and proposal for a diagnostic algorithm. Neurol. Sci. 2006, 27, 303–311. [Google Scholar] [CrossRef]
- Kyriakides, T.; Angelini, C.; Schaefer, J.; Sacconi, S.; Siciliano, G.; Vilchez, J.J.; Hilton-Jones, D. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur. J. Neurol. 2010, 17, 767–773. [Google Scholar] [CrossRef]
- Berger, K.I.; Chan, Y.; Rom, W.N.; Oppenheimer, B.W.; Goldring, R.M. Progression from respiratory dysfunction to failure in late-onset Pompe disease. Neuromuscul. Disord. 2016, 26, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Mellies, U.; Stehling, F.; Dohna-Schwake, C.; Ragette, R.; Teschler, H.; Voit, T. Respiratory failure in Pompe disease: Treatment with noninvasive ventilation. Neurology 2005, 64, 1465–1467. [Google Scholar] [CrossRef] [PubMed]
- Boentert, M.; Prigent, H.; Várdi, K.; Jones, H.N.; Mellies, U.; Simonds, A.K.; Wenninger, S.; Cortés, E.B.; Confalonieri, M. Practical Recommendations for Diagnosis and Management of Respiratory Muscle Weakness in Late-Onset Pompe Disease. Int. J. Mol. Sci. 2016, 17, 1735. [Google Scholar] [CrossRef]
- DeRuisseau, L.R.; Fuller, D.D.; Qiu, K.; DeRuisseau, K.C.; Donnelly, W.H.; Mah, C.; Reier, P.J.; Byrne, B.J. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc. Natl. Acad. Sci. USA 2009, 106, 9419–9424. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.I.I.; Van Der Beek, N.A.M.E.; Van Doorn, P.A.; Vletter, W.B.; Nemes, A.; Van Dalen, B.M.; Cate, F.J.T.; Van Der Ploeg, A.T.; Geleijnse, M.L. Cardiac involvement in adults with Pompe disease. J. Intern. Med. 2008, 264, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.; Cope, H.; Li, J.S.; Kishnani, P.S. Severe Cardiac Involvement Is Rare in Patients with Late-Onset Pompe Disease and the Common c.-32-13T>G Variant: Implications for Newborn Screening. J. Pediatr. 2018, 198, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Qiu, W.-J.; Lee, J.; Chien, Y.-H.; Hwu, W.-L.; Zschocke, J.; Gibson, K.M. Hypertrophic Cardiomyopathy in Pompe Disease Is Not Limited to the Classic Infantile-Onset Phenotype. JIMD Rep. 2014, 17, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, S.; Wahbi, K.; Theodore, G.; Garcia, J.; Salviati, L.; Bouhour, F.; Vial, C.; Duboc, D.; Laforêt, P.; Desnuelle, C. Atrio-ventricular block requiring pacemaker in patients with late onset Pompe disease. Neuromuscul. Disord. 2014, 24, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Bailey, L.A.; Estrada, J.; Rehder, C.W.; Li, J.S.; Rogers, J.G.; Bali, D.S.; Buckley, A.F.; Kishnani, P.S. Severe Cardiomyopathy as the Isolated Presenting Feature in an Adult with Late-Onset Pompe Disease: A Case Report. JIMD Rep. 2016, 31, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Walczak-Galezewska, M.; Skrypnik, D.; Szulinska, M.; Musialik, K.M.; Skrypnik, K.; Bogdanski, P. Late-onset Pompe disease in a 54 year-old sportsman with an episode of syncope: A case report. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3665–3667. [Google Scholar]
- Hossain, M.A.; Miyajima, T.; Akiyama, K.; Eto, Y. A Case of Adult-onset Pompe Disease with Cerebral Stroke and Left Ventricular Hypertrophy. J. Stroke Cerebrovasc. Dis. 2018, 27, 3046–3052. [Google Scholar] [CrossRef]
- Wens, S.C.A.; Kuperus, E.; Mattace-Raso, F.U.S.; Kruijshaar, M.E.; Brusse, E.; van Montfort, K.C.A.G.M.; de Boer, M.S.; Sijbrands, E.J.G.; van der Ploeg, A.T.; van Doorn, P.A. Increased aortic stiffness and blood pressure in non-classic Pompe disease. J. Inherit. Metab. Dis. 2014, 37, 391–397. [Google Scholar] [CrossRef]
- van der Beek, N.; Soliman, O.; van Capelle, C.; Geleijnse, M.; Vletter, W.; Kroos, M.; Reuser, A.; Frohn-Mulder, I.; van Doorn, P.; van der Ploeg, A. Cardiac evaluation in children and adults with Pompe disease sharing the common c.−32-13T>G genotype rarely reveals abnormalities. J. Neurol. Sci. 2008, 275, 46–50. [Google Scholar] [CrossRef]
- Bembi, B.; Cerini, E.; Danesino, C.; Donati, M.A.; Gasperini, S.; Morandi, L.; Musumeci, O.; Parenti, G.; Ravaglia, S.; Seidita, F.; et al. Diagnosis of glycogenosis type II. Neurology 2008, 71, S4–S11. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Steiner, R.D.; Bali, D.; Berger, K.; Byrne, B.J.; Case, L.E.; Crowley, J.F.; Downs, S.; Howell, R.R.; Kravitz, R.M.; et al. Pompe disease diagnosis and management guideline. Genet. Med. 2006, 8, 267–288. [Google Scholar] [CrossRef]
- Musumeci, O.; Toscano, A. Diagnostic tools in late onset Pompe disease (LOPD). Ann. Transl. Med. 2019, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Ozdamar, S.E.; Koc, A.F.; Tekce, H.D.; Kotan, D.; Ekmekci, A.H.; Sengun, I.S.; Yuceyar, A.N.; Uluc, K. Expert opinion on the diagnostic odyssey and management of late-onset Pompe disease: A neurologist’s perspective. Front. Neurol. 2023, 14, 1095134. [Google Scholar] [CrossRef] [PubMed]
- Er, T.-K.; Chen, C.-C.; Chien, Y.-H.; Liang, W.-C.; Kan, T.-M.; Jong, Y.-J. Development of a feasible assay for the detection of GAA mutations in patients with Pompe disease. Clin. Chim. Acta 2014, 429, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Savarese, M.; Torella, A.; Musumeci, O.; Angelini, C.; Astrea, G.; Bello, L.; Bruno, C.; Comi, G.P.; Di Fruscio, G.; Piluso, G.; et al. Targeted gene panel screening is an effective tool to identify undiagnosed late onset Pompe disease. Neuromuscul. Disord. 2018, 28, 586–591. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, J.C.; Kruijshaar, M.E.; Harlaar, L.; Rizopoulos, D.; van der Beek, N.A.M.E.; van der Ploeg, A.T. Long-term follow-up of 17 patients with childhood Pompe disease treated with enzyme replacement therapy. J. Inherit. Metab. Dis. 2018, 41, 1205–1214. [Google Scholar] [CrossRef]
- Kuperus, E.; Kruijshaar, M.E.; Wens, S.C.; de Vries, J.M.; Favejee, M.M.; van der Meijden, J.C.; Rizopoulos, D.; Brusse, E.; van Doorn, P.A.; van der Ploeg, A.T.; et al. Long-term benefit of enzyme replacement therapy in Pompe disease: A 5-year prospective study. Neurology 2017, 89, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Parini, R.; De Lorenzo, P.; Dardis, A.; Burlina, A.; Cassio, A.; Cavarzere, P.; Concolino, D.; Della Casa, R.; Deodato, F.; Donati, M.A.; et al. Long term clinical history of an Italian cohort of infantile onset Pompe disease treated with enzyme replacement therapy. Orphanet J. Rare Dis. 2018, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- van der Ploeg, A.T.; Clemens, P.R.; Corzo, D.; Escolar, D.M.; Florence, J.; Groeneveld, G.J.; Herson, S.; Kishnani, P.S.; Laforet, P.; Lake, S.L.; et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N. Engl. J. Med. 2010, 362, 1396–1406. [Google Scholar] [CrossRef]
- Strothotte, S.; Strigl-Pill, N.; Grunert, B.; Kornblum, C.; Eger, K.; Wessig, C.; Deschauer, M.; Breunig, F.; Glocker, F.X.; Vielhaber, S.; et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J. Neurol. 2009, 257, 91–97. [Google Scholar] [CrossRef]
- van Capelle, C.I.; Poelman, E.; Frohn-Mulder, I.M.; Koopman, L.P.; Hout, J.M.v.D.; Régal, L.; Cools, B.; Helbing, W.A.; van der Ploeg, A.T. Cardiac outcome in classic infantile Pompe disease after 13 years of treatment with recombinant human acid alpha-glucosidase. Int. J. Cardiol. 2018, 269, 104–110. [Google Scholar] [CrossRef]
- Lecis, M.; Rossi, K.; Guerzoni, M.E.; Mariotti, I.; Iughetti, L. Enzyme Replacement Therapy (ERT) on Heart Function Changes the Outcome in Patients with Infantile-Onset Pompe Disease: A Familial History. Case Rep. Pediatr. 2023, 2023, 8470341. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-F.; Yang, C.C.; Liao, H.-C.; Huang, L.-Y.; Chiang, C.-C.; Ho, H.-C.; Lai, C.-J.; Chu, T.-H.; Yang, T.-F.; Hsu, T.-R.; et al. Very Early Treatment for Infantile-Onset Pompe Disease Contributes to Better Outcomes. J. Pediatr. 2016, 169, 174–180.e1. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.A.; Almalik, M.H.; Mirghani, H.M. Early administration of enzyme replacement therapy for Pompe disease: Short-term follow-up results. J. Inherit. Metab. Dis. 2008, 31 (Suppl. S2), 431–436. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.P.F.; Hout, J.M.P.V.D.; Kamphoven, J.H.J.; Disseldorp, J.A.M.; Remmerswaal, M.; Arts, W.F.M.; Loonen, M.C.B.; Vulto, A.G.; Van Doorn, P.A.; De Jong, G.; et al. Enzyme replacement therapy in late-onset Pompe’s disease: A three-year follow-up. Ann. Neurol. 2004, 55, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Molnár, M.J.; Oláh, B.B.; Visy, K.V.; Grosz, Z.; Sebők, Á.; Dézsi, L.; Almássy, Z.; Kerényi, L.; Jobbágy, Z.; Jávor, L.; et al. A késői kezdetű Pompe-kórban szenvedők enzimpótló kezelésének hosszú távú követése [The long-term follow-up of enzyme replacement treatment in late onset Pompe disease]. Ideggyogy Sz 2020, 73, 151–159. (In Hungarian) [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; von Landenberg, C.; Kuchenbecker, K.; Reimann, J.; Kornblum, C. Long-term effects of enzyme replacement therapy in an elderly cohort of late-onset Pompe disease. Neuromuscul. Disord. 2022, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Semplicini, C.; De Antonio, M.; Taouagh, N.; Béhin, A.; Bouhour, F.; Echaniz-Laguna, A.; Magot, A.; Nadaj-Pakleza, A.; Orlikowski, D.; Sacconi, S.; et al. Long-term benefit of enzyme replacement therapy with alglucosidase alfa in adults with Pompe disease: Prospective analysis from the French Pompe Registry. J. Inherit. Metab. Dis. 2020, 43, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Parenti, G.; Andria, G.; Ballabio, A. Lysosomal Storage Diseases: From Pathophysiology to Therapy. Annu. Rev. Med. 2015, 66, 471–486. [Google Scholar] [CrossRef]
- van Gelder, C.M.; Hoogeveen-Westerveld, M.; Kroos, M.A.; Plug, I.; van der Ploeg, A.T.; Reuser, A.J.J. Enzyme therapy and immune response in relation to CRIM status: The Dutch experience in classic infantile Pompe disease. J. Inherit. Metab. Dis. 2014, 38, 305–314. [Google Scholar] [CrossRef]
- Douillard-Guilloux, G.; Raben, N.; Takikita, S.; Ferry, A.; Vignaud, A.; Guillet-Deniau, I.; Favier, M.; Thurberg, B.L.; Roach, P.J.; Caillaud, C.; et al. Restoration of muscle functionality by genetic suppression of glycogen synthesis in a murine model of Pompe disease. Hum. Mol. Genet. 2009, 19, 684–696. [Google Scholar] [CrossRef]
- Xiao, J.; Westbroek, W.; Motabar, O.; Lea, W.A.; Hu, X.; Velayati, A.; Zheng, W.; Southall, N.; Gustafson, A.M.; Goldin, E.; et al. Discovery of a Novel Noniminosugar Acid α Glucosidase Chaperone Series. J. Med. Chem. 2012, 55, 7546–7559. [Google Scholar] [CrossRef]
- Clayton, N.P.; Nelson, C.A.; Weeden, T.; Taylor, K.M.; Moreland, R.J.; Scheule, R.K.; Phillips, L.; Leger, A.J.; Cheng, S.H.; Wentworth, B.M. Antisense Oligonucleotide-mediated Suppression of Muscle Glycogen Synthase 1 Synthesis as an Approach for Substrate Reduction Therapy of Pompe Disease. Mol. Ther. Nucleic Acids 2014, 3, e206. [Google Scholar] [CrossRef]
- Borie-Guichot, M.; Tran, M.L.; Génisson, Y.; Ballereau, S.; Dehoux, C. Pharmacological Chaperone Therapy for Pompe Disease. Molecules 2021, 26, 7223. [Google Scholar] [CrossRef] [PubMed]
- Kroos, M.A.; Pomponio, R.J.; Hagemans, M.L.; Keulemans, J.; Phipps, M.; DeRiso, M.; Palmer, R.E.; Ausems, M.G.; Van der Beek, N.A.; Van Diggelen, O.P.; et al. Broad spectrum of Pompe disease in patients with the same c.-32-13T->G haplotype. Neurology 2007, 68, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Boentert, M.; Florian, A.; Dräger, B.; Young, P.; Yilmaz, A. Pattern and prognostic value of cardiac involvement in patients with late-onset pompe disease: A comprehensive cardiovascular magnetic resonance approach. J. Cardiovasc. Magn. Reson. 2016, 18, 91. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).